Introduction
Central dopaminergic hyperactivity continues to be one of the key hypotheses for the pathophysiology of schizophrenia (Howes & Kapur, Reference Howes and Kapur2009; Howes & Murray, Reference Howes and Murray2014; Seeman, Lee, Chau-Wong, & Wong, Reference Seeman, Lee, Chau-Wong and Wong1976). Excess transmission at dopamine receptors and blockade of these receptors to treat psychosis were the primary focus in initial formulations in the 1970s (Matthysse, Reference Matthysse1973; Snyder, Reference Snyder1976). Later in the 1990s, a modified dopamine hypothesis of schizophrenia was proposed (Davis, Kahn, Ko, & Davidson, Reference Davis, Kahn, Ko and Davidson1991) based on, for example, neuronal lesions in the prefrontal cortex in rats resulting in increased levels of dopamine and in greater dopamine D2 receptor density in the striatum (Pycock, Kerwin, & Carter, Reference Pycock, Kerwin and Carter1980). The hyper-function of the striatal dopamine system has been suggested to underlie the pathophysiology of the positive symptoms of schizophrenia (Abi-Dargham et al., Reference Abi-Dargham, Kegeles, Zea-Ponce, Mawlawi, Martinez, Mitropoulou and Siever2004; Davis et al., Reference Davis, Kahn, Ko and Davidson1991; Howes et al., Reference Howes, Montgomery, Asselin, Murray, Valli, Tabraham and Grasby2009b; McCutcheon, Abi-Dargham, & Howes, Reference McCutcheon, Abi-Dargham and Howes2019; Snyder, Reference Snyder1976). Positive symptoms may be induced by the increased synaptic release of dopamine in the striatum (Breier et al., Reference Breier, Su, Saunders, Carson, Kolachana, de Bartolomeis and Pickar1997; Buchsbaum et al., Reference Buchsbaum, Christian, Lehrer, Narayanan, Shi, Mantil and Mukherjee2006; de Haan et al., Reference de Haan, Lavalaye, van Bruggen, van Nimwegen, Booij, van Amelsvoort and Linszen2004; McCutcheon, Beck, Jauhar, & Howes, Reference McCutcheon, Beck, Jauhar and Howes2018; Schmitt et al., Reference Schmitt, la Fougere, Dresel, Frodl, Hahn, Moller and Meisenzahl2008; Yang et al., Reference Yang, Yu, Yeh, Chiu, Chen and Lee2004). Furthermore, patients also show a significant elevation in striatal synaptic dopamine levels compared to healthy controls (Kegeles et al., Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper, Slifstein and Laruelle2010; McCutcheon et al., Reference McCutcheon, Beck, Jauhar and Howes2018; Slifstein & Abi-Dargham, Reference Slifstein and Abi-Dargham2018).
Neurochemical imaging techniques single-photon emission computed tomography (SPECT) and positron emission tomography (PET) provide in vivo indices of the different stages of dopamine neurotransmission, including its pre-synaptic synthesis, release into the synapse, and the levels of receptors and transporters (Howes & Kapur, Reference Howes and Kapur2009; McCluskey, Plisson, Rabiner, & Howes, Reference McCluskey, Plisson, Rabiner and Howes2020).
Elevated dopamine synthesis capacity has been consistently reported in 6-fluoro-(18F)-L-3,4-dihydroxyphenylalanine ([18F]-DOPA) and L-[b-11C]-3,4-dihydroxyphenylalanine ([11C]-DOPA) PET studies in schizophrenia, including in first-episode patients (Hietala et al., Reference Hietala, Syvälahti, Kuoppamäki, Hietala, Syvälahti, Haaparanta and Salokangas1995; Jauhar et al., Reference Jauhar, Veronese, Nour, Rogdaki, Hathway, Natesan and Howes2019) and has been shown to predate the onset of schizophrenia in individuals with prodromal psychotic symptoms (Howes et al., Reference Howes, Montgomery, Asselin, Murray, Valli, Tabraham and Grasby2009b). Synaptic dopamine can be studied using challenge approaches which stimulate its release or deplete synaptic dopamine levels (Egerton, Demjaha, McGuire, Mehta, & Howes, Reference Egerton, Demjaha, McGuire, Mehta and Howes2010). These approaches are based on the competition between dopamine and radioligands such as raclopride and [123I] iodobenzamide (IBZM) for binding to dopamine receptors (Laruelle, Reference Laruelle2000), although recent evidence indicates the process is more complex than suggested by a simple competition model (Guo et al., Reference Guo, Guo, Kralikova, Jiang, Schieren, Narendran and Rayport2010). Studies using challenge approaches have found evidence of increased radiotracer displacement in patients with untreated schizophrenia compared to controls, indicating greater dopamine release (Laruelle, Abi-Dargham, Gil, Kegeles, & Innis, Reference Laruelle, Abi-Dargham, Gil, Kegeles and Innis1999), and increased synaptic dopamine levels (Abi-Dargham et al., Reference Abi-Dargham, Rodenhiser, Printz, Zea-Ponce, Gil, Kegeles and Laruelle2000; Kegeles et al., Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper, Slifstein and Laruelle2010; McCutcheon et al., Reference McCutcheon, Beck, Jauhar and Howes2018). The dopamine hypothesis of schizophrenia has been revised in light of this neurochemical imaging evidence (Howes & Murray, Reference Howes and Murray2014; McCutcheon et al., Reference McCutcheon, Abi-Dargham and Howes2019).
Indeed, the earlier formulations were built on the findings that antipsychotics work by blocking D2/3 receptors, and drugs such as amphetamine which activate the dopamine system, can trigger psychotic symptoms (Abi-Dargham, Reference Abi-Dargham2004; Berman, Kuczenski, McCracken, & London, Reference Berman, Kuczenski, McCracken and London2009; Curran, Byrappa, & McBride, Reference Curran, Byrappa and McBride2004; Howes et al., Reference Howes, Egerton, Allan, McGuire, Stokes and Kapur2009a). Based on early findings of an elevation in striatal D2/3 receptor availability in schizophrenia (Wong et al., Reference Wong, Wagner, Tune, Dannals, Pearlson, Links and Gjedde1986), there was an initial focus on the D2 receptor. However, subsequent studies of D2/3 receptor availability in schizophrenia have been inconsistent, whilst there is no difference v. controls in dopamine transporter availability (Chen et al., Reference Chen, Yang, Howes, Lee, Landau, Yeh and Bramon2013; Howes et al., Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012). One factor in many of the studies that could explain the inconsistent findings is prior antipsychotic treatment, which may upregulate D2/3 receptor levels and increase variability in patients (Brugger et al., Reference Brugger, Angelescu, Abi-Dargham, Mizrahi, Shahrezaei and Howes2020). There is therefore a need for large studies of medication-naïve first-episode patients to determine whether dopamine receptor availability abnormalities are associated with the onset of the illness.
Cognitive impairments have consistently been found in patients with schizophrenia compared to healthy individuals (Elvevag, Weinberger, Suter, & Goldberg, Reference Elvevag, Weinberger, Suter and Goldberg2000; Lencz et al., Reference Lencz, Knowles, Davies, Guha, Liewald, Starr and Malhotra2014; Ranlund et al., Reference Ranlund, Calafato, Thygesen, Lin, Cahn, Crespo-Facorro and Bramon2018) and may be considered a core aspect of the clinical syndrome. There is growing evidence that cognition is a pathway through which genetic variation influences schizophrenia risk (Calafato & Bramon, Reference Calafato and Bramon2019; Toulopoulou et al., Reference Toulopoulou, Zhang, Cherny, Dickinson, Berman, Straub and Weinberger2019). Indeed, a genome-wide association meta-analysis of human cognition including over 129 000 participants showed that intelligence has a strong protective effect on schizophrenia risk (Savage et al., Reference Savage, Jansen, Stringer, Watanabe, Bryois, de Leeuw and Posthuma2018). The Wisconsin Card Sorting Test (WCST) and Continuous Performance Task (CPT) are commonly used to evaluate the aspects of patients' cognitive functions (Bellani & Brambilla, Reference Bellani and Brambilla2008; Elvevag et al., Reference Elvevag, Weinberger, Suter and Goldberg2000; Everett, Lavoie, Gagnon, & Gosselin, Reference Everett, Lavoie, Gagnon and Gosselin2001; Green, Satz, Ganzell, & Vaclav, Reference Green, Satz, Ganzell and Vaclav1992). The WCST was found to relate to genetic variation in dopamine receptors (Rybakowski et al., Reference Rybakowski, Borkowska, Czerski, Kapelski, Dmitrzak-Weglarz and Hauser2005, Reference Rybakowski, Borkowska, Czerski, Dmitrzak-Weglarz, Skibinska, Kapelski and Hauser2006), and CPT was influenced by the estimates of dopamine release in patients with schizophrenia (Braver, Barch, & Cohen, Reference Braver, Barch and Cohen1999; Cohen & Servan-Schreiber, Reference Cohen and Servan-Schreiber1993). Medication-free patients with schizophrenia show reduced prefrontal cortical dopamine release while performing cognitive tasks such as WCST, which supports the frontal hypo-dopaminergic hypothesis of cognitive symptoms in schizophrenia, and suggests a differential regulation of striatal dopamine release in associative regions (Rao et al., Reference Rao, Northoff, Tagore, Rusjan, Kenk, Wilson and Mizrahi2019; Slifstein et al., Reference Slifstein, van de Giessen, Van Snellenberg, Thompson, Narendran, Gil and Abi-Dargham2015). We hypothesize that patients with higher striatal D2/3 receptor availability, which indicates higher dopamine release, should have better cognitive performance (Fagerlund et al., Reference Fagerlund, Pinborg, Mortensen, Friberg, Baaré, Gade and Glenthøj2013).
Our study focused on post-synaptic dopamine regulation using [123I] IBZM SPECT. We set out to compare D2/3 post-synaptic receptor availability between 53 healthy controls and 21 drug-naïve patients with recent-onset schizophrenia. The relationships between D2/3 receptor availability and both cognitive function and clinical symptoms were also investigated.
Methods
Sample
All study participants were living in Tainan City, the fifth largest in Taiwan with a population of 1 880 906 (Bureau of civil affairs, Tainan city government, 2019). A total of 21 medication-naïve first-episode patients with schizophrenia were recruited from the psychiatric outpatient clinic of the National Cheng Kung University Hospital. This included 11 patients and one control from our previous study (Yang et al., Reference Yang, Yu, Yeh, Chiu, Chen and Lee2004). Fifty-three healthy community residents of Tainan City were recruited as volunteers through research advertisements. All participants were right-handed. Patients were recruited from August 2001 to April 2005 and controls were recruited from June 1999 to April 2005. All participants including controls were interviewed by senior psychiatrists who have been practicing for more than 10 years, using the Chinese version of the Mini International Neuropsychiatric Interview (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998), to ensure that the controls were free of any Axis I or Axis II psychiatric disorders and to confirm the diagnosis for patients. The Positive and Negative Syndrome Scale (PANSS) of patients with schizophrenia was rated by one psychiatrist. Brain magnetic resonance images (MRI) and blood biochemical profiles of all controls were assessed and showed no abnormalities. The mean duration of illness was 10.7 months (s.d. = 20.7, median = 2.0, interquartile range = 11.8 months).
Before any procedure was performed, written informed consent was obtained from each of the participants after a complete explanation of the study. The Ethical Committee for Human Research at the National Cheng Kung University Hospital approved the study. Inclusion criteria: (i) patients should fulfill DSM-IV criteria for schizophrenia; (ii) age between 17 and 60; (iii) no physical illness and with stable vital signs; (iv) participants never received any antipsychotic or antidepressant treatment and were free of any psychotropic medication at the time of testing. Exclusion criteria for all participants: (i) other co-morbid psychiatric illnesses, or neurological illnesses; (ii) evidence of substance abuse/dependence as assessed during the clinical interview with the research psychiatrist, at time of enrollment; (iii) intellectual disability; (iv) all female participants of child-bearing age had to take an acceptable form of contraceptive throughout the study, in order to be included and underwent an instant urine pregnancy test prior to starting the experiments; (iv) all patients who were deemed at risk of acute suicide/self-harm were excluded.
Assessment battery
Before receiving any treatment, patients underwent the baseline assessments within 7 days of entering the study including SPECT, psychopathology scales and cognitive testing. Healthy controls received the same assessments. Baseline assessments are described below.
Measurement of striatal dopamine D2/3 receptor density
Before SPECT examination with [123I] IBZM, the thyroid gland was protected with 9 ml of Lugol's solution. For brain SPECT imaging, each subject was intravenously administered 185 MBq (injected mass of IBZM: 8.2 ng; specific radioactivity 8900 MBq/nmol) of [123I] IBZM (Institute of Nuclear Energy Research, Lungtan, Taiwan) in a quiet environment approximately 10 min after setting the intravenous lines. The imaging was initiated approximately 120 min later, and 30 min of imaging data were collected during the procedure. To avoid tilt and misalignment, we carefully positioned participants and monitored them during scanning, and used a head holder to further reduce movement artifacts. Participants were informed of the necessity to avoid head movement. Sinograms were reviewed blind to diagnosis to determine whether post-acquisition correction for head movements was needed. Movement correction was conducted using the motion correction software ICON (Siemens, version 8.5 KB21).
We used a triple-headed rotating γ camera (Multispect 3; Siemens, Hoffman Estates, IL, USA) with ultra-high-resolution fan-beam collimators, which yields an image resolution of approximately 8.5 mm for the full width half maximum (FWHM). The SPECT images were acquired over a circular 360° rotation, with 120 steps, at a rate of 50 s per step, in a 128 × 128 × 16 matrix. The images were then reconstructed using Butterworth and Ramp filters (Friston et al., Reference Friston, Frith, Liddle, Dolan, Lammertsma and Frackowiak1990) (cut-off frequency = 0.3 Nyquist, power factor = 7), with attenuation according to Chang's method (Chang, Reference Chang1978). The reconstructed transverse images were realigned parallel to the canthomeatal line; slice thickness = 2.89 mm. For semi-quantitative analyses, six consecutive transverse slices on which the striatum was best visualized were combined to obtain a 17.34 mm-thick slice. Then regions of interest (ROIs) were placed over the striatum and the frontal cortex (see Fig. 1). The ROIs were drawn directly on the SPECT images by an experienced nuclear-medicine physician who was blind to the participants' clinical status and data. During this process, the participants' MRIs (GE, SIGNA CV-I, 1.5T, WI, USA), obtained within 2 weeks after SPECT examination, were used as a visual reference to determine the areas of the ROI. The sizes of all ROI were at least twice those of the FWHM. The specific striatal [123I] IBZM binding was calculated as the mean count in the striatal ROI minus the mean count in the frontal region divided by the mean count in the frontal region [(St−F)/F] and this ratio represents striatal D2/3 availability (Brucke et al., Reference Brucke, Podreka, Angelberger, Wenger, Topitz, Kufferle and Deecke1991; Toyama, Ichise, Ballinger, Fornazzari, & Kirsh, Reference Toyama, Ichise, Ballinger, Fornazzari and Kirsh1993).

Fig. 1. The regions of interest (ROIs) were placed over the striatum and the frontal cortex. The ROIs were drawn directly on the SPECT images by an experienced nuclear-medicine physician who was blind to the participants' clinical status and data.
Psychopathology ratings
On the day of recruitment, we collected standardized psychopathology ratings using the Global Assessment of Functioning (GAF), which ranges 0–100 from poorest to optimal functioning (Hopper & Wanderling, Reference Hopper and Wanderling2000) and the PANSS, with 30 psychotic symptom and general psychopathology items (range 30–210) from least to most severely symptomatic (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987).
Cognitive function assessments
Patients' executive function and attention/vigilance were assessed using the Wisconsin Card Sorting Test (WCST) and the Continuous Performance Test (CPT), respectively.
Wisconsin Card-Sorting Test (WCST)
A computerized version of the WCST was administered by an experienced clinical neuropsychologist. Definitions of indices were as described in the WCST manual (Heaton, Chelune, Talley, Kay, & Curtiss, Reference Heaton, Chelune, Talley, Kay and Curtiss1993). The number of categories completed and perseverative errors were used to assess the performance (Stratta et al., Reference Stratta, Daneluzzo, Prosperini, Bustini, Mattei and Rossi1997; Volkow et al., Reference Volkow, Gur, Wang, Fowler, Moberg, Ding and Logan1998).
Continuous Performance Test (CPT)
The CPT is a psychological test that primarily measures attention (Chen, Hsiao, Hsiao, & Hwu, Reference Chen, Hsiao, Hsiao and Hwu1998; Hsieh et al., Reference Hsieh, Chu, Yang, Yang, Yeh, Lee and Chen2005). In this version, the critical stimulus was a particular sequence of two stimuli out of the available set (AX task: subjects were asked to respond whenever the number ‘9’ was preceded by the number ‘1’). Each test session began with a 2 min practice. During the test, numbers from 0 to 9 were randomly presented for 50 ms each, at a rate of one per second. A total of 331 trials, 34 (10%) of which were target stimuli, were presented over 5 min. Subject responses were recorded automatically (Sunrise Systems, version 2.20, Pembroke, MA, USA) (Smid, de Witte, Homminga, & van den Bosch, Reference Smid, de Witte, Homminga and van den Bosch2006).
Statistical analyses
The main aim of our study was to assess the differences between patients and controls in the specific striatal [123I] IBZM binding, considering both the left and right striatum measures. Linear mixed modeling was used to compare [123I] IBZM binding between the two participant groups. To allow for possible group differences between left and right sites, we tested a group by laterality interaction. On the basis of previous literature, we considered that age, sex, and tobacco smoking are potential confounders and therefore included these as covariates in the analysis (Chen et al., Reference Chen, Yang, Lee, Yeh, Lee, Chiu and Chu2005; Kuikka, Tiihonen, Karhu, Bergstrom, & Rasanen, Reference Kuikka, Tiihonen, Karhu, Bergstrom and Rasanen1997; Volkow et al., Reference Volkow, Gur, Wang, Fowler, Moberg, Ding and Logan1998; Yang et al., Reference Yang, Yao, Yeh, Lee, Chen, Lu and Chiu2008). The models included subject-varying intercepts to acknowledge the correlation between the two repeated measures per participant. We expanded the above model to test the interaction of group by age or group by sex, respectively.
Our subsidiary aim was to explore the association between the specific striatal [123I] IBZM binding and psychopathology ratings or cognitive performance in patients using Spearman's ρ correlations.
Demographic differences between patients and controls were examined with χ2 tests for categorical variables or with Student's t tests for continuous variables. For the latter, Levene's test was used to assess the assumption of the equality of variances. Diagnostic plots as well as one-sample Kolmogorov–Smirnov tests were used to test for normality.
We report uncorrected p values for all analyses. However, since we examined the influence of striatal [123I] IBZM binding on 10 key parameters (clinical group, four PANSS scores, four cognitive measures, and GAF), we adjusted for multiple testing and our statistical significance was established at p < 0.005 (statistical trend p < 0.01). We used SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA) for all analyses.
Results
The demographic characteristics of the patient and control groups are summarized in Table 1. Patients and controls had a similar sex distribution and tobacco smoking habits. However, compared to the controls, the patients were significantly younger (t = −2.33, df = 72; p = 0.02), less likely to be married (χ2 = 7.71; p = 0.005), and had fewer years of education (t = −3.44, df = 71; p = 0.001). The ratio of specific striatal binding in both patients and controls was normally distributed (Kolmogorov–Smirnov test was not significant and diagnostic plots showed no departure from normality).
Table 1. Demographic characteristics of participants

M, married or living with a partner; S, single, divorced or separated.
*p < 0.05; **p < 0.01.
After controlling for age, sex, and tobacco smoking, the mean specific striatal binding showed no significant difference between patients and controls (estimated difference = 0.001; 95% CI −0.11 to 0.11; F = 0.00, df = 1, 69; p = 0.99). These results are summarized in Table 2. In the same, there was a significant main effect of laterality, whereby the right side had a higher ratio of the specific striatal binding than the left side (estimated difference = 0.03; 95% CI 0.007–0.048; F = 7.28, df = 1, 73; p = 0.009). The group by laterality interaction was not significant and was therefore dropped from our model (F = 0.01, df = 1, 72; p = 0.91). There was no significant effect of sex (estimated difference = −0.01; 95% CI −0.12 to 0.10; F = 0.03, df = 1, 69; p = 0.86). Similarly, tobacco smoking did not have a significant influence on IBZM binding (estimated difference = 0.06; 95% CI −0.08 to 0.19; F = 0.65, df = 1, 69; p = 0.42). Finally, there was a highly significant effect of age whereby IBZM binding declined with advancing age (estimated binding ratio change per decade of age = −0.01; 95% CI −0.01 to −0.004; F = 11.5, df = 1, 69; p = 0.001). Of note there was no significant interaction between age and group (F = 0.02, df = 1, 68; p = 0.89) or between group and sex (F = 0.17, df = 1, 68; p = 0.68), indicating that the age decline in IBZM was similar in patients and controls as well as in both sexes. These findings are summarized in Table 2 and Fig. 2.
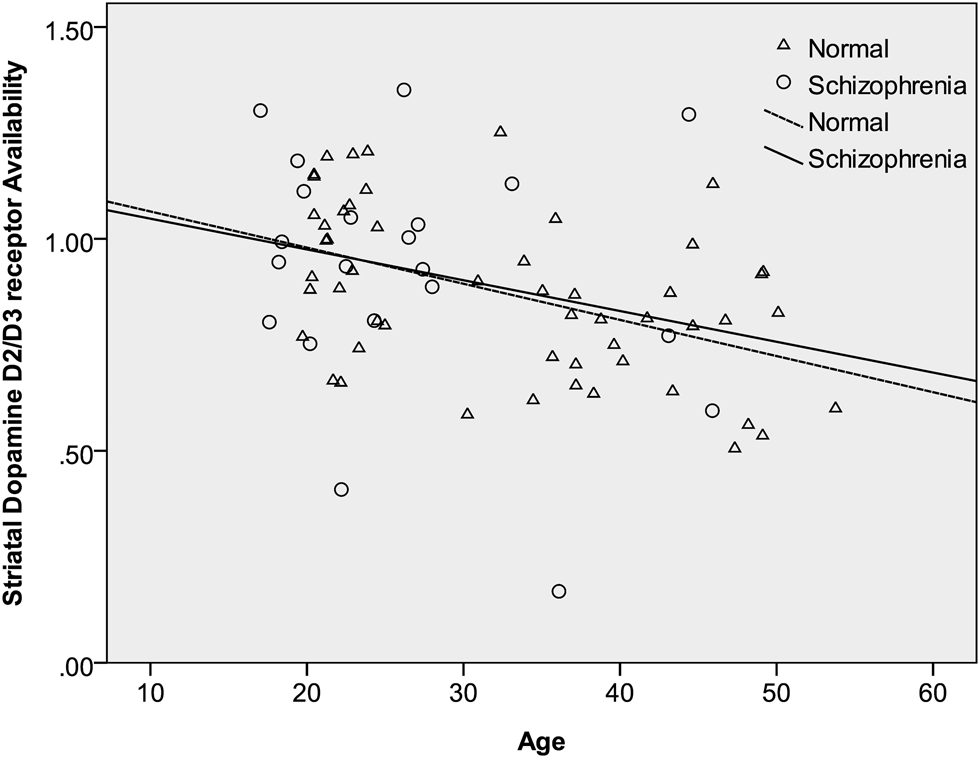
Fig. 2. The relation between age and striatal dopamine D2/3 receptor availability [(St−F)/F] in patients with schizophrenia and controls. As described in the main analyses, having adjusted for the main effects of group, sex, and tobacco smoking, there was a significant decline of IBZM binding with advancing age but no significant interaction between the age and group. This graph shows regression lines describing the relationship between [123I] iodobenzamide binding and age within patients and controls separately. The almost parallel lines illustrate the similar rates of decline in the two groups.
Table 2. Dopamine D2/3 receptor availability [(St−F)/F] by group and sex
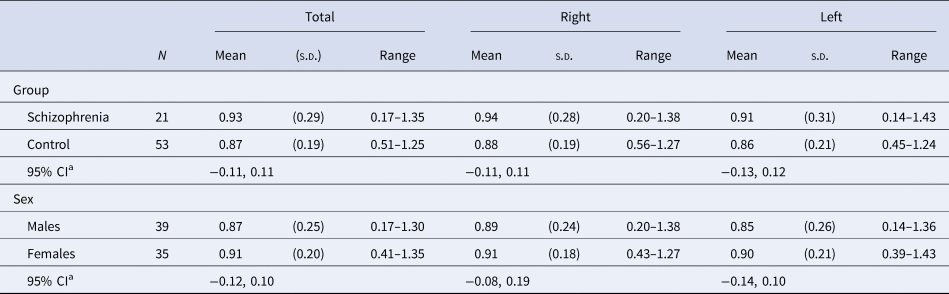
[(St−F)/F]: mean count in the striatal region minus the mean count in the frontal region divided by the mean count in the frontal region.
a Independent variables included group, age, sex, and tobacco smoking.
Among the 21 patients included, 19 patients completed the GAF, and 19 patients completed the PANSS scales. No significant correlations were found between the mean specific striatal binding and psychopathological rating scores. A total of 17 patients completed WCST and 16 patients completed CPT. There was suggestive evidence for a positive correlation between the mean specific striatal binding and the scores of the number of categories completed in WCST (ρ = 0.54, p = 0.02) (Table 3), however this did not survive correction for multiple testing.
Table 3. Spearman's ρ correlations of D2/3 receptor availability [(St−F)/F] and psychopathology in the schizophrenia group
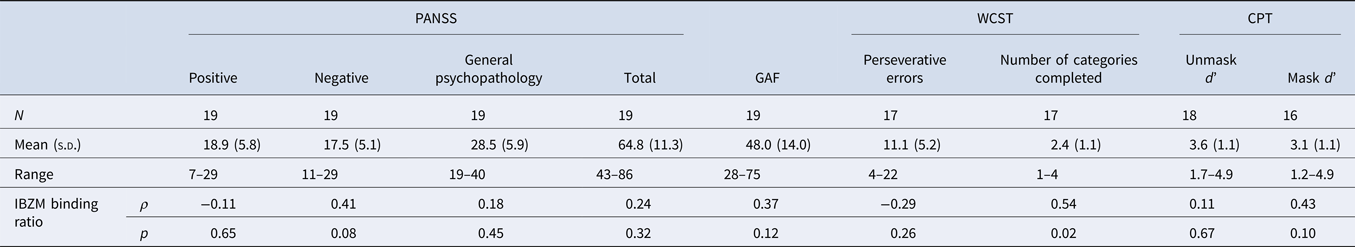
IBZM, [123I] iodobenzamide; PANSS, Positive and Negative Syndrome Scale; GAF, Global Assessment of Functioning; WCST, Wisconsin Card Sorting Test; CPT, Continuous Performance Task.
[(St−F)/F]: mean count in the striatal region minus the mean count in the frontal region divided by the mean count in the frontal region.
*p < 0.005, adjusted for multiple testing.
We used the median, 0.95, of IBZM binding ratio [(St−F)/F, D2/3 receptor availability] as the cut-off point to divide patients into low and high dopamine binding groups with no differences in age, sex, tobacco smoking (high: n = 10, low: n = 11) (Tanaka, Reference Tanaka2006) based on the concept of hyperdopaminergic/normo-dopaminergic subtyping in schizophrenia (Howes & Kapur, Reference Howes and Kapur2014). We found no evidence of group differences in the masked d’ between the high dopamine binding group (n = 9) and the low dopamine binding group (n = 7) (Mann–Whitney U = −1.74; p = 0.08). Similarly, no significant difference was found in WCST performance between the two groups.
There were no significant differences between patients recruited in 2004 (n = 11) and after 2004 (n = 10) in demographic or imaging measures or PANSS scores (positive, negative, general psychopathology, sum, p = 0.08, 0.48, 0.02, 0.06). We found weak evidence for better WCST scores (perseveration errors and number of categories completed, p = 0.07 and 0.06), and CPT unmask d’ score (p = 0.07) in patients recruited after 2004 (online Supplementary Table S1).
In conclusion, these findings provide some support for our hypothesis that, amongst people with schizophrenia, a higher dopamine release is associated with better cognitive performance (Fagerlund et al., Reference Fagerlund, Pinborg, Mortensen, Friberg, Baaré, Gade and Glenthøj2013).
Discussion
Our study shows that the specific striatal binding ratio of medication-naive patients with schizophrenia was not significantly different from that of healthy controls, and we found no evidence of changes in D2/3 receptor availability. Our findings are consistent with previous studies conducted at baseline before treatment. Of note, not all the patients in these preceding experiments were medication-naïve, but those patients recruited in the study of Wulff et al. were completely medication-naïve (Abi-Dargham et al., Reference Abi-Dargham, Rodenhiser, Printz, Zea-Ponce, Gil, Kegeles and Laruelle2000; Corripio et al., Reference Corripio, Escarti, Portella, Perez, Grasa, Sauras and Alvarez2011; Wulff et al., Reference Wulff, Pinborg, Svarer, Jensen, Nielsen, Allerup and Glenthøj2015).
Previous literature on D2 receptor availability showed diverse findings. Indeed, a meta-analysis found a small (Cohen's d = 0.26) yet significant elevation of D2/3 receptors in schizophrenia. It also showed D2/3 receptor upregulation is not detected in antipsychotic-naïve patients (Howes et al., Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012), which is fully consistent with our data. Other studies reported no evidence of major alterations in dopamine D2/3 receptors in patients with schizophrenia (Kegeles et al., Reference Kegeles, Abi-Dargham, Frankle, Gil, Cooper, Slifstein and Laruelle2010; Slifstein & Abi-Dargham, Reference Slifstein and Abi-Dargham2018). Thus, while previous imaging studies were inconsistent with regards to D2/3 dysfunction and varied depending on clinical characteristics and imaging methods, in light of our findings in medication-naïve patients, these D2/3 availability abnormalities are likely to be confounded by antipsychotic medication.
Our research has shown that aging has a powerful influence on both pre- and post-synaptic dopaminergic function. In a previous study, we showed that the specific uptake of [99mTc]-TRODAT-1, a radiotracer for the dopamine transporter, decreases with advancing age, and that this aging decline was observed both in controls and patients, but was faster amongst the antipsychotic-naïve patients with first-episode schizophrenia (Chen et al., Reference Chen, Yang, Howes, Lee, Landau, Yeh and Bramon2013). Furthermore, the density of striatal dopamine D2/3 receptors also declines with age in healthy individuals (Chen et al., Reference Chen, Yang, Lee, Yeh, Lee, Chiu and Chu2005). Finally, in this current study, our methodology was sensitive enough to identify a highly significant effect of age whereby IBZM binding declines with advancing age and it does so at a similar rate in both patients with schizophrenia and controls.
We found suggestive evidence that those patients with higher dopamine release may have better cognition. Our findings support the concept of hyperdopaminergic/normo-dopaminergic subtyping in schizophrenia (Howes & Kapur, Reference Howes and Kapur2014) that proposed there are differences in the dopamine system between patients who respond to antipsychotic drugs and those who do not. Although there is a consistent alteration in dopaminergic function in schizophrenia, evidence of heterogeneity is also reported (Howes et al., Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012), higher baseline dopamine metabolite levels are generally associated with good subsequent response to antipsychotic treatment, whereas lower dopamine metabolite levels are associated with poor response (Yoshimura, Ueda, Shinkai, & Nakamura, Reference Yoshimura, Ueda, Shinkai and Nakamura2003). Those medication-naïve hyperdopaminergic patients showed tentative evidence of better CPT performance, which suggests cognitive differences could exist in medication-naïve patients with different D2/3 receptor availability beside the severity of symptoms. There was also some evidence that a better performance in WCST correlated with a higher mean specific striatal binding (dopamine D2/3 receptor availability) in our study, thus also supports the aforementioned findings. Our results did not show significant correlations between the mean specific striatal binding (dopamine D2/3 receptor availability) and psychopathological rating scores, which is consistent with our previous study of striatal dopamine transporter availability (Chen et al., Reference Chen, Yang, Howes, Lee, Landau, Yeh and Bramon2013). We hypothesize that could be influenced by the complex dysfunction of a specific dorsal fronto-striato-thalamic circuit, linking different brain areas across different stages of psychosis, so simultaneously also affecting cognitive functions (Dandash, Pantelis, & Fornito, Reference Dandash, Pantelis and Fornito2017; Fagerlund et al., Reference Fagerlund, Pinborg, Mortensen, Friberg, Baaré, Gade and Glenthøj2013; Okubo et al., Reference Okubo, Suhara, Suzuki, Kobayashi, Inoue, Terasaki and Toru1997). Increased striatal dopamine signaling and impaired integration of cortical inputs into the striatum could affect cognitive components involved in the behaviors of patients with schizophrenia (Conn, Burne, & Kesby, Reference Conn, Burne and Kesby2020), but may not completely reflect the whole clinical symptoms. Our results support the hypothesis that dysfunction of cortico-striato-thalamic circuits influences the pathogenesis of psychosis, which may also implicate global cognitive deficits in schizophrenia (Dandash et al., Reference Dandash, Pantelis and Fornito2017).
A strength of our study is that we recruited a relatively large and clinically homogenous sample, where all the patients were medication-naive and at a similar early stage of their illness. Our controls recruited from the community had a similar gender distribution, but were significantly older, and had spent significantly more time in education. These differences are similar to the previous studies of patients with schizophrenia and other psychotic disorders compared to healthy controls (Loughland et al., Reference Loughland, Draganic, McCabe, Richards, Nasir, Allen and Carr2010).
Decreases in either brain volume or dopamine transporter availability in the striatum have been reported in tobacco smokers (Brody et al., Reference Brody, Olmstead, London, Farahi, Meyer, Grossman and Mandelkern2004; Yang et al., Reference Yang, Yao, Yeh, Lee, Chen, Lu and Chiu2008). Our patient and control groups were not significantly different in smoking status, and our previous study has shown no significant difference in striatal dopamine receptor availability between smokers and controls (Yang et al., Reference Yang, Yao, McEvoy, Chu, Lee, Chen and Chiu2006); hence, smoking is unlikely to have confounded our results. Nevertheless, all our analyses were adjusted for tobacco smoking in this study.
One potential limitation of our study is that the ROIs were manually drawn directly on the SPECT image. While there is the potential for observer bias using this approach, we avoided this by ensuring that the ROIs were delineated independently of the clinical assessment and blind to the subject's clinical group. We delineated the ROI on the SPECT image rather than on the MRI because this does not require the ROI to be transformed from MRI to SPECT space. However, studies have found similar results using both approaches (Inoue et al., Reference Inoue, Katsumi, Hayashi, Mukai, Ishizu, Hashikawa and Fukuyama2004; Wang et al., Reference Wang, Volkow, Levy, Fowler, Logan, Alexoff and Schyler1996). Furthermore, we used the frontal cortex as the reference region, since no direct evidence indicates cortical dopaminergic alterations in patients with schizophrenia (Kambeitz, Abi-Dargham, Kapur, & Howes, Reference Kambeitz, Abi-Dargham, Kapur and Howes2014). Another potential limitation is that patients recruited in our study for SPECT imaging had to be relatively cooperative in order to complete the procedure. This is an issue for most imaging studies and may potentially affect the generalizability of our findings to those with more florid disorder. We did not differentiate the subtypes of patients with schizophrenia in our study, which could limit information for further evaluation between specific clinical symptomatology and striatal dopamine D2/3 receptor availability (Hietala et al., Reference Hietala, Syvälahti, Vilkman, Vuorio, Räkköläinen, Bergman and Salokangas1999; McCutcheon, Krystal, & Howes, Reference McCutcheon, Krystal and Howes2020).
Intriguingly, the evidence from molecular genetics continues to implicate post-synaptic D2 receptors in schizophrenia. A recent meta-analysis found that genetic variants coding for D2 receptors (C957T polymorphism) constitute a risk factor for schizophrenia especially in Caucasian populations (Liu et al., Reference Liu, Fan, Ding, Hu, Cai, Wang and Pan2014). Similarly, a landmark genome-wide association study identifying over 100 loci associated with schizophrenia found significant hits implicating the dopamine D2 receptor gene indicating its possible role in the etiology of the disease (Flint & Munafo, Reference Flint and Munafo2014; Ripke et al., Reference Ripke, Neale, Corvin, Walters, Farh and Holmans2014; Ripke, Walters, & O'Donovan, Reference Ripke, Walters and O'Donovan2020).
Future research will need to characterize the mechanisms through which genetic variants in dopamine receptors influence schizophrenia susceptibility and imaging biomarkers obtained through PET or SPECT, especially when used to study unaffected relatives and populations with increased risk for the disease. Finally, as suggested by Howes et al. (Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012) and consistent with this study, future schizophrenia treatments should target the presynaptic control of dopamine synthesis and release rather than focus exclusively on post-synaptic receptors, and further focus on other striatal neurochemistry such as non-dopaminergic neurotransmitter systems that may contribute to dopaminergic dysfunction (McCutcheon et al., Reference McCutcheon, Abi-Dargham and Howes2019).
In conclusion, our imaging evidence does not support a major dopaminergic abnormality in schizophrenia affecting post-synaptic dopamine receptors, although in this study, we did not investigate pre-synaptic synthesis capacity and release (Abi-Dargham, van de Giessen, Slifstein, Kegeles, & Laruelle, Reference Abi-Dargham, van de Giessen, Slifstein, Kegeles and Laruelle2009; Howes et al., Reference Howes, Kambeitz, Kim, Stahl, Slifstein, Abi-Dargham and Kapur2012). Both the previous literature and these findings suggest that any enhanced post-synaptic D2/3 receptor availability is likely to be secondary to antipsychotic treatment rather than the illness itself.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720005413
Acknowledgement
This work was supported by the grants of the National Science Council of Taiwan (NSC 91-2314-B-006-074, NSC 92-2314-B-006-111, NSC 93-2314-B-006-107, NSC 95-2314-B-006-115-MY2, and NSC 99-2314-B-006-019-MY3) and Atomic Energy Council of Taiwan (NSC 91-NU-7-006-002 and NSC 92-NU-7-006-004). The authors would like to thank Ching Lin Chu, Tsai Hua Chang, and Chien Ting Lin for their administrative support.
Financial support
This research was funded by the National Science Council of Taiwan (NSC 91-2314-B-006-074, NSC 92-2314-B-006-111, NSC 93-2314-B-006-107, NSC 95-2314-B-006-115-MY2, and NSC 99-2314-B-006-019-MY3) and Atomic Energy Council of Taiwan (NSC 91-NU-7-006-002 and NSC 92-NU-7-006-004).
Conflict of interest
The funding institutions of this study had no further role in the study design, the collection, analysis and interpretation of data, the writing of this paper, or the decision to submit for publication. The authors report no financial relationships with commercial interests.








