Introduction
River-dwelling cetaceans are particularly vulnerable to human pressures in many forms because they cannot escape them. Almost everywhere they live, river dolphins are subject to frequent and persistent anthropogenic threats, including chemical pollution, fragmentation of waterways, reduced availability of food resources because of fishing and damming, hunting, and accidental entrapment in fishing nets (Reeves & Martin, Reference Reeves, Martin, Würsig, Thewissen and Kovacs2018). Consequently, the Yangtze river dolphin Lipotes vexillifer was the first cetacean to be rendered extinct by humans (Turvey et al., Reference Turvey, Pitman, Taylor, Barlow, Akamatsu and Barrett2007), and the remaining two taxa of river dolphins in Asia, Platanista gangetica and Platanista minor, are now categorized as Endangered on the IUCN Red List (Braulik et al., Reference Braulik, Smith and Chaudhry2012; Smith et al., Reference Smith, Braulik and Sinha2012).
In South America, the boto or Amazon river dolphin Inia geoffrensis is also categorized as Endangered (da Silva et al., Reference da Silva, Trujillo, Martin, Zerbini, Crespo, Aliaga-Rossel and Reeves2018b). The species has a wide geographical distribution that extends from the Andes to the Atlantic, including hundreds of rivers with varying levels of connectivity in Brazil, Colombia, Ecuador, Bolivia and Venezuela. Some of the more isolated populations have evolved distinct characteristics that may justify recognition as separate species or subspecies. Although the detailed taxonomy of the Amazon river dolphin is not yet resolved, it is certain that there is not one homogenous meta-population (Gravena et al., Reference Gravena, Farias, da Silva, da Silva and Hrbek2014; Hrbek et al., Reference Hrbek, da Silva, Dutra, Gravena, Martin and Farias2014; Siciliano et al., Reference Siciliano, Valiati, Emin-Lima, Costa, Sartor and Dorneles2016).
A continuous 25-year study of botos in and near the Mamirauá Reserve, Amazonas state, Brazil (Fig. 1), has resulted in estimates of key reproductive parameters and annual survival rates (Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013; Martin & da Silva, Reference Martin and da Silva2018). Data from the Reserve show a prolonged and steep population decline (da Silva et al., Reference da Silva, Freitas, Dias and Martin2018a), probably driven by harmful fishery practices. These practices are widespread, and pressures outside the Reserve could be worse, not only because regulations applicable within the Reserve may offer some protection, but because some threats (e.g. damming and gold mining) have no known impact in the Mamirauá region (da Silva et al., Reference da Silva, Freitas, Dias and Martin2018a).
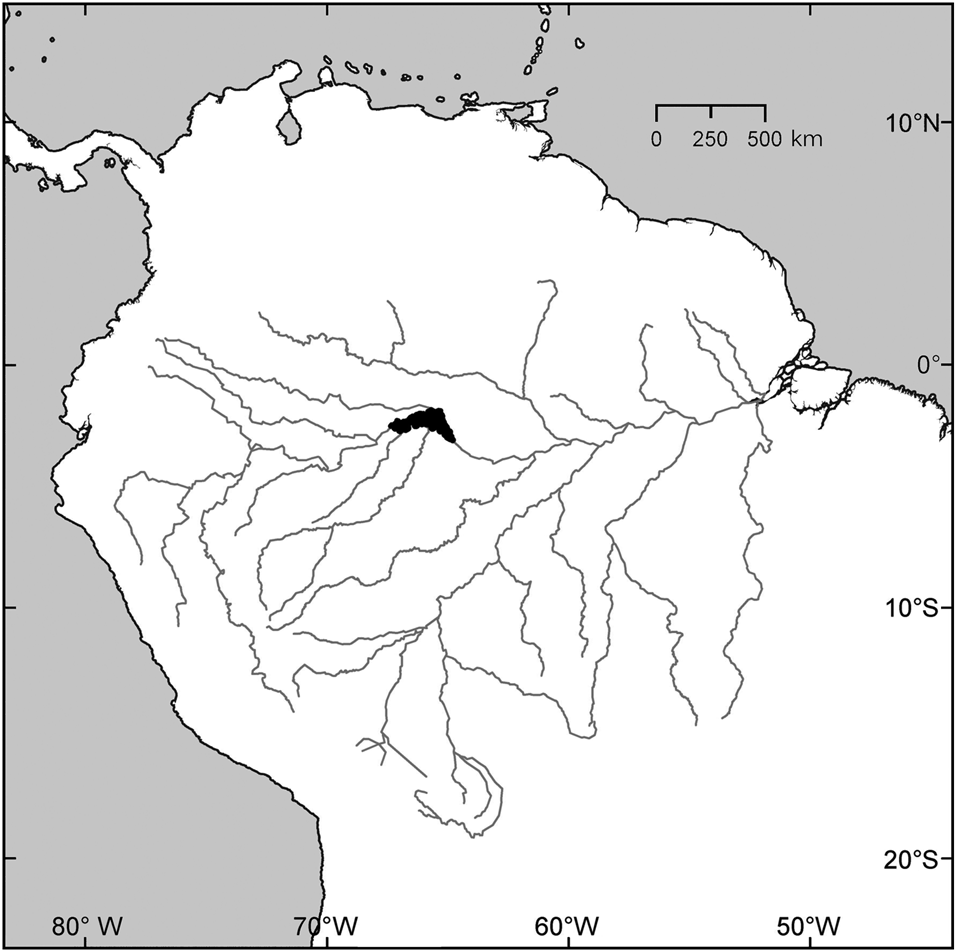
Fig. 1 Northern South America, with the Amazon River and its main tributaries. The study area, the Mamirauá Sustainable Development Reserve, is shown in black.
Estimates of the key reproductive parameters (Martin & da Silva, Reference Martin and da Silva2018) and survival rates (Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013) of botos from Mamirauá provide an opportunity to test whether these population characteristics are consistent with the decline in animal numbers observed during 1994–2017 (da Silva et al., Reference da Silva, Freitas, Dias and Martin2018a).
Study area and species
We collected data in an area of seasonally flooded lowland forest, and in the rivers that drain this landscape, one of which is the mainstem of the Amazon River (Fig. 1). The focus of the work was the Mamirauá Sustainable Development Reserve, Amazonas state, Brazil.
Even the most resident of the botos occurring in the Mamirauá Reserve were often not observed for several consecutive months, and some animals were not recorded for several years before returning. Trained observers were on the water for 7 hours nearly every day throughout the year, thus any marked individual not observed for several weeks was probably absent from the study area, which has a diameter of c. 90 km and an area of > 6,000 km2. Botos marked in Mamirauá were routinely encountered tens of km away from the Reserve, and the prolonged absence of some individuals suggests that they range over an extensive area. This is supported by data obtained via satellite tracking of Amazon river dolphins in Brazil, Colombia and Bolivia, which demonstrated that the animals routinely move tens or hundreds of km (Mosquera Guerra et al., Reference Mosquera Guerra, Trujillo, da Costa, Marmontel, Armenteras and Usma Oviedo2018). Botos occurring within the Mamirauá Reserve on any particular day therefore represent a sample of a population that ranges over at least tens of thousands of km2 of the Brazilian Amazon.
Methods
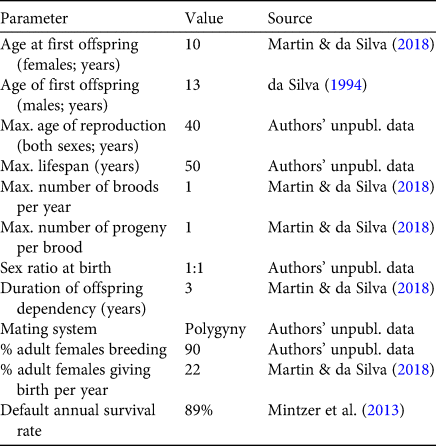
We used Vortex 10.3.5.7 (Lacy & Pollak, Reference Lacy and Pollak2017) to predict population trajectories using reproductive and survival data collected in and near the Mamirauá Reserve during 1994–2018 (Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013; Martin & da Silva, Reference Martin and da Silva2018). Vortex is a Monte Carlo simulation package that models the effects of deterministic factors and demographic, environmental and genetic stochasticity on population dynamics. We used 1,000 model runs (Lacy et al., Reference Lacy, Miller and Traylor-Holzer2015) in each of six scenarios that differed only in the value for the annual survival rate (three options) and whether or not breeding depression was assumed at low population numbers (two options: yes or no). A stable age distribution was assumed for all models. For the survival rate we used the estimate for this population of 89% from Mintzer et al. (Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013) and two more conservative but plausible values of 88 and 86%. Model runs assumed that all input parameters remain constant over time, and predicted the boto population size over a period of 200 years (Lacy et al., Reference Lacy, Miller and Traylor-Holzer2015), which represents > 9 generations (Moore et al., Reference Moore, Martin and da Silva2018). The current population size of the species is not known. For the simulation we set the initial population size at 50,000, and defined extinction as the point when one sex was eliminated. No additional harvest of river dolphins was added to the model. Carrying capacity was assumed to be considerably greater than the initial population size. To be conservative, no inbreeding depression was allowed for, and the Allee parameter (A) and steepness parameter (B) were set to default values of 1.0 and 2.0, respectively (Lacy et al., Reference Lacy, Miller and Traylor-Holzer2015). Input parameters for the model are shown in Table 1. In simulations 1–3, no allowance was made for possible density-dependent impacts on breeding. In simulations 4–6, the assumption was made that the percentage of animals involved in breeding is reduced to 50% when the population is severely depleted.
Table 1 Input values for the Vortex model. All values derive from the authors’ long-term study of > 650 marked animals in and near the Mamirauá Reserve, Amazonas state, Brazil.
Results
Outputs of the model runs are shown in Table 2. The year of population extinction was conservatively defined as the point at which 95% of model iterations predicted extinction. This value is little affected by the estimated initial population size; in Simulation 1 (Table 2) it increases from year 165 to year 173 of the model run (a 5% difference) when initial population size is doubled to 100,000. Because the current population size is not known, thresholds of low population levels were expressed as percentages of original population size (5 and 1%).
Table 2 Results of model runs. The values in the bottom three rows represent the number of years from the start of the model run when the stated condition was reached. For example, in Simulation 4 the model predicted that 1% of the starting population would remain after 50 years.

Figure 2 illustrates the variation in predicted population size over 120 years when the model is run 1,000 times. In this scenario, annual survival is set at 89% and no corrections are made for depressed breeding at low population levels (i.e. as represented in Simulation 1).
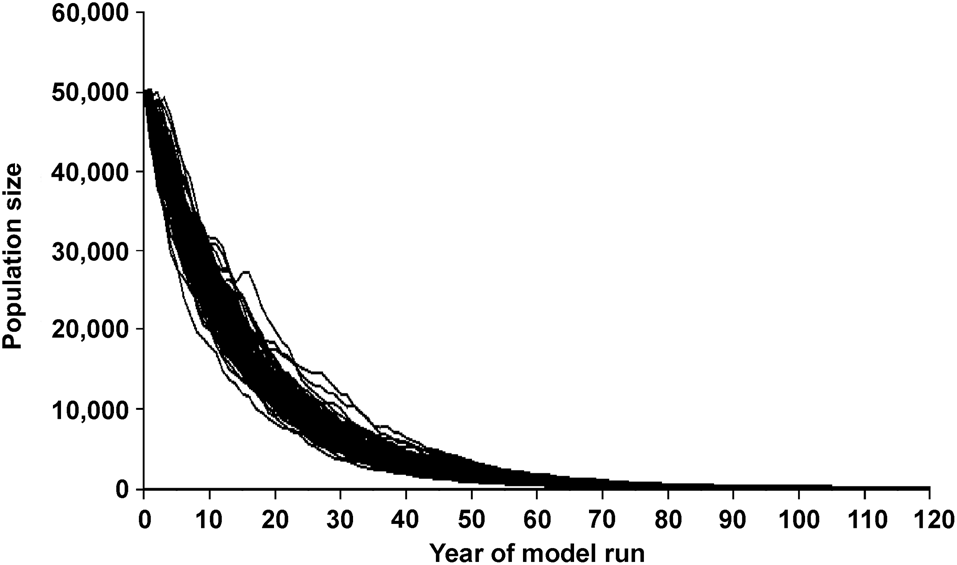
Fig. 2 Predicted population size of the Amazon river dolphin Inia geoffrensis over 120 years when the starting population is 50,000, the survival and reproductive rates are as published by Mintzer et al. (Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013) and Martin & da Silva (Reference Martin and da Silva2018), and no corrections are made for depressed breeding at low population levels (as represented in Simulation 1). This graph illustrates the variation in predicted values generated by the Vortex package over 1,000 model runs.
Figure 3 shows the estimated mean and standard deviation of population size over time in Simulations 1 and 3 (Table 2). The two curves illustrate the range of results obtained from the 6 simulation runs. The upper curve (Simulation 1) is based on published data from the Mamirauá study (Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013; Martin & da Silva, Reference Martin and da Silva2018) and shows the likely population trend if conditions for dolphins do not worsen with time.
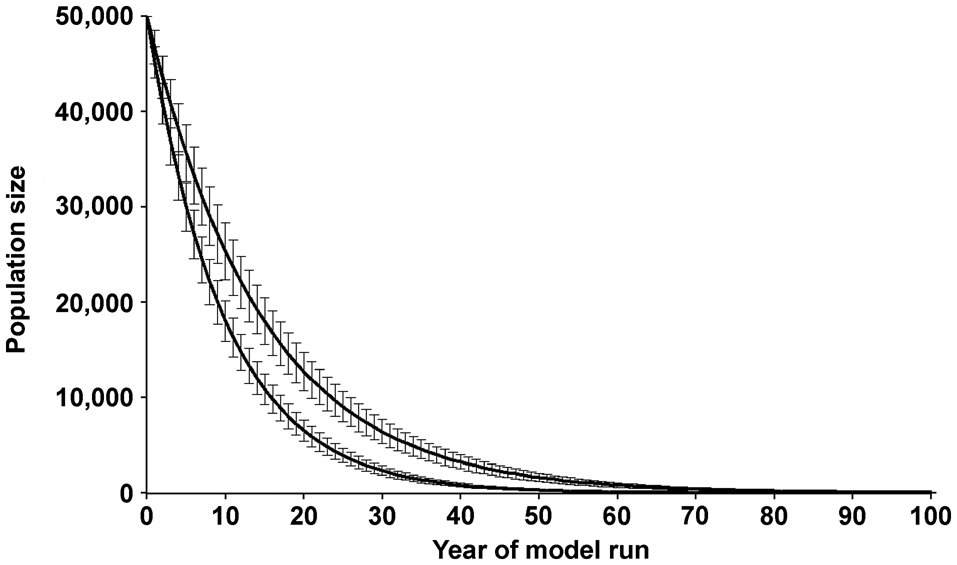
Fig. 3 Predicted population size of the Amazon river dolphin over 100 years, with annual survival rates of 89% (Simulation 1, upper curve: Mintzer et al., Reference Mintzer, Martin, da Silva, Barbour, Lorenzen and Frazer2013) and 86% (Simulation 3, lower curve). The mean values and standard deviation of 1,000 model runs are shown. The upper curve represents the same model runs as in Fig. 2. No allowance was made for possible breeding depression at low population numbers. For clarity, only two of the six model runs are shown here, but the remaining four are close to the curves shown.
Discussion
The annual rate of population decline predicted by the model with default input (Simulation 1) was 5.5%. This is similar to the average 5.48% annual decline of botos observed over 22 years (da Silva et al., Reference da Silva, Freitas, Dias and Martin2018a). Our findings demonstrate that three independent data sources (standardized counts, estimates of survival of marked animals and estimates of reproductive parameters) lead to the same conclusion. In this population, fewer botos are being recruited into the breeding population than are being removed from it, and the consequent decline is rapid and persistent (Figs 2 & 3). In each of the six simulation scenarios (Table 2), all of which make the optimistic and perhaps unrealistic assumption that environmental conditions will not become less suitable for this species, the initial population is reduced by at least 95% in < 50 years.
Although the observed and predicted rates of population change are consistent, and it is therefore reasonable to assume that the model is working with accurate input parameters, the long-term predictions of population size may not be accurate because circumstances are likely to change. Most importantly, the number of people using these waterways as a source of food and/or for transport, or whose activities pollute the water with sewage, noise and chemical or physical waste, is increasing. At the current rate, the human population of the state of Amazonas, which covers 22% of the Amazon basin, is expected to double every 79 years (IBGE, 2019). It is therefore likely that the number of gillnets, in which dolphins often drown, will increase, and that the density of fish, including those species that the dolphins feed on, will decrease. Although new dams are not planned for the area around Mamirauá, 227 new hydro-electric dams are planned for the Amazon basin as a whole (Castello & Macedo, Reference Castello and Macedo2016; Forsberg et al., Reference Forsberg, Melack, Dunne, Barthem, Goulding and Paiva2017), almost all of which can be expected to have a negative impact on river dolphins, directly or indirectly (Araújo & Wang, Reference Araújo and Wang2015). As a consequence of these changes, annual survival rates of dolphins are likely to decrease. This model predicts that the impact of reducing annual survival by just 1% brings forward the point at which 95% of model iterations predict extinction to year 141 of the model run. If survival is reduced by 3% this happens in year 110 even if no breeding depression at low population levels is assumed. The equivalent figures with breeding depression are year 108 and 88, respectively.
Predicting when extinction is likely to occur is dependent upon a number of assumptions that cannot be tested, but the models leave no reasonable doubt that extinction will occur unless key population parameters improve. Reproductive characteristics are unlikely to be influenced by human-induced change at present, but survival rates can be. Although many different anthropogenic factors negatively affect boto populations, fishing practices cause the greatest damage. Botos are drowned in fishing nets, especially monofilament gillnets. Despite laws prohibiting it, they are also deliberately killed, especially for use as fish bait (da Silva et al., Reference da Silva, Martin and do Carmo2011; Iriarte & Marmontel, Reference Iriarte and Marmontel2013a,Reference Iriarte and Marmontelb; Brum et al., Reference Brum, da Silva, Rossoni and Castello2015). Enforcement of existing fishery laws throughout the species’ range would increase its chances of survival. In addition, measures to reduce mortality in gillnets are necessary, particularly during the calving season. There is evidence to suggest that a large proportion of calves die in the first few months of life (Martin & da Silva, Reference Martin and da Silva2018), and drowning because of entanglement in gillnets is probably a major contributor to this mortality. The peak of calving and early calf care in September–November coincides with low water levels, when most botos are confined to edges of the main rivers and when high densities of large-mesh gillnets are set to catch fish there (Martin & da Silva, Reference Martin and da Silva2018). Neonate botos are neither sufficiently experienced to avoid gillnets, nor strong enough to escape them once entangled. A single encounter with a gillnet during the first 3 months of life would thus likely be fatal.
Another important threat to the long-term survival of boto populations is the segmentation of rivers by dams across their range. These structures have profoundly damaging impacts on the ecological functioning of rivers (Lees et al., Reference Lees, Peres, Fearnside, Schneider and Zuanon2016; Forsberg et al., Reference Forsberg, Melack, Dunne, Barthem, Goulding and Paiva2017), and harm dolphins by disrupting fish migration and preventing genetic exchange between dolphins either side of the dam. The scale of dam building planned for the Amazon basin (Forsberg et al., Reference Forsberg, Melack, Dunne, Barthem, Goulding and Paiva2017), if realized, is likely to increase the rate of decline of botos and advance the date of their extinction.
Although the present population trajectory points towards extinction, there is still time to reverse the decline. Botos are still in sufficient abundance for reproduction to occur normally, and as yet relatively few rivers are dammed to the extent that animal movement is constrained, so genetic interchange is continuing. But the results of this study demonstrate that effective action must soon be taken by the range states of the Amazon river dolphin if this iconic animal is to survive. One important first step was taken by the government of Brazil: the banning of a commercial fishery for a scavenging catfish, the piracatinga Calophysus macropterus, for which large numbers of botos were killed for use as bait. This ban (IBAMA, 2014) commenced in 2015 for a 5-year period, and was largely, although not entirely, effective. Given the rate of decline of boto populations, the renewal of the ban on a permanent basis is crucial; a necessary, but not sufficient, measure to address anthropogenic dolphin mortality. Effective control of the use of the most damaging gillnet types will also be needed urgently if boto calves are to survive in sufficient numbers to replace adult losses.
Acknowledgements
This study was part of Projeto Boto, a cooperative agreement between the National Amazon Research Institute (Instituto Nacional de Pesquisas da Amazônia; INPA/MCTIC) and the Mamirauá Sustainable Development Institute (MSDI-OS/MCTIC). We thank the many interns of Projeto Boto who contributed to data collection, and Admisson M. Carvalho, who participated in 90% of the surveys. Funding was provided by INPA/Ministério da Ciência, Tecnologia, Inovações e Comunicações (MCTIC), and Associação dos Amigos do Peixe-boi (AMPA)/Petrobras Socioambiental Program.
Author contributions
Study design, fieldwork: ARM, VMFdS; data analysis, writing: ARM.
Conflicts of interest
None.
Ethical standards
The research abided by the Oryx guidelines on ethical standards, and was approved by the Ethical Standards Committee of the Brazilian Government Institute under which it was carried out (Instituto Nacional de Pesquisas da Amazônia, Manaus).







