The prevalence of overweight and obesity has risen in recent years, causing a worldwide public health problem due to an increased risk for the metabolic syndrome and development of type 2 diabetes, CVD and cancer. This risk can be significantly lowered by losing weight( Reference Bessesen 1 ). Usually, losing weight can be readily achieved, but sustaining the lower weight is problematic. Successfully maintaining weight loss, defined as ‘keeping off an intentional loss of at least 10 % body weight for at least one year’( Reference Wing and Hill 2 ), is proven to be difficult. In general, up to 80 % of the people are unsuccessful( Reference Wu, Gao and Chen 3 , Reference Barte, ter Bogt and Bogers 4 ) and their risk for metabolic complications after weight regain appears even higher than that at the start of the weight-loss period( Reference Blomain, Dirhan and Valentino 5 , Reference Delahanty, Pan and Jablonski 6 ). It is therefore of great importance to understand the mechanisms that influence the risk for weight regain. Many studies have already shown the involvement of various psychosocial and lifestyle factors in weight maintenance such as motivation to lose weight, social support, physical activity and eating habits( Reference Wu, Gao and Chen 3 , Reference Elfhag and Rossner 7 ). Other studies have shown effects of physiological and molecular parameters on weight maintenance – for example, increased insulin sensitivity after weight loss is associated with weight regain( Reference Yost, Jensen and Eckel 8 , Reference Wing 9 ). In addition, fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) are associated with weight regain( Reference Wong, Holst and Astrup 10 ). Wang et al.( Reference Wang, Menheere and Astrup 11 ) showed that men with the metabolic syndrome at baseline were more at risk for weight regain than men without this condition. Further, it has been shown that a higher protein intake after weight loss improves weight maintenance( Reference Aller, Larsen and Claus 12 ). Irisin levels decrease when body weight is reduced but returns to baseline levels in subjects regaining the lost weight( Reference Crujeiras, Pardo and Arturo 13 ). All these findings indicate the involvement of various factors in weight regain or maintenance after weight loss. Studies have pointed at special roles of sex hormones( Reference Wang, Menheere and Astrup 11 ), metabolic factors( Reference Wang, Menheere and Astrup 11 , Reference Wang, Holst and Wodzig 14 ), hunger and satiety hormones( Reference Crujeiras, Goyenechea and Abete 15 ) as well as epigenetic modifications such as methylation of the neuropeptide Y and pro-opiomelanocortin gene promoters in maintenance of weight loss( Reference Crujeiras, Campion and Diaz-Lagares 16 ). The present study focused particularly on the adipose tissue as a key player for weight regain or maintenance after weight loss.
Baseline BMI, fat mass and plasma leptin concentrations are associated with increased risk for weight regain, indicating an important role for adipocytes( Reference Vogels, Diepvens and Westerterp-Plantenga 17 , Reference Vogels and Westerterp-Plantenga 18 ). Mauriège et al.( Reference Mauriège, Imbeault and Doucet 19 ) showed that metabolic parameters of the subcutaneous adipose tissue are related to weight regain. Change of lipoprotein lipase activity during weight loss was negatively related to weight regain in women, whereas change of alpha 2 adrenergic receptor (α2-AR) was positively related to weight regain in men. The latter effect seems to occur despite the fact that adrenaline-stimulated lipolysis returns to pre-diet levels during the weight-maintenance phase( Reference Koppo, Siklova-Vitkova and Klimcakova 20 ). In a weight loss-maintenance study, Verhoef et al.( Reference Verhoef, Camps and Bouwman 21 ) observed that the change in weight during follow-up was related to a change in the levels of the lipolytic enzyme adipose triglyceride lipase in adipose tissue during weight loss. Compared with lean people, the subcutaneous adipose tissue of obese people shows higher endoplasmic reticulum (ER) stress at the level of proteins and gene expression( Reference Boden, Duan and Homko 22 ). Sharma et al.( Reference Sharma, Das and Mondal 23 ) reported a positive correlation between BMI and activating transcription factor 67 (ATF67)-induced ER stress markers. Human individuals losing weight after a gastric bypass showed decreased levels of ER stress in the adipose tissue, linking weight changes directly to ER stress( Reference Gregor, Yang and Fabbrini 24 ). Although those findings suggest that cellular stress is a consequence of weight gain, it might well be that cellular stress is also a factor that stimulates accumulation of fat. In fact, reactive oxygen species promote the initiation of adipogenesis as well as the terminal differentiation of adipocytes( Reference Krautbauer, Eisinger and Hader 25 , Reference Liu, Chan and Higuchi 26 ). In this regard, we hypothesised that cellular stress of adipocytes could also play a role in the risk for weight regain after weight loss. In order to investigate this, we have compared levels of stress-related proteins in the adipose tissue from overweight men and women during weight loss and follow-up. In addition, we compared in vivo findings with observations from in vitro cultured adipocytes after glucose restriction. As stress markers, we selected eight different proteins, which are all involved in different aspects of cellular stress; these were β-actin, binding Ig protein (BiP), calnexin, heat shock protein (HSP) 27, HSP60, HSP70, superoxide dismutase (SOD) 1 and SOD2. β-Actin is a component of actin microfilaments that provide structural support and mediate cellular motion( Reference Shawlot, Deng and Fohn 27 ). BiP is involved in translocation, folding and assembly of secretory and transmembrane proteins within the ER( Reference Kleizen and Braakman 28 ). Calnexin is a Ca-binding protein involved in proper folding of glycoproteins in the ER( Reference Guerin, Arseneault and Dumont 29 , Reference Bousette, Abbasi and Chis 30 ). HSP27 activates the proteasome to degrade unnecessary or damaged proteins( Reference Garrido 31 , Reference Horman, Galand and Mosselmans 32 ), whereas HSP60 acts as a chaperonin for proteins to be transported to the mitochondrion( Reference Boden, Duan and Homko 22 ). HSP60 is also released because of inflammatory stress to exert autocrine/paracrine effects on adipocytes( Reference Marker, Sell and Zillessen 33 ). HSP70 binds to misfolded proteins after stress-induced protein damage( Reference Mayer, Ciechanover and Rechsteiner 34 ). SOD 1 and 2 catalyse conversion of superoxide radicals into peroxide and O2 to defend the cell against oxidative stress( Reference Toichi, Yamanaka and Furukawa 35 ). These specific proteins have been selected because several studies have already shown that they can be regarded as markers of cellular stress, such as ER stress and oxidative stress( Reference Shawlot, Deng and Fohn 27 – Reference Bousette, Abbasi and Chis 30 , Reference Marker, Sell and Zillessen 33 , Reference Toichi, Yamanaka and Furukawa 35 ). Furthermore, based on proteomics observations, Wang et al.( Reference Wang, Bouwman and Mariman 36 ) have suggested an association between these proteins and cellular stress.
Our objective was to test the hypothesis that altered expressions of the above-mentioned proteins during weight loss and follow-up are related to the risk for weight regain.
Methods
Subjects and study design
The eighteen subjects of the present study (nine females, nine males), aged 20–55 years with a BMI of 27–39 kg/m2, were selected from a larger cohort, who underwent a weight loss-maintenance intervention( Reference Verhoef, Camps and Bouwman 21 ). This study was conducted according to the Declaration of Helsinki guidelines and is registered on ClinicalTrials.gov (registration number: NCT01015508). All procedures involving human participants were approved by the Central Committee on Human Research and by the Medical Ethical Committee of Maastricht University, The Netherlands. Written informed consent was obtained from all the subjects.
Selection of the eighteen subjects was based on their weight change throughout the intervention. In short, subjects were healthy, non-smokers and were not using medications (except for women using oral contraceptives). All subjects maintained a stable weight for three months before the intervention. Participants followed an 8-week, very low-energy diet with about 2·1 MJ/d (Modifast; Nutrition et Santé Benelux). The diet provided 50 g carbohydrates, 52 g proteins, 7 g fat and a micronutrient content, which met the Dutch recommended daily intake. Following weight loss, subjects were instructed to maintain their new body weight for a period of 10 months without following a prescribed diet. However, subjects did receive advice on monitoring and limiting food intake. At three time points – before diet (t0), after diet (t2) and after a 10-month follow-up (t12) – adipose tissue biopsies and plasma samples were obtained. Body weight was measured in underwear after overnight fasting using a calibrated scale of the BodPod®.
Test subjects were classified into two groups according to the percentage weight loss during the diet and percentage weight regain during follow-up: weight maintainers (WM) and weight regainers (WR). Participants were categorised as WM if there was a weight reduction of at least 10 % but then regained <6 % weight during follow-up. Participants were categorised as WR if there was a weight reduction of at least 10 % but then regained 6 % or more weight.
Adipose tissue biopsies and protein isolation
Abdominal subcutaneous adipose tissue biopsies were obtained by needle biopsy under local anaesthesia (2 % lidocaine; Fresenius Kabi) after an overnight fast. Tissue samples were immediately rinsed in saline, frozen in liquid N2 and stored at −80°C until protein isolation.
About 350 mg of frozen adipose tissue was ground in a mortar with liquid N2. The powder was dissolved in 200 μl of 8 m-urea, 2 % (w/v) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and 65 mm-dithiothreitol/100 mg biopsy and vortexed for 5 min. The homogenate was centrifuged for 30 min at 14 000 rpm at 10°C. The supernatant was carefully collected, aliquoted and stored at −80°C until Western blotting. Protein concentrations were determined by a Bradford-based protein assay (Bio-Rad).
In vitro cell culture experiments
Human Simpson–Golabi–Behmel syndrome (SGBS) cells were cultured and differentiated as described previously( Reference Wabitsch, Brenner and Melzner 37 ). In brief, SGBS pre-adipocytes were cultured in a T25 flask till 90 % confluence was achieved in Dulbecco's modified Eagle's medium: Nutrient Mixture F-12 (DMEM/F12) medium supplemented with 1 % penicillin/streptomycin (Life Technologies), 10 % fetal bovine serum (Bodinco), 66 nm-biotin and 33 nm-d-pantothenic acid (Sigma-Aldrich). Confluent pre-adipocytes were split into two 150-mm petri dishes: a starvation dish and a control dish. In parallel, the two dishes were cultured until 80–90 % confluence was achieved in the same medium as described above. The medium was changed every 2–3 d. To induce differentiation, confluent pre-adipocytes were washed with PBS buffer and the medium was changed to serum-free DMEM/F12 medium containing 0·5 mm-3-isobutyl-1-methylxanthine (IBMX), 25 nm-dexamethasone, 2 µm-rosiglitazone, 0·01 mg/ml human transferrin, 20 nm-insulin, 100 nm-cortisol and 0·2 nm-triiodothyronine (Sigma-Aldrich). After 4 d, cells were further cultured in serum-free DMEM/F12 medium containing 20 nm-insulin, 100 nm-cortisol, 0·01 mg/ml human transferrin and 0·2 nm-triiodothyronine. Every 2 d, the medium was refreshed. After 14 d, 65–80 % of the pre-adipocytes differentiated into mature adipocytes.
For starvation experiments, mature adipocytes were cultured in DMEM/F12 medium without glucose and supplemented with 1 % penicillin/streptomycin, 20 nm-insulin and 0·55 mm-glucose for a period of 96 h as glucose restriction to lose fat( Reference Renes, Rosenow and Roumans 38 ). As control, mature adipocytes, originating from the same pre-adipocyte as the starved adipocytes, were cultured in the same medium with 17·5 mm-glucose. After 96 h, cells were lysed using radioimmunoprecipitation assay buffer, and protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Fisher). Samples were stored at −80°C until Western blotting. This entire procedure was performed three times in parallel to create three separate experiments.
Western blotting
A total of 15 µg of protein from in vitro or in vivo samples was separated on a 12 % SDS-polyacrylamide Criterion gel (Bio-Rad) at 180 V. After electrophoretic separation, proteins were transferred to 0·45-µm nitrocellulose membranes in a trans-blot turbo transfer system (30 min at 25 V; Bio-Rad). Subsequently, the membranes were stained with Ponceau S to check for protein bands. Following destaining, blots were blocked for 1 h in Tris-buffered saline containing 0·1 % Tween 20 (TBST) and 5 % non-fat dry milk powder. Thereafter, blots were incubated overnight at 4°C with primary antibodies against β-actin (1:1000 dilution; Santa Cruz), BiP (1:300 dilution; R&D Systems), calnexin (1:1000 dilution; Cell signaling), HSP27 (1:1000 dilution; Cell signaling), HSP70 (1:1000 dilution; R&D Systems), SOD1 (1:800 dilution; R&D Systems) and SOD2 (1:1000 dilution; R&D Systems) in TBST containing 5 % non-fat dry milk powder. After incubation with primary antibodies, membranes were washed three times for 10 min with TBST and incubated for 1·5 h with a 1:10 000 dilution of horseradish peroxidase-conjugated secondary antibody (DAKO) in TBST containing 5 % non-fat dry milk powder. After washing, bands were visualised using a chemiluminescent substrate (SuperSignal CL; Thermo Fischer Scientific) and a charge-coupled device (CCD) camera (XRS system; Bio-Rad). Data were quantified using Image Lab™ 4.0 Software (Bio-Rad).
A pooled sample was used to correct for differences between blots. Blots were normalised using all protein bands seen with Ponceau S as a measure of total protein in the sample instead of a housekeeping protein. Generally used housekeeping proteins such as β-actin are influenced by dieting as previously shown in the 2D-gel electrophoresis analysis by Bouwman et al.( Reference Bouwman, Claessens and van Baak 39 ), whereas Ponceau S has recently been indicated to be a suitable alternative for housekeeping proteins( Reference Gilda and Gomes 40 ).
HSP60 plasma concentrations
Plasma samples were stored at −80°C after collection. HSP60 concentrations were determined by ELISA (Cusabio Biotech) following the manufacturer’s instructions. Absorbance was read by a spectrophotometer at 450 nm.
Statistical analyses
Independent T test was carried out for baseline comparisons between WM and WR. ANOVA repeated measures were carried out to determine possible differences over time within a group for the human intervention study. For the in vitro measurements, a dependent T test was carried out to determine differences between control cells and glucose-restricted cells. The dependent T test was used because control cells and glucose-starved cells originate from the same cultured pre-adipocytes. For the human study, fold changes during weight loss and during the whole study were evaluated by ratio of the values in t2:t0 and t12:t0, respectively. Fold change comparisons between WM and WR were performed by using independent T test.
Pearson’s R and Spearman’s ρ correlation coefficients were calculated for relationships between parameters during the dieting period. Only correlations found significant with both tests were reported, this was done to make the analysis more stringent and reliable. The Spearman rank correlation might be the preferred method as we do not a priori know whether the protein changes are in a linear relationship. On the other hand, when proteins are closely functionally interacting, a linear relationship might be expected. Therefore, we decided to select only values that were significant (P<0·01) with both methods. Statistical analyses were carried out using SPSS 20.0 for Windows (SPSS Inc.). For all statistical tests, P<0·05 was considered to be statistically significant, except for correlations (P<0·01). Variation in the number of participants between analyses is due to the exclusion of subjects with missing data. All variables were checked for normal distribution, and variables with a skewed distribution were ln-transformed to satisfy conditions of normality. Extreme outliers (values higher than 3× interquartile range calculated with SPSS) influencing the data were removed during statistical analyses. Data are presented as mean values with their standard errors, unless otherwise indicated.
Results
Subject characteristics
No significant difference was observed between the WM and the WR group for baseline weight and BMI. Body weight was significantly reduced after diet in both the groups, and during follow-up weight gain was significant in the WR group (P<0·001), but not in the WM group (Table 1). The WR group decreased weight by 14·3 % and regained 8·2 % of the weight at t2. The WM group decreased weight by 14·4 % and only regained 0·9 %. Fig. 1 shows individual weight changes of the WM and WR groups.

Fig. 1 Body weight progression during the course of the study for weight maintainers (WM; n 9) and weight regainers (WR; n 9). Each line represents the body weight (kg) of an individual measured before 8 weeks of a very low-energy diet (t0), after the very low-energy diet (t2), and after 10 months of follow-up (t12).
Table 1 Subject characteristic of the two groups (weight regainers (WR), weight maintainers (WM)) at time points t0, t2 and t12Footnote * (Mean values with their standard errors)
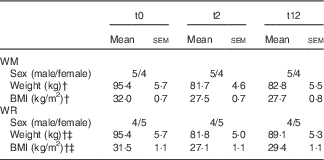
* Repeated-measures ANOVA between time points.
† P<0·001 (t0–t2).
‡ P<0·001 (t2–t12).
Stress-related proteins in adipose tissue after energy restriction
After weight loss, β-actin, BiP, calnexin, HSP27, HPS60 and HSP70 levels decreased in the WM group, whereas an increase was observed in the WR group as shown in Table 2. Fold changes between two time points were calculated and compared between the WR and the WM group (Fig. 2). During the weight loss period (t0–t2), the WR group differed from the WM group with respect to β-actin (trend), calnexin (trend), HSP27 (trend), HSP60 and HSP70 (Fig. 2(a)). Levels of stress proteins changed only during weight loss, as during the follow-up period (t2–t12) no change for any protein was observed (data not shown). After the complete intervention (t0–t12), the WR group still differed from the WM group with respect to HSP27 (trend) and HSP70 (Fig. 2(b)). BiP, SOD1 and SOD2 did not significantly change during weight loss. After dieting, no significant difference in plasma HSP60 levels was observed between the WR and the WM group. In addition, plasma HSP60 levels did not reflect HSP60 concentrations in adipose tissue (data not shown).

Fig. 2 Fold changes in stress-related proteins during (a) the weight loss-phase (after 8 weeks of weight loss (t2):baseline (t0)) and (b) the whole study (after 10 months of follow-up (t12):t0). Each box plot shows the median and interquartile range without outliers of the fold change in each protein. Weight maintainers (![]() ; n 9) and weight regainers (
; n 9) and weight regainers (![]() ; n 9). The difference between the two groups was analysed by independent t test on ln-transformed values. P values below 0·05 are considered significantly different. BiP, binding immunoglobulin protein; HSP, heat shock protein; SOD, superoxide dismutase.
; n 9). The difference between the two groups was analysed by independent t test on ln-transformed values. P values below 0·05 are considered significantly different. BiP, binding immunoglobulin protein; HSP, heat shock protein; SOD, superoxide dismutase.
Table 2 Protein abundance levels measured by Western blot at three time pointsFootnote * (Mean values with their standard errors)

WM, weight maintainers; WR, weight regainers; BiP, binding Ig protein; HSP, heat shock protein; SOD, superoxide dismutase.
* Repeated-measures ANOVA between time points.
† P>0·05–P<0·1 (t0–t2).
‡ P<0·05 (t2–t12).
§ P<0·05 (t0–t12).
|| P<0·05 (t0–t2).
During the weight loss phase, changes in stress proteins were correlated in both groups to be able to gain more insight into the mechanistic regulation of weight loss-induced cellular stress. In online Supplementary Fig. S1, the correlation plots can be observed. On the basis of these significant correlations, we have drawn interaction maps for the WM and WR groups (Fig. 3). In both groups, the similar regulation of HSP60 with HSP70 is obvious; however, although in the WM group a link between β-actin and HSP27 exists, in the WR group these proteins seem to follow the regulation of HSP60.

Fig. 3 Schematic overview of the correlated proteins within the weight maintainers (WM) and weight regainers (WR) groups during the diet phase (t0–t2). Significant correlations (P<0·01) found with both Pearson R and Spearman ρ are represented by the connecting lines with the correlation coefficients (r). SOD, superoxide dismutase; HSP, heat shock protein; BiP, binding immunoglobulin protein.
Stress-related proteins in adipocytes after low glucose starvation
The in vivo results were based on adipose tissue biopsies, which in addition to adipocytes also contain stromal vascular cells. To investigate whether there is an adipocyte-specific stress response to energy restriction, we performed an in vitro experiment. Mature SGBS adipocytes received glucose-restricted medium for 4 d causing around 17 % loss of TAG content from the cells as previously reported by Renes et al.( Reference Renes, Rosenow and Roumans 38 ). In parallel, mature SGBS adipocytes cultured with control medium did not show loss of TAG. Cells were then harvested and proteins were isolated. The relative amounts of the eight proteins were measured, but SOD1 expression appeared to be too low to measure. Similar to the WR group, expressions of all measured stress proteins were increased during energetic restriction with statistical significance for β-actin and HSP60 (Fig. 4).

Fig. 4 Stress protein levels of Simpson–Golabi–Behmel syndrome adipocytes after glucose restriction measured with Western blotting. Glucose restriction medium containing 20 nM-insulin and 0·55 mM-glucose (![]() ). The control group received medium with 20 nM-insulin and 17·5 mM-glucose (
). The control group received medium with 20 nM-insulin and 17·5 mM-glucose (![]() ). All groups consist of n 3 measured in duplicate. Values are means, with standard errors represented by vertical bars. * P<0·05 with dependent t test. BiP, binding immunoglobulin protein; HSP, heat shock protein; SOD, superoxide dismutase.
). All groups consist of n 3 measured in duplicate. Values are means, with standard errors represented by vertical bars. * P<0·05 with dependent t test. BiP, binding immunoglobulin protein; HSP, heat shock protein; SOD, superoxide dismutase.
Discussion
The results of the present study suggest that adipocyte stress is a biological risk factor for weight regain after weight loss. In the WR group, the levels of β-actin, calnexin, HSP27, HSP60 and HSP70 were increased after weight loss compared with the WM group. Correlation analysis indicated that changes in β-actin, HSP70 and HSP27 are linked to changes in HSP60 as a possible key factor contributing to weight regain. Increased levels of β-actin and HSP60 were also observed after 4 d of glucose restriction of SGBS adipocytes, indicating that the in vivo observations reside in the mature adipocytes of the adipose tissue.
Our findings show that during weight loss stress proteins increase in the adipose tissue of individuals who are at risk for weight regain. One of those is β-actin, a component of actin filaments( Reference Shawlot, Deng and Fohn 27 ). During weight loss, adipocytes change shape due to shrinking, which requires re-allocation of cellular components. When parts of the cell need to be moved, 10–30 actin filaments assemble into so-called stress-fibres, which can perform mechanical traction. Accordingly, in our in vitro experiment with cultured SGBS adipocytes, we observed a significant up-regulation of β-actin after glucose restriction in parallel to the loss of TAG and shrinking of the cells. In the adipose tissue of the WR subjects after weight loss, we observed an up-regulation of β-actin, indicating that more stress-fibres are formed and more mechanical stress exists in the adipocytes of the WR group than in those of the WM group. Unfortunately, we were unable to quantify stress fibres directly. Nevertheless, our β-actin results are in line with those of Mutch et al.( Reference Mutch, Pers and Temanni 41 ) who detected an up-regulation of β-actin gene expression in the WR group and a down-regulation in the WM group.
HSP27 showed a trend for change in adipose tissue after weight loss and there was no change after glucose restriction in the in vitro cultured adipocytes. On the other hand, in the WM group, we found a strong correlation between HSP27 and β-actin, suggesting a functional link between these proteins. It has been shown that HSP27 binds to actin filaments (F-actin) and that under cell stress HSP27 becomes phosphorylated, which enhances binding affinity for F-actin( Reference Clarke and Mearow 42 ). In this regard, HSP27 appears to be involved in the regulation of actin filament dynamics. In renal cells, HSP27 provides protection against the consequences of ATP depletion and this function is dependent on HSP70( Reference Sreedharan, Riordan and Thullin 43 ), which is known to form complexes with HSP60( Reference Sarto, Binz and Mocarelli 44 ). In the present study, our correlation map of the WR group showed a link between changes over the diet period for β-actin, HSP27 and HSP70 via HSP60 (Fig. 3). Altogether, these data in combination with our in vitro response to energy depletion strongly suggest that weight regain relates to cell stress and involves the regulation of actin filament dynamics.
Calnexin increased in the adipose tissue in the WR group after weight loss, whereas in the other group the level seemed to drop. Calnexin retains newly synthesised N-glycosylated proteins inside the ER to ensure proper folding with the help of folding factor ERp57( Reference Guerin, Arseneault and Dumont 29 , Reference Bousette, Abbasi and Chis 30 ). Wrongly folded proteins may enter into a cycle of unfolding and refolding or may be broken down by the ER-associated degradation pathway( Reference Helenius and Aebi 45 ), whereas correctly folded glycoproteins are transported out of the cell. Interestingly, N-glycoproteins are important for the formation of the extracellular matrix (ECM) of adipocytes( Reference Jones and Jones 46 ). Our results showed relatively high levels of calnexin after dieting in the WR group, which seems to be in line with the up-regulation of genes with a focal adhesion function in the WR group of the study of Mutch et al.( Reference Mutch, Pers and Temanni 41 ) and their down-regulation in the WM group.
Besides mechanical stress, other forms of cellular stress might be involved in the risk for weight regain after weight loss. HSP70 is a marker for ER stress, although it may also have a protective function against ER stress-induced apoptosis( Reference Mosser, Caron and Bourget 47 ), possibly by inhibiting key stress kinases( Reference Simar, Jacques and Caillaud 48 ). Our results show increased concentrations of HSP70 after dieting in the WR group. This implies that ER stress is present in the adipose tissue of those subjects and as such might be related to the risk of weight regain after weight loss.
In this study, we found that the WR group had elevated levels of HSP60 in the adipose tissue after dieting compared with WM subjects. HSP60 is present in the circulation of people with type 2 diabetes( Reference Dasu, Devaraj and Park 49 ) and increased levels are observed in the adipose tissue of obese subjects compared with lean subjects( Reference Boden, Duan and Homko 22 ). In starved 3T3-L1 adipocytes, an up-regulation of HSP60 is shown compared with non-starved cells( Reference Renes, Bouwman and Noben 50 ), which complies with our present in vitro observations in human SGBS adipocytes. HSP60 stimulates the release of pro-inflammatory adipokines from the adipose tissue, promoting inflammation and, as such, may support the development of insulin resistance. This suggests that WR subjects are at higher risk for obesity-related complications. Interestingly, as HSP60 is a chaperone for mitochondrial proteins, an increase in HSP60 after weight loss indicates a dysregulation of the mitochondrial metabolic processes in WR subjects. However, a possible role for such impairment in the risk of weight regain requires further investigation.
A limitation of the present study is the relatively small number of participants. In this group, the age varied between 20 and 55 years and the BMI between 27 and 39 kg/m2. Thus, it might be that we included pre- and post-menopausal woman as well as overweight and obese individuals. Nevertheless, this study produced new insights that can form the basis for further studies in larger cohorts. For the moment, it is unclear why some people show increased adipose cellular stress during weight loss while others do not. One explanation might be genetic predisposition. Alternatively, adipocyte size at baseline might play a role. A general model for accumulation of cellular stress during weight loss states that when adipocytes lose fat and shrink, the surrounding ECM is unable to follow the size reduction( Reference Kolehmainen, Salopuro and Schwab 51 – Reference Mariman 53 ). Consequently, mechanical-type cellular stress rises, which can be neutralised most easily by renewed lipid uptake and storage. Indeed, measuring the adipocyte molecular metabolism using proteomics technologies indicates that after returning to energy balance adipocytes rapidly prepare for re-storing TAG( Reference Bouwman, Claessens and van Baak 39 , Reference Bouwman, Wang and van Baak 54 ). Moreover, during weight loss, the plasma leptin level decreases dramatically, out of proportion with the loss of fat mass, which implies that, after weight loss, an extra impulse is given to energy intake to warrant the re-storing of fat and release of cellular stress( Reference Maffei, Halaas and Ravussin 55 ).
In conclusion, analyses of subcutaneous adipose tissue show increased cellular stress after an 8-week diet in a group of subjects who regained most of their weight during follow-up. In vitro cultured mature adipocytes subjected to energy restriction showed similar protein changes. These findings support the idea that adipocyte stress plays a role as a biological risk factor for weight regain after weight loss and suggest involvement of mechanical stress with dynamics of stress fibres.
Acknowledgements
The authors acknowledge the participants for cooperation in this study. The authors thank Ping Wang for helpful discussions and advice.
This work was supported by the Netherlands Organisation for Scientific Research TOP (grant number 200500001). The Netherlands Organisation for Scientific Research had no role in the design, analyses or writing of this article.
E. C. M. M. and N. J. T. R. designed the study. N. J. T. R., S. G. C., J. R. and F. G. B. collected the data. N. J. T. R. analysed the data and wrote the manuscript. E. C. M. M. and K. R. W. contributed to the interpretation of the data and reviewed the manuscript. The study was executed under the supervision of E. C. M. M. All the authors read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515005139









