Hearing loss is highly prevalent in older people and can reduce quality of life substantially( Reference Dalton, Cruickshanks and Klein 1 , Reference Lotfi, Mehrkian and Moossavi 2 ), with detrimental effects on emotional health( Reference Li, Zhang and Hoffman 3 – Reference Boi, Racca and Cavallero 5 ) and on cognitive function( Reference Lin, Yaffe and Xia 6 – Reference Lin, Thorpe and Gordon-Salant 8 ). Hearing loss is often described as an inevitable decline that occurs during the ageing process( Reference Dalton, Cruickshanks and Klein 1 , Reference Williamson and Fried 9 ), however, emerging research suggests that potentially modifiable risk factors, including risk factors previously related to CVD risk( Reference Gopinath, Flood and Rochtchina 10 , Reference Torre, Cruickshanks and Klein 11 ), may be associated with a decreased or increased risk of hearing loss. This has prompted investigation into the possibility that certain nutrients and foods may also be associated with the incidence of hearing loss. Higher intake of n-3 fatty acids( Reference Gopinath, Flood and Rochtchina 10 , Reference Curhan, Eavey and Wang 12 ), oily fish( Reference Gopinath, Flood and Rochtchina 10 , Reference Curhan, Eavey and Wang 12 , Reference Rosenhall, Idrizbegovic and Hederstierna 13 ), Mg( Reference Choi, Miller and Tucker 14 ), vitamin intake, including vitamins A( Reference Gopinath, Flood and McMahon 15 ), B12 Reference Houston, Johnson and Nozza (16 – Reference Péneau, Jeandel and Déjardin 18 ) C( Reference Choi, Miller and Tucker 14 ) and E( Reference Gopinath, Flood and McMahon 15 ), antioxidant intake, including β-carotene( Reference Choi, Miller and Tucker 14 ), and moderate alcohol consumption( Reference Popelka, Cruickshanks and Wiley 19 ) have been associated with a lower incidence of hearing loss. Conversely, Shargorodsky et al. ( Reference Shargorodsky, Curhan and Eavey 20 ) found that the overall intake of vitamins C, E and β-carotene was not associated with the incidence of hearing loss in men. Additionally, a high cholesterol intake( Reference Gopinath, Flood and Teber 21 ), a high consumption of food with a high glycaemic load( Reference Gopinath, Flood and McMahon 22 ) and excessive alcohol consumption( Reference Rosenhall, Sixt and Sundh 23 ) have been associated with an increased incidence of hearing loss. However, some of these studies have used only self-reported measures of hearing loss rather than objective measures which are preferable in this context( Reference Shargorodsky, Curhan and Eavey 20 , Reference Curhan, Stankovic and Eavey 24 , Reference Curhan, Eavey and Wang 25 ). Since associations have been reported between the above dietary factors and hearing loss, it seems plausible that certain dietary patterns (DP) may also be associated with hearing loss.
In recent years, DP analysis has become a widely used method of investigating the relationship between diet and disease( Reference Hu 26 ). This technique adopts a holistic approach of examining diet and can be a useful complementary approach when examining specific individual nutrients, to more effectively capture the interaction between various nutrients( Reference Hu 26 , Reference Ambrosini, O’Sullivan and de Klerk 27 ). Two main types of DP analysis can be carried out: a priori and a posteriori methods. A priori methods include dietary indices, such as the Mediterranean diet score, which are based on nutritional recommendations and guidelines( Reference Bountziouka, Tzavelas and Polychronopoulos 28 ), whilst a posteriori methods use multivariate statistical techniques such as factor analysis, principal component analysis (PCA) and cluster analysis( Reference Hu 26 ) to derive the patterns within the dietary data( Reference Bountziouka, Tzavelas and Polychronopoulos 28 ). For this analysis, PCA was performed to derive a posteriori DP.
To the best of our knowledge, there have been no previous studies that examined a posteriori DP in relation to hearing loss. Spankovich et al. ( Reference Spankovich and Le Prell 29 ) carried out a priori analysis and examined the Healthy Eating Index in relation to audiological thresholds, demonstrating an association between a healthy eating pattern and lower high-frequency thresholds in adults aged 20–69 years. However, a posteriori analysis may hold some advantages over a priori analysis, in that this approach is data driven and does not rely on scientific guidelines or nutritional recommendations( Reference Kastorini, Papadakis and Milionis 30 ). The a posteriori approach gives a true representation of what the DP of a given population actually are, however, the DP only represents the cohort they were derived from and may not be generalisable to other populations. In this study, we investigated the association between a posteriori DP and hearing loss in men aged 45–59 years enrolled in the Caerphilly Prospective Study (CaPS)( 31 ). Prevalence of hearing loss was carried out 5 years after dietary assessment. We hypothesised that a healthier DP would be associated with a reduced prevalence of hearing loss.
Methods
Study population
CaPS began recruitment between 1979 and 1983 (baseline). The original cohort consisted of 2512 men who were aged between 45 and 59 years and lived in Caerphilly or the surrounding villages in Wales, UK. No exclusion criteria were applied. Subsequent examinations took place at 5-year intervals( Reference Bainton, Miller and Bolton 32 ). At phase 2, 561 men were lost to follow-up, therefore, an additional 447 men were included in the study, giving a new total of 2398 men( Reference Mertens, Markey and Geleijnse 33 ). The initial aims of the study were to investigate ischaemic heart disease and the associations with various factors including cholesterol, fibrinogen, plasma viscosity, testosterone and insulin( 34 ). The baseline sample of 2512 men were the focus of this study.
Ethics
All CaPS participants gave written informed consent and the study had the approval of the local research ethics committee and adhered to the guidelines contained within the Declaration of Helsinki of 1975 as revised in 1983.
Dietary data
A self-administered, semi-quantitative fifty-six-item FFQ was completed during baseline examination( Reference Yarnell, Fehily and Milbank 35 ). The FFQ measured usual consumption of a range of foods. Frequency and quantity of intake of breads, fats, dairy products, sugar, alcohol, coffee and tea were asked for. For some of these foods, such as fats, dairy products and sugar, average intake per week for the family was requested. For such items, in order to estimate the individual intake, the family intake stated for the FFQ was divided by a ‘total family score’. To obtain this score, an adult or toddler aged 5 years or older was assigned a value of 1, a child aged between 1 and 4 years was given a value of 0·5, and an infant under the age of 1 year was assigned a value of 0 as their contribution to the family intake was assumed to be negligible( Reference Yarnell, Fehily and Milbank 35 ). Frequency alone was measured for cereals, fruits, vegetables, eggs, meat, fish, confectionery and drinks. A 30 % subsample of the men also completed a 7-d weighed food intake (WI) to validate the FFQ as a more robust measure of dietary intake( Reference Yarnell, Fehily and Milbank 35 ). Pearson correlation coefficients were 0·3–0·4 across food items (alcohol 0·75), representing weak but statistically significant correlations( Reference Yarnell, Fehily and Milbank 35 ). Of the 764 men who completed the WI, only 665 men (87 %) had maintained a satisfactory record and were included in this analysis( Reference Elwood, Strain and Robson 36 ). FFQ data were available for the full cohort (2512 men) and WI data were available for 665 men.
Auditory assessment
Pure-tone unaided binaural threshold (dBA) was assessed at four different frequencies (0·5, 1, 2 and 4 kHz) with a Bosch audiometer and sound-reducing cups during the second phase, 5 years after the baseline examination. Audiometric assessment was performed in a community clinic environment where background ambient noise was approximately 50 dBA Reference Gallacher, Ilubaera and Ben-Shlomo (37 ). Assessment began at 0·5 kHz and 50 dBA. The sound level was decreased by 10 dBA until the sound could no longer be detected, and the sound was then increased and decreased by 5 dBA until a threshold level was found. This technique was repeated for other frequencies( Reference Gallacher, Ilubaera and Ben-Shlomo 37 ). The pure-tone average (PTA) threshold was calculated as the average of the four frequencies (0·5, 1, 2 and 4 kHz) and both ears. Hearing loss was defined as PTA0·5–4 kHz >25dB( Reference Gallacher, Ilubaera and Ben-Shlomo 37 ). Audiometric data were available for 1886 men.
Assessment of covariates
Anthropometry parameters, including height and weight, were measured using a Holtain stadiometer and a beam balance, respectively. Blood pressure was recorded by one observer using a Hawksley random zero mercury sphygmomanometer and a full twelve-lead electrocardiogram was performed and Minnesota coded by two experienced readers. The men were invited to attend a clinic the following morning, after fasting overnight, to obtain a venous blood sample from which blood cholesterol was assessed( Reference Yarnell, Patterson and Thomas 38 ). Physical activity at work was estimated using the Health Insurance Plan questionnaire, and leisure time activity was assessed using the Minnesota Leisure Time Activity questionnaire. For the current analysis, a physical activity level (PAL) score was calculated by summing the activity levels at work, activity levels to and from work and leisure time physical activity. Two points were awarded if very active at work, one point was given if occasionally active at work, one point was given if one or more miles were travelled to work through cycling or walking and one point was scored if consistent leisure activity was completed( Reference Yu, Patterson and Yarnell 39 ). Participants were classified as non-smokers, ex-smokers or current smoking, which included pipe or cigar smokers, light cigarette smokers (<15/d), moderate cigarette smokers (15–24/d) or heavy cigarette smokers (≥25/d). Social class was determined according to occupation; manual or non-manual, according to the classification of occupations and coding index (1980)( Reference Fehily, Phillips and Yarnell 40 , 41 ).
Statistical analysis
SPSS Statistics for Windows version 20 (IBM Corp.) was used for all statistical analyses, including t tests, χ 2 tests, logistic and ordinal logistic regression models and PCA. All variables were normally distributed. To carry out PCA, all food and drink items in the FFQ and WI separately were condensed into thirty-two groups (online Supplementary Table S1). In the initial stages of data analysis, to decide which method would give more accurate results, the WI food items were condensed into the same thirty-two food groups as the FFQ food items but also into forty-three food groups, as the WI comprised a larger number of food items. However, it was decided to condense the WI food items into the same thirty-two food groups as the FFQ items for ease of comparison of DP and as a validation of the FFQ.
The process of PCA aggregates food items/groups according to how well they correlate with one another. The decision of how many factors to retain in the analysis was made by examining the break in the scree plot, along with eigenvalues >1 and the interpretability of the factors obtained. Orthogonal varimax rotation was then used to create a simpler structure with greater interpretability( Reference Michels and Schulze 42 ). The DP generated were then named according to the positive and negative factor loadings of food and drink items. Factor loadings <–0·2 in magnitude were considered low and factor loadings >0·2 in magnitude were considered high, which aided naming of the patterns.
Multiple logistic and ordinal logistic regression models were used to examine the association between the DP with hearing loss. DP were divided into fifths and each participant received a quintile ranking for each of the three DP. Hearing loss was first assessed as a dichotomous variable with a cut point of >25 dB PTA threshold to compare hearing loss with no hearing loss (≤25 dB PTA threshold) and examined using logistic regression. Hearing loss was also assessed as an ordinal variable according to the categories: no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB and severe hearing loss >60 dB and examined using ordinal logistic regression.
The models were adjusted for potential confounding factors. These included factors associated with both hearing loss and DP in the current data set. This was assessed by linear regression analysis. Other possible confounders from the current literature were also considered. The models were first adjusted for age (model 1), then again adjusted for occupation (model 2) (in the form of social class; manual or non-manual ( Reference Gallacher, Ilubaera and Ben-Shlomo 37 )) and then further adjusted for the continuous variables: BMI, systolic blood pressure (SBP), PAL score, total cholesterol and the categorical variable; smoking (non-smokers, ex-smokers, pipe or cigar smokers, light cigarette smokers, moderate cigarette smokers or heavy cigarette smokers) (model 3). Other models were investigated, including adjustment for age, occupation, smoking and PAL score, as it is possible the other variables may be intermediate markers rather than confounders; however, the effect sizes were similar, therefore, the model proposed above (model 3) was maintained. Height was also explored as a possible confounder but did not significantly influence the models and therefore was not considered for further analysis. For the WI data, further adjustment for energy intake was explored but did not significantly affect the models, therefore data are not presented. Statistical significance was defined as P<0·05.
Results
Baseline characteristics of all 2512 men recruited for CaPS are shown in Table 1. The men were also grouped into two categories: those with hearing loss (PTA >25 dB) and those with no hearing loss (≤25 dB PTA) (Table 1). Compared with men without hearing loss, men with hearing loss were significantly older, shorter and lighter and were more likely to be current smokers and manual workers. Those with hearing loss and those with no hearing loss had similar BMI, PAL score and total cholesterol levels.
Table 1 Baseline characteristics of all men aged 45–59 years in the Caerphilly Prospective Study grouped according to having hearing loss (pure-tone average (PTA) >25 dB) or no hearing loss (PTA ≤25 dB)*† (Numbers and percentages; mean values and standard deviations)

WI, weighed food intake.
* Independent samples t tests were used for continuous variables and χ 2 tests for categorical variables.
† n-value for WI data no hearing loss n max 236 and hearing loss n max 279.
Three DP were determined by PCA using the FFQ data – traditional, healthy, high sugar/low alcohol. Together, these three DP explained 23·4 % of the total variance. The traditional pattern had high factor loading scores for the following foods: red meat, vegetables, processed meat, whitefish/shellfish, eggs, organ meat, poultry, lard, butter, potato, cheese, milk, oily fish, grains, fried potato, beer, sugar, tap water, soft drinks, tea and confectionery. The healthy pattern was characterised by high positive loadings for cereals, fruit, high-fibre bread, confectionery, vegetables, natural juices, margarine, milk and cream and negative loadings for beer, lard and butter. The high-sugar/low-alcohol pattern had high factor loading scores for tea, sugar, milk, white bread, confectionery, fried potato and negative loadings for wine, other alcoholic drinks, coffee, natural juices, high-fibre bread and beer (online Supplementary Table S2).
Logistic regression analysis of the FFQ data, using a cut point of 25 dB for hearing loss, showed a protective effect for the healthy DP after adjustment for age (OR per fifth=0·78, 95 % CI 0·73, 0·83; P<0·001) and a detrimental effect for the high-sugar/low-alcohol pattern model (OR per fifth=1·12, 95 % CI 1·05, 1·20; P=0·001). The former association was hardly altered after adjustment for potential confounding factors (OR per fifth=0·83; 95 % CI 0·77, 0·90; P<0·001) but was consistent with chance variability for the high-sugar/low-alcohol pattern as this association became insignificant (P=0·16) (Table 2). Similarly, only the healthy DP remained significantly inversely associated with hearing loss after adjustment for potential confounding factors in the ordinal logistic regression analysis of the FFQ data (OR per fifth=0·87, 95 % CI 0·81, 0·94; P<0·001) (Table 3).
Table 2 Logistic regression analysis of the association between hearing loss (pure-tone average >25 dB) and quintiles (Q) of dietary pattern factor score for FFQ data from the Caerphilly Prospective StudyFootnote * (Odds ratios and 95 % confidence intervals)
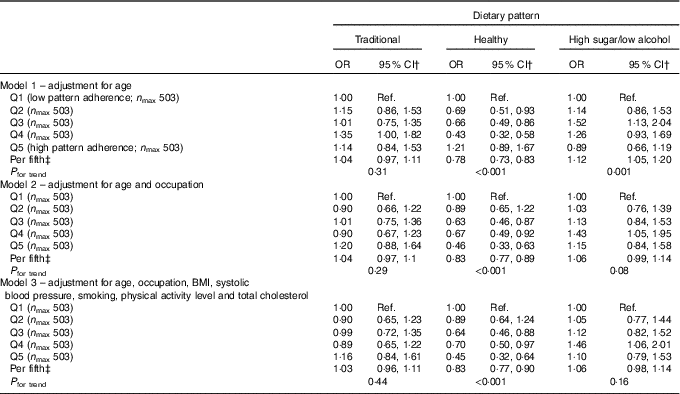
Ref., reference.
* Hearing loss defined as PTA >25 dB.
† OR for hearing loss in comparison to Q1 (reference).
‡ OR for hearing loss per fifth of dietary pattern factor score.
Table 3 Ordinal logistic regression analysis of the association between categories of hearing loss (no hearing loss ≤25dB, mild >25–40 dB, moderate >40–60 dB, severe >60 dB) and quintiles (Q) of dietary pattern factor score for FFQ data from the Caerphilly Prospective StudyFootnote * (Odds ratios and 95 % confidence intervals)
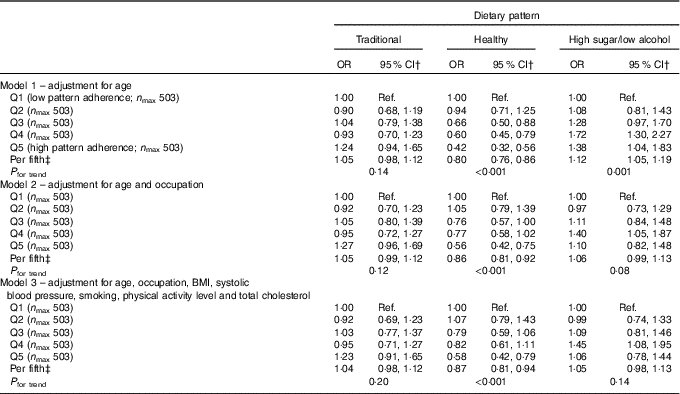
DP, dietary pattern; Ref., reference.
* Categories of hearing loss: no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB and severe >60 dB.
† OR of having greater hearing loss (reference; severe hearing loss) for those in each quintile of DP (reference; Q1).
‡ OR for a one-unit increase in hearing loss category for each higher quintile of DP.
For the ordinal logistic regression, the categories of hearing loss were no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB and severe >60 dB. Data are presented with OR of having greater hearing loss (reference; severe hearing loss) for those in each quintile of DP (reference; Q1). The OR as a trend are also presented which represents a one-unit increase in hearing loss category for each higher quintile of DP.
Three similar DP were determined using factor analysis with the WI data – traditional, healthy, high sugar/low alcohol. Together, these three DP explained 20·2 % of the total variance. The traditional pattern was characterised by high loadings for butter, white bread, lard, sugar, tea, potatoes, red meat and eggs and negative loadings for cooking oil, high-fibre bread, cereals and fruit juices. The healthy pattern had high factor loadings for fruit, wine, confectionery, vegetables, other soft drinks, fruit juices, cheese, high-fibre bread, other alcoholic drinks, cream, coffee and red meat and negative loadings for white bread, tea, sugar and cooking oil. The high-sugar/low-alcohol pattern was characterised by high factor loadings for milk, cereals, tea, confectionery, cream and sugar and negative loadings for beer, organ meat, eggs, red meat, potatoes fried, coffee, oily fish and lard (online Supplementary Table S3).
After adjustment for potential confounding factors using the WI data, logistic regression analysis demonstrated a significant association between the traditional pattern and greater risk of hearing loss (OR per fifth=1·18; 95 % CI 1·02, 1·35; P=0·02) and between the healthy pattern and reduced risk of hearing loss (OR per fifth=0·85; 95 % CI 0·73, 0·99; P=0·03) (Table 4). Ordinal logistic regression analysis of the WI data showed that the traditional DP remained significantly associated with hearing loss after adjustment for potential confounding factors (OR per fifth=1·17, 95 % CI 1·03, 1·33; P=0·02). The healthy DP only lost statistical significance once the model was fully adjusted (OR per fifth=0·87, 95 % CI 0·76, 1·00; P=0·06) (Table 5).
Table 4 Logistic regression analysis of the association between hearing loss (pure-tone average >25 dB) and quintiles (Q) of dietary pattern factor score for weighed food intake data from the Caerphilly Prospective StudyFootnote * (Odds ratios and 95 % confidence intervals)

Ref., reference.
* Hearing loss defined as PTA >25 dB.
† OR for hearing loss in comparison to Q1 (reference).
‡ OR for hearing loss per fifth of dietary pattern factor score.
Table 5 Ordinal logistic regression analysis of the association between categories of hearing loss (no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB, severe >60 dB) and quintiles (Q) of dietary pattern factor score for weighed food intake data from the Caerphilly Prospective StudyFootnote * (Odds ratios and 95 % confidence intervals)

DP, dietary pattern; Ref., reference.
* Categories of hearing loss; no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB, severe >60 dB.
† OR of having greater hearing loss (reference; severe hearing loss) for those in each quintile of DP (reference; Q1).
‡ OR for a one-unit increase in hearing loss category for each higher quintile of DP.
Table 6 provides a summary of the results of the logistic and ordinal logistic regression analyses using both FFQ and WI data. This table shows that for the healthy DP, three out of four analyses showed a statistically significant inverse association with hearing loss, whereas the traditional DP demonstrated a significant positive association with hearing loss in two out of four analyses. The high-sugar/low-alcohol DP was not significantly associated with hearing loss in any analyses in the fully adjusted models.
Table 6 Summary table of logistic and ordinal logistic regression analyses of the association between hearing loss (HL) (pure-tone average (PTA) >25 dB for logistic regression and categories of hearing loss; no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB and severe >60 dB for ordinal logistic regression) and quintiles (Q) of dietary pattern factor score (Q1–Q5) after full adjustment for potential confounding factorsFootnote * for FFQ and weighed food intake (WI) data from the Caerphilly Prospective Study (Odds ratios and 95 % confidence intervals)

* Adjustment for age, occupation, BMI, systolic blood pressure, smoking, physical activity level and total cholesterol.
† Hearing loss defined as PTA >25 dB for logistic regression.
‡ OR for hearing loss per fifth of dietary pattern factor score.
§ Categories of hearing loss; no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB, severe >60 dB.
Discussion
The main finding from this study was that a healthy DP was significantly inversely associated with prevalence of hearing loss. This was found using hearing loss as a dichotomous variable (cut point of 25 dB) for both FFQ and WI data, and using hearing loss as an ordinal variable (no hearing loss ≤25 dB, mild >25–40 dB, moderate >40–60 dB, severe >60 dB) for the FFQ data, with the WI healthy DP only losing statistical significance (P=0·06). Prevalence of hearing loss was assessed 5 years after dietary assessment; however, the findings presented are cross-sectional, therefore, the possibility of reverse causality cannot be discounted.
There was limited evidence showing that a traditional DP was associated with an increased prevalence of hearing loss using logistic and ordinal logistic regression analyses of the WI data but not with the FFQ data. The high-sugar/low-alcohol DP was significantly associated with hearing loss in age-adjusted models for both logistic and ordinal logistic regression analyses of the FFQ data; but after further adjustment, this statistical significance was lost.
Very few studies investigated DP and hearing loss previously. Spankovich et al. ( Reference Spankovich and Le Prell 29 ) investigated the Healthy Eating Index and hearing loss and found that a more healthy diet was associated with better hearing at high frequencies in adults aged 20–69 years. Diet quality has also been examined in relation to concurrent vision and hearing impairment. Individuals in the lowest quintile compared to the highest quintile of dietary score had double the risk of having concurrent vision and hearing impairment( Reference Gopinath, Schneider and Flood 43 ). To our knowledge, a posteriori DP have not previously been examined in relation to hearing loss.
A greater number of studies demonstrated significant associations between dietary factors and hearing loss. A diet high in oily fish( Reference Gopinath, Flood and Rochtchina 10 , Reference Curhan, Eavey and Wang 12 , Reference Rosenhall, Idrizbegovic and Hederstierna 13 ), n-3 fatty acids( Reference Gopinath, Flood and Rochtchina 10 , Reference Curhan, Eavey and Wang 12 ), vitamins and antioxidants( Reference Choi, Miller and Tucker 14 , Reference Houston, Johnson and Nozza 16 – Reference Péneau, Jeandel and Déjardin 18 , Reference Curhan, Stankovic and Eavey 24 ) and moderate alcohol consumption( Reference Popelka, Cruickshanks and Wiley 19 ) has been associated with a reduced risk of hearing loss, which supports our finding that a healthier diet is associated with reduced risk of hearing loss. Conversely, a high cholesterol intake( Reference Gopinath, Flood and Teber 21 ) and a high intake of foods with a high glycaemic load( Reference Gopinath, Flood and McMahon 22 ) have been associated with an increased risk of hearing loss.
Our study has examined DP in relation to hearing loss and not only individual dietary factors. Examining individual nutrients or foods may not be optimal, due to the interaction between nutrients. DP analysis, on the other hand, investigates the synergistic effects of nutrients and foods, producing a more comprehensive approach of examining the overall diet( Reference Ambrosini, O’Sullivan and de Klerk 27 ). DP may be more likely to produce a significant association with the risk of morbidity when compared with single nutrients( Reference Ambrosini, O’Sullivan and de Klerk 27 ). Also, since fewer statistical tests are carried out in DP analysis, there is less likelihood of obtaining results due to chance (type I error)( Reference Ambrosini, O’Sullivan and de Klerk 27 ).
There is the possibility that the associations found between dietary factors and hearing loss are due to the association between CVD risk factors and age-related hearing loss( Reference Torre, Cruickshanks and Klein 11 , Reference Park, Johnson and Shea Miller 45 , Reference Rosenhall and Sundh 46 ). Elevated TAG levels, elevated resting heart rate, low level of HDL-cholesterol, high SBP, a history of smoking and increased BMI have been associated with an increased risk of hearing loss( Reference Torre, Cruickshanks and Klein 11 , Reference Helzner, Patel and Pratt 44 – Reference Park 47 ). It has been hypothesised that the link between CVD risk factors and hearing loss may be due to reduced blood flow to the cochlea of the inner ear, which will reduce the ability to hear optimally( Reference Torre, Cruickshanks and Klein 11 ). Since the above studies( Reference Torre, Cruickshanks and Klein 11 , Reference Helzner, Patel and Pratt 44 – Reference Park 47 ) have shown that there could be a link between CVD risk factors and hearing loss, there is the possibility that certain dietary changes, which are likely to reduce CVD risk, may also have a beneficial influence on hearing loss.
Key strengths of our investigation include its large cohort size of 2512 men. Also, although an FFQ was used to obtain dietary data from the full cohort, a 7-d WI was also completed in a 30 % subset. This robust dietary assessment method is unusual in epidemiological studies. In a previous validation study of the FFQ using the WI, Pearson correlation coefficients were 0·3–0·4 across food items (alcohol 0·75), representing weak but statistically significant correlations( Reference Yarnell, Fehily and Milbank 35 ). Furthermore, hearing loss was measured using audiometry, in contrast to many other studies that have used only self-reported measures( Reference Shargorodsky, Curhan and Eavey 20 , Reference Curhan, Stankovic and Eavey 24 , Reference Curhan, Eavey and Wang 25 ).
Nevertheless, there are several limitations to consider. Only men were investigated in the study and these men were all living in the town of Caerphilly, or surrounding villages in Wales, therefore, the generalisability of the results of this study to the wider population may be limited. Further limitations were that men with conductive hearing loss, otosclerosis etc. were not excluded from the analysis, information on hearing aid use was not collected and information was not available on individuals who were lost to follow-up.
The FFQ was also limited for some food groups, particularly for fruits. This may have resulted in some measurement error in the DP found from the FFQ( Reference Reedy, Wirfält and Flood 48 ). The weighed intake also has some negatives as people may change their dietary habits when they know they are weighing their food and assessing their dietary intake. Also, auditory assessment was not conducted in an optimal setting (i.e. in a soundproof booth), but the technique used was validated in seventy participants with full clinical assessment using a soundproof booth. Good correlations were found between procedures at each of the four frequencies (r 0·69–0·93)( Reference Gallacher, Ilubaera and Ben-Shlomo 37 ). The DP analysis was conducted in the full cohort of 2512 men, whilst audiometric assessment was only carried out in 1886 men. Ideally, the DP analysis would be carried out only in those with audiometric assessment.
Furthermore, although adjustment was made for numerous potential confounding factors, there is still the possibility of residual confounding. In particular, exposure to noise, socio-economic status and CVD risk factors may not have been fully accounted for. Information on chronic conditions such as heart disease, stroke, diabetes and hypertension were collected, however, we did not have access to these data for the current analysis. Occupation (in the form of social class) was adjusted for, which would have accounted for some workplace noise exposure as well as socio-economic status. There is, however, a possibility of residual confounding by exposure to noise as part of employment not accounted for by the social class variable. Several CVD risk factors were also adjusted for, however, these factors may not have been fully accounted for in the current analysis. We determined which factors were associated with both hearing loss and DP to ensure that true confounding factors were adjusted for, and we adjusted for similar potential confounding factors which have been previously used in the literature when examining diet and hearing loss( Reference Gopinath, Flood and Rochtchina 10 , Reference Choi, Miller and Tucker 14 , Reference Gopinath, Flood and McMahon 15 , Reference Shargorodsky, Curhan and Eavey 20 , Reference Gopinath, Flood and McMahon 49 ). Various models were investigated further since it is possible that BMI, SBP and total cholesterol could actually be intermediate markers rather than confounders and that we therefore over-adjusted the models. A model of adjustment for age, occupation, smoking and PAL score was also investigated, excluding possible intermediate markers; however, effect sizes were very similar. Height was also explored in the models as a factor that cannot be affected by recent dietary intake, whereas BMI can; however, this did not greatly influence the models and therefore was not included in any further analyses.
DP analysis can also be relatively subjective and a number of arbitrary decisions were made, including how to best group food items into food groups, the number of patterns to be kept for the final analysis and the naming of the identified patterns( Reference Michels and Schulze 42 , Reference Reedy, Wirfält and Flood 48 ). As an example, we decided to maintain several alcoholic beverage groups, instead of merging them, since health benefits of alcohol beverages have been suggested to differ depending on the type of beverage and the associated pattern of consumption; therefore, these groups were kept separate as we were interested in determining whether these might affect the produced DP.
An advantage of our analysis is the use of both FFQ and WI data, which produced similar DP. Spearman’s correlation coefficients between the DP found using the FFQ and the WI ranged from 0·32 for the traditional patterns and healthy patterns, to 0·4 for the correlation between the high-sugar/low-alcohol patterns, which indicated a fairly weak to moderate but statistically significant correlation.
A recent study that carried out DP analysis in the same CaPS cohort as in this study found some similarities but also some differences( Reference Mertens, Markey and Geleijnse 33 ). They did not name their patterns. Indeed, it was difficult to name the DP in this study, which is one of the limitations of a posteriori DP analysis( Reference Reedy, Wirfält and Flood 48 ). Mertens et al. ( Reference Mertens, Markey and Geleijnse 33 ) found a mainly similar traditional pattern as in our study. Their second DP was different with a high intake of pulses as well as meat, fish, rice, pasta, fruit, vegetables and eggs. Our healthy pattern was similar in some ways with high factor loadings for cereals, vegetables and fruits. There was also a similar third pattern which was characterised by confectionery and sweet foods as well as avoidance of alcohol( Reference Mertens, Markey and Geleijnse 33 ). This analysis, however, differed from our analysis as they used dietary questionnaires from phases 2 and 3 of the CAPS, which took place over a period of 5 years, to generate their DP, hence similar DP would not necessarily be expected( Reference Mertens, Markey and Geleijnse 33 ). A limitation of our study is the derivation of DP from data that are over 40 years old; therefore, as found in the study by Mertens et al., DP would have changed since then( Reference Mertens, Markey and Geleijnse 33 ).
The issue of whether to adjust for energy intake in DP analysis is debatable( Reference Newby and Tucker 50 , Reference Willett, Howe and Kushi 51 ). Energy requirements vary according to age, sex, BMI and PAL; therefore, as the overall food intake will differ by these factors, it is generally considered more appropriate to adjust for energy intake in analyses of dietary intake. Energy adjustment is required for a number of reasons; to control for confounding, to give a measure of dietary composition, not just dietary intake, and to mitigate the effects of measurement error( Reference Gopinath, Flood and McMahon 49 , Reference Willett, Howe and Kushi 51 ). In this current analysis, adjustment for energy intake of the WI data did not appreciably alter effect sizes. In previous studies, adjustment for energy intake did slightly alter factor loadings and derived DP but did not greatly alter the associations with variables such as birthweight( Reference Balder, Virtanen and Brants 52 , Reference Northstone, Ness and Emmett 53 ).
Furthermore, there is a relatively small amount of variance in dietary data explained by the three DP. This could be perhaps due to other DP also being present in this cohort. It was also not possible to measure change in diet or hearing loss as these both were assessed only once. This is a limitation particularly because auditory assessment was assessed only once, that is, 5 years after dietary assessment.
Conclusion
Hearing loss can have a significant detrimental impact on a person’s emotional health and can be very isolating and debilitating. We found that a healthy DP was significantly and inversely associated with the prevalence of hearing loss in older men aged 45–59 years in the CaPS. This demonstrates that a healthy diet may contribute to a reduced risk of hearing loss. However, the role of dietary factors in hearing loss remains to be fully established and warrants further investigation using robust exposure and end point assessment.
Acknowledgements
The present analysis was completed by N. E. G while at Queen’s University, funded by a PhD studentship from the Department for Employment and Learning Funding, Northern Ireland, UK.
J. Y., J. E. G., Y. B.-S. and A. F. designed research; J. Y., J. E. G., A. F. acquisition of data; N. E. G., C. C. P., C. E. N. and N. L. analysed data; N. E. G. wrote paper; C. E. N., C. C. P., J. Y., N. E. G., Y. B.-S. and J. V. W. critical revision of manuscript; and N. E. G. and J. V. W. had primary responsibility for final content.
All authors read and approved the final manuscript. Authors have no potential conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000175









