Vitamin A (VA) deficiency is usually prevented with high-dose capsules of VA( Reference Bloem, Hye and Wijnroks 1 ) or food fortification( Reference Dary and Mora 2 ). Supplementing at-risk populations significantly reduces child mortality( Reference Fawzi, Chalmers and Herrera 3 ). VA supplementation programmes are cost-effective, but programmes are difficult to sustain and many people at risk do not receive supplements( Reference Stein, Sachdev and Qaim 4 ).
Supplementation programmes are most expensive, and typically have the least coverage, in rural areas( Reference Stein, Sachdev and Qaim 4 , Reference Ruel 5 ). Growing staple crops that are rich in β-carotene (BC) is an attractive alternative to providing supplements, especially in rural areas( Reference Ruel 5 ). Cassava is a staple food for millions of people in Africa and South America( Reference Nhassico, Muquingue and Cliff 6 ). African countries where cassava is a staple have a high incidence of VA deficiency (17–69 %), as defined by serum retinol concentrations ≤0·70 µmol/l( 7 ). It grows well in poor soils, is resistant to insect and animal pests and is less labour intensive to grow compared with other staples such as maize( Reference Montagnac, Davis and Tanumihardjo 8 , Reference Udoh and Kormawa 9 ). Cassava is a good source of energy, but is poor in protein and lacking in BC( Reference Montagnac, Davis and Tanumihardjo 8 ). Multinational non-governmental organisations (especially HarvestPlus and BioCassava+) are biofortifying cassava to increase its BC content and have successfully developed yellow-orange-fleshed cassava cultivars with moderately high (8–12 µg/g) concentrations of BC.
Unfortunately, cassava contains cyanogenic glycosides (linamarin and lotaustralin) in its plant cell vacuoles, and an enzyme (linamarase) in its cell walls( Reference Nhassico, Muquingue and Cliff 6 ). When cassava is cut or crushed, the compartmentalisation of the enzyme is destroyed, and cyanogenic glycosides and linamarase combine and are hydrolysed to cyanide. Attempts to remove cyanogenic glycosides from cassava reduce its viability and result in greater susceptibility of the root to animals and insects( Reference Siritunga and Sayre 10 ). Acute cyanide poisoning is rare among cassava eaters, but prolonged exposure can result in disabilities such as Konzo, an irreversible paralysis; and tropical ataxic neuropathy in the elderly( Reference Nhassico, Muquingue and Cliff 6 ). Moderate processing techniques such as drying or soaking may be able to remove cyanide while preserving carotenoids( Reference Padmaja 11 ).
The BC content, the BC to VA conversion ratio and cyanide content data upon which this model is partially based are derived from a study conducted by our laboratory in which both typical and BC-enhanced cassava were fed to a small group of American women( Reference La Frano, Woodhouse and Burnett 12 ). These data were combined with the FAO cassava consumption data to model the daily VA intake and cyanide exposure of African women. The model simulates the BC intake and cyanide exposure of 5000 women, based on per capita consumption for each of six African countries, assuming that all cassava was replaced by currently available biofortified cassava; that this replacement did not influence the health, genetic and microflora status of the consumer or the status of other nutrients or SLAMENGHI factors( Reference De Pee and West 13 ); and that the conversion efficiency of BC to VA did not change as VA stores increased. This model allows the estimation of the percentage of women who would attain their recommended dietary intakes of VA. It also estimates the percentage of women who would exceed the reference dose (RfD) for cyanide exposure. The US Environmental Protection Agency documents risk for health effects (other than cancer and gene mutations) from chronic chemical exposure through the RfD( 14 ). The RfD is the ‘estimate, with uncertainty spanning perhaps an order of magnitude, of a daily oral exposure to the human population (including sensitive subgroups) that is likely to be without an appreciable risk of deleterious effects during a lifetime’. This information allows one to estimate the risk–benefit possibilities of biofortified cassava consumption on a countrywide basis.
Experimental methods
Study design overview
The following materials and methods are described in more detail elsewhere( Reference La Frano, Woodhouse and Burnett 12 ). Briefly, ten American women participated in a randomised cross-over trial, consuming the following treatments: 100 g biofortified cassava; 100 g biofortified cassava with 15 ml added oil; and 100 g typical white cassava with 15 ml oil plus a retinyl palmitate (RP) tracer, with 2-week washouts between treatments. RP and BC concentrations in the TAG-rich layer (TRL) of plasma increased with both biofortified cassava treatments. Changes in RP in the TRL plasma fraction were combined with FAO consumption data to model the daily BC intake and RP formation of African women that could be expected if all of the cassava in their diet was replaced with the variety of biofortified cassava used in our study. Cyanide data from the second and third soaking procedures were used to simulate the effects of marginal processing, such as occurs during drought or in populations unfamiliar with cassava, and was also combined with FAO consumption data to model the daily cyanide exposure of African women from consumption of the current variety of biofortified cassava.
Subjects
A total of ten women (aged 18–45 years) participated in this study. The following procedures were conducted for each woman: height, weight and blood pressure measurements; pregnancy prescreening; and administration of health questionnaires. Subject demographics and inclusion and exclusion procedures are described in more detail elsewhere( Reference La Frano, Woodhouse and Burnett 12 ). As expected, RP formation from biofortified cassava in these women varied greatly. Also as usual, this variation did not depend on demographic characteristics of the subjects such as body weight, BMI or age. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of California, Davis Institutional Review Board no. 20108133, 28 June 2010–26 May 2011; no. 223773, 25 May 2011–16 March 2013. Written informed consent was obtained from all the subjects.
Preparation of cassava
Biofortified cassava (GM 905-69) bred to contain elevated BC concentrations was provided by the International Center for Tropical Agriculture. Typical white cassava, which contained negligible amounts of BC, was acquired from the Las Montanas Supermarket. Biofortified cassava was placed on dry ice and air freighted to San Francisco, and then transported by road to the Western Human Nutrition Research Center, Davis, CA, USA. The roots were washed, peeled and flash frozen, and then stored in a food-safe freezer at −20°C in the Metabolic Kitchen and Human Feeding Laboratory. Before preparation, the roots were thawed overnight at 4°C, and then rinsed twice with deionised (DI) water. Tips from the distal and proximal ends were cut (1–2 cm) and the peel removed. Four volumes of DI water were added to the chopped roots and mixed. It was then refrigerated overnight for 12 h, and then drained. Four volumes of DI water were added to the cassava, mixed and changed every 2 h for 8 h. Following the last draining, the cassava was rinsed with DI water, and simmered (95°C) in ten volumes of DI water for 30 min, until it was just tender. Boiled cassava was drained, cooled and aliquots were stored in 50 ml polypropylene centrifuge tubes wrapped in aluminium foil under N2 at −20°C, in a food-safe freezer. As a precaution to preserve carotenoids, all procedures were conducted under dim light to minimise light exposure.
Cassava meal preparation
The cassava meal has been described elsewhere( Reference La Frano, Woodhouse and Burnett 12 ). Briefly, 100 g cassava were mixed with quick-cooking oatmeal (40 g), imitation butter flavouring (0·5 g), pears canned in light syrup (150 g), salt (0·5 g), honey (21 g), raisins (18·9 g) and unfortified rice milk (245·5 g). The porridge, total weight 577 g, was microwaved until a temperature of 165°F was reached. Two types of BC-enhanced porridge were prepared. The first contained no added oil, but had 6 g of fat in the meal. This meal had a very low fat content, representing the fat content from a monotonous staple diet. The second porridge had 15 ml of added oil, for a total of 20 g fat. This meal had a fat content representing a diversified diet. However, the 20 g fat porridge reflects a less typical fat intake for a single meal (breakfast) of 41 %, which is almost half the average daily per capita fat intake for sub-Saharan Africa( Reference Abrahams, McHiza and Steyn 15 ).
Cyanide analysis
Each step of cassava preparation was analysed for cyanogenic glycosides using a La Motte Cyanide in Water Test Kit (LaMotte). Cassava preparations (1 g) were added to a 15 ml test tube, were mashed with a mortar, and then mixed with 7 ml of DI water by vortexing for 1 min. Preparations were left at room temperature (22°C) for 20 min and then vortexed for 20 s before filtering through a 0·2 µm Pall Gelman Acrodisc (Sigma Aldrich) into a LaMotte test tube. The water extracts were tested by following the manufacturer's specifications. To confirm the results, the final prepared samples were analysed by Applied Specialization and Consulting LLC, Bothwell, WA, using alkaline hydrolysis, distillation and ion chromatography with pulsed amperometric detection.
Diet
Subjects ate a low-fat, low-carotenoid, low-VA diet for 7 d prior to each intervention. For the first 4 d, subjects were asked to avoid dark green, red, orange and yellow fruits and vegetables (which are good sources of carotenoids), liver, giblets, herring, VA-fortified cereals and multivitamins. For the last 3 d prior to each cassava treatment, subjects came to the Western Human Nutrition Research Center and were given a take-home diet that was low fat, low carotenoid and low VA( Reference La Frano, Woodhouse and Burnett 12 ).
Each cassava treatment consisted of one large bowl of cassava porridge, described earlier. This cassava meal was given at breakfast, at about 07·30 hours. The subjects ate no other meals that day until dinner time, approximately 10·5 h later. At dinner time, they were given a low-carotenoid, low-VA, low-fat dinner. Each subject served as her own control and was given all three treatments. Treatments were randomly assigned, with researchers blinded to the treatments given.
Blood collections and analysis
Blood collection and analysis have been described elsewhere( Reference La Frano, Woodhouse and Burnett 12 ). Blood samples were collected from the subjects at baseline and five more times postprandially (at 2, 3·5, 5, 7·25 and 9·5 h). A 1 ml sample of plasma was added to a 2·2 ml polyallomer ultracentrifuge tube (Beckman Coulter) and overlaid with a prepared NaCl salt solution (density = 1·006 kg/l). This was ultracentrifuged in a Beckman Coulter Optima TLX containing a swing-out rotor type Beckman TLA 100 ultracentrifuge at 130 000 g for 20 min at 4°C. The tube was then placed in a Beckman Centritube Slicer and sliced at a fixed position to isolate the TRL plasma fraction( Reference La Frano, Woodhouse and Burnett 12 ).
Extraction of carotenoids and vitamin A
Carotenoids and VA were extracted from 1 g homogenised samples of the cassava porridges and the pre-test day diet using a liquid–liquid extraction method described previously( Reference La Frano, Woodhouse and Burnett 12 ). Liquid–liquid extraction was used to isolate carotenoids and VA within the TRL plasma fraction. Methanol (1 ml) was added to the TRL plasma fraction, and then compounds were extracted twice with 1 ml hexane. Extractions were performed immediately after each blood collection.
Analytical procedure
Plasma and food sample injections (20 µl) were analysed by reversed-phase HPLC with electrochemical detection( Reference La Frano, Woodhouse and Burnett 12 ). The system contained an ESA model 582 solvent delivery system (Dionex (ESA)), an ESA model 542 autosampler and an ESA model 5600 Coularray electrochemical detector. Carotenoids and retinoids were separated with an ESA MD-150 column (150 mm × 3·2 mm, 3 µm pore size), using a CH30 Eppendorf column heater (Eppendorf) at 37°C. Gradient elution was with methanol–0·2 m-aqueous ammonium acetate (90:10, v/v) and methanol–isopropanol–ammonium acetate (78:20:2, by vol.)( Reference La Frano, Woodhouse and Burnett 12 ), at a flow rate of 0·8 ml/min. Cell potentials were set at 400 mV for carotenoids and 700 mV for retinoids. Retention times were 3·2, 12·6, 14·8 and 20·2 min for retinol, echinenone (internal standard), RP and BC, respectively. Samples were analysed in duplicate. Inter-assay precision was monitored using a spiked pooled plasma sample purchased from UTAK. Precision was 6·8 % for RP and 10·8 % for BC.
Model design
Subjects were aged 29 (sd 2) years, had a BMI of 23·1 (sd 7) kg/m2 and normal cholesterol, TAG, glucose, Hb and haematocrit. RP concentrations were 87 (sem 13·5) and 74·9 (sem 11·5) nmol × h/l for biofortified cassava with added oil, and biofortified cassava without added oil, respectively( Reference La Frano, Woodhouse and Burnett 12 ). Initial cyanide concentrations varied from 280 to 5·51 parts per million (ppm), decreasing to 4·04 ppm after the second wash and 0·61 ppm after the third wash. Cyanide was undetectable after five washes.
Data from the feeding study were combined with FAO cassava consumption estimates to model the BC intake, the BC to VA conversion and cyanide exposure of African women. Equations (1)–(3) describe the methodology:
R s is the ratio of VA formed from ingested BC for the sth subject; s ranges from one to ten; it represents each of the ten subjects in the feeding study. RPB s and RPR s are the AUC (nmol × h) of the newly digested RP in the plasma TRL fractions for the biofortified and white cassava with RP tracer, respectively, for the sth subject. RP nmol/l plasma × 0·0427 litre × kg body weight was used for the sth subject to convert from RP/l to RP/total blood plasma volume prior to calculating AUC.
AUC were calculated with the PK package( Reference Jaki and Wolfsegger 16 ). RD is the RP tracer dose (nmol). MW is the molecular weight of VA (286·5 g/mol). D is the mean ingested BC dose from the biofortified cassava, which was 2020 µg.
Equation (1) is adapted from Li et al. ( Reference Li, Nugroho and Rocheford 17 ):
VA ij represents the VA formed (μg/d) for the ith individual, where i ranges from one to 5000 to represent the number of simulated individuals; and for the jth country, where j ranges from one to six to represent each of the six African countries with the highest cassava consumption.
At present, only mean cassava consumption data are available for these countries. The mean consumption data used were from the following six African countries: Angola (378 g/d), Central African Republic (371 g/d), Congo (763 g/d), Ghana (551 g/d), Mozambique (591 g/d) and Nigeria (313 g/d)( 18 ).
C j represents the per capita daily cassava consumption (g) consumed in the jth country. Y is the ratio of the weight of cooked cassava (g)/raw cassava (g), which was 0·71. B is the mean BC content of the cooked cassava (μg/g). R i is the amount of VA formed from the ingested BC, calculated as a sampled ratio derived from a log-normal distribution, which was developed with the means and standard deviations of the log-transformed vector of R s values. R s is described in equation (1) earlier; for the ith individual, with i representing the number of simulated individual ratios (which are indexed from one to 5000).
The number of individuals simulated was increased progressively to 5000 where a stable pattern developed: a 1 % or less change in the means and standard deviations for replicate runs. The log-normal distribution was employed to account for the inter-individual variability of the formation of VA from BC in larger groups of women.
The third equation
evaluates cyanide exposure. Y jp is the cyanide exposure (mg/kg per d) from consumption of the cassava porridge in the jth country, j ranges from one to six (representing the six African countries with the greatest intakes of cassava); and from the pth soak, where p ranges from one to two. This represents the data obtained from the second and third cassava soakings in our preparation procedure. Data from these soakings simulate suboptimal processing( 19 , Reference Montagnac, Davis and Tanumihardjo 20 ). C j represents the daily per capita cassava consumption (g) consumed in the jth country. Y is the cooked cassava yield of 0·71, explained in equation (2) earlier. W p is the cyanide concentration in the raw cassava (mg/kg) after the pth soak. F is a 55·5 % retention factor for cyanide after the cassava is boiled( Reference Montagnac, Davis and Tanumihardjo 20 ). M is the mean body weight (kg) of the ten subjects. Loss of cyanide from microwaving was assumed to be negligible.
Vitamin A and cyanide benchmarks
The following benchmarks were used in the evaluation of intake estimates (μg/d) and exposure estimates (mg/kg per d): the recommended dietary reference intake for VA of 500 µg/d for females 19–65 years of age( 21 ); the safe upper limit (for preformed VA) of 3000 µg/d( 22 ); the RfD for cyanide exposure of 6 × 10−4 (mg/kg per d)( 23 ).
The ratio of cyanide exposure to RfD was employed in the comparison of exposure for different countries. All computational tasks were performed using the R Programming Language and Environment for Statistical Computing (R version 2.13.0, http://cran.r-project.org/).
Results
The control cassava porridge, representing the cassava currently available for use, contained a negligible amount of BC and thus it was estimated that no VA was formed from white cassava. VA intake (μg/d) estimates from consumption of both types of biofortified cassava porridge (6 g fat and 20 g fat) were compared (Fig. 1). Generally, the presence of added fat increased the absorption of BC and its conversion to VA. However, these differences were non significant, probably due to the small number of subjects tested and the large variations between subjects.

Fig. 1. Distribution of individuals' daily vitamin A (VA; μg in log base 10 scale) intake from consumption of both types of porridge: porridge with 6 g fat (![]() ) and porridge with 20 g fat (
) and porridge with 20 g fat (![]() ). Each boxplot indicates: bottom line of box – 1st quartile; centre line of box – median; top line of box – 3rd quartile, with dots showing outliers.
). Each boxplot indicates: bottom line of box – 1st quartile; centre line of box – median; top line of box – 3rd quartile, with dots showing outliers.
The percentage of each country's individual daily intake of VA (μg/d) meeting the FAO VA recommendations of 500 µg/d is presented (Table 1). Biofortified cassava, fed at the average daily intake of people in the respective countries, is estimated to meet the nutrient recommendations of 91·56–99·82 % (6 g fat porridge) and 84·84–97·54 % (20 g fat porridge) of the population of these countries, from the lowest cassava consumption (Nigeria) to the highest cassava consumption (Congo).
Table 1. Summary of individuals meeting vitamin A (VA) recommendations and exceeding the upper limit (UL) for preformed VA

The impact of soaking cassava on cyanide is shown (Table 2). If the cassava was prepared as in our study, with six soaks and rinses, then there would be no detectable cyanide present in the cassava. The soaking method used in our study was developed so that we could monitor the amounts of cyanide and BC during preparation. It is not a common processing method in Africa, although it resembles the common method of soaking cassava in a stream or other sources of running water for several hours. It shows that careful preparation of the biofortified cassava results in a non-toxic product that retains most of its BC. However, water and food scarcities can lead to poorer processing methods( Reference Nzwalo and Cliff 24 , Reference Telos 25 ). Therefore, we estimated the cyanide exposure (μg/kg per d) from consumption of the biofortified cassava variety used, as if it were soaked two or three times. Although the processing procedures differ, the cyanide residues left after two or three soakings are similar to those left after the common African processing methods of heap fermentation or soaking in still water( Reference Padmaja 11 ). After two and three soakings, the cassava retains some residual cyanide (Table 2). The ratio (exposure to RfD) values (Table 3) demonstrated a marked reduction in cyanide exposure between the second and the third soaking: 13·01–31·71 after the second soaking; and 1·96–4·79 after the third soaking; as expected, the lowest exposure would theoretically be in Nigeria, the highest in Congo.
Table 2. Cyanide residue according to the number of soakings
(Mean values and standard deviations)
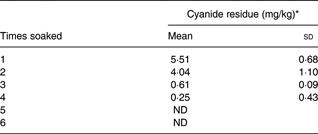
ND, not detected.
* mg cyanide/kg raw biofortified cassava.
Table 3. Summary of cyanide exposure

RfD, reference dose.
* Exposure (μg/kg per d) = mg cyanide/kg raw biofortified cassava multiplied by a 55·5 % boiling retention factor for cyanide( 19 ) multiplied by the consumed cooked cassava yield (g) for each country, all divided by the mean body weight (kg) of the ten subjects.
† Exposure (μg/kg per d)/RfD of 6 × 10−4 (mg/kg per d)( 23 ).
Discussion
This study modelled the effectiveness of BC-enhanced cassava as a means of increasing BC intake in specific African countries with VA deficiency. If the current biofortified cassava variety replaced 100 % of the cassava currently consumed, then it would provide sufficient BC to meet the recommended dietary intake of VA for almost all women. BC intake from the consumption of typical white cassava would be negligible.
Currently, when the potential impact of a biofortified food on VA status is estimated at all, it is by using conversion factors to calculate the mean retinol equivalents or retinol activity equivalents that are formed by the intervention. The conversion factor used by the United States Institute of Medicine to calculate retinol activity equivalents is 12 µg BC:1 µg VA( 22 ). If this conversion factor is used to estimate VA intake, then individuals eating the average amounts of cassava in Angola, Central African Republic, Congo, Ghana, Mozambique and Nigeria would consume 451, 443, 910, 657, 705 and 373 µg/d, respectively. Our distributional analysis (Fig. 1) should be a superior method of analysis since it addresses inter-individual variation. Thus, it estimates the range of VA that individuals would attain from this intervention and the number of individuals meeting or failing to meet recommendations. To our knowledge, this is the first distributional analysis of the potential impact of a biofortified food on VA status in any population.
Mean VA intake from the consumption of biofortified cassava met dietary recommendations, on average (500 µg/d), for the six countries for 96 % of the individuals eating the lower-fat cassava (6 g fat porridge). Despite the fact that added oil increased the mean intake estimates of VA in every country, it also increased the variability in the amount ingested. Because of this increase in variability, the average percentage of people meeting their dietary recommendation actually decreased slightly, to 92 % (20 g fat porridge). Thus, the present results suggest that the low intakes of fat typical in Africa are sufficient to convert the BC in biofortified cassava to VA.
Our calculations suggest that the amount of VA formed from biofortified cassava would greatly exceed recommendations for some women who replaced white cassava with biofortified cassava in their diets (Fig. 1). However, the upper limit for VA is for preformed VA, and not for VA formed from carotenoids( 22 ). Although many aspects of carotenoid metabolism remain obscure, it is known that high carotenoid concentrations are poorly absorbed and converted to VA. For example, ingesting too much preformed VA can cause acute toxicity, even death( Reference Adams 26 , Reference Castaño, Etchart and Sookoian 27 ). High carotenoid intakes do not cause these acute symptoms even when intakes are prolonged. In fact, carotenoids were originally believed to be essentially non-toxic even at very high dosages, and no upper limit for carotenoids has ever been set. Unfortunately, results from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study and the Beta-Carotene and Retinol Efficacy Trial (CARET) lung cancer trials have demonstrated that smokers who ingested pharmacological doses of BC increased their risk for cancer, suggesting that it is useful to take extra caution when consuming higher than recommended carotenoid intakes( 22 , Reference Rautalahti, Albanes and Virtamo 28 , Reference Omenn, Goodman and Thornquist 29 ). Because food-based interventions are intended to reach all populations including young children and pregnant women, we believe it is appropriate to take a conservative approach. Furthermore, these equations can be used for other nutrients and toxicants, where estimating the upper safe limit of intake is more useful.
The present results suggest that food-based interventions in which biofortified cassava is substituted for typical cassava should be monitored for signs of carotenoid overconsumption. Fortunately, this can be monitored easily since consuming large amounts of carotenoids can lead to carotenodermia( Reference Roe 30 , Reference Micozzi, Brown and Taylor 31 ), which appears as a yellowing of the skin.
For all the ten subjects, the conversion ratio of BC to VA varied 3400 % (0·3–10·6:1) for the 20 g porridge compared with 900 % (1·4–12·1:1) for the 6 g fat porridge; this greater variation is also depicted by a greater interquartile range for the 20 g fat porridge as compared with the 6 g fat porridge (Fig. 1). The wide range of conversion ratios of BC to VA seen in this study are as expected; similar ranges are seen in other studies, even those that provide BC as purified supplements instead of food( Reference Ho, de Moura and Kim 32 , Reference Hickenbottom, Follett and Lin 33 ). In fact, subjects living under controlled conditions, with very similar demographic characteristics and health histories, have widely divergent values for carotenoid absorption and conversion( Reference Ho, de Moura and Kim 32 – Reference Burri and Park 34 ). The reasons for this variation are not completely understood, although several genetic polymorphisms appear to be important( Reference Lietz, Oxley and Leung 35 – Reference Lietz, Lange and Rimbach 37 ). Since demographic characteristics are not predictive of BC absorption and metabolism, they are not included in our model.
The cassava consumed in this study contributed no cyanide to the diet. We prepared the cassava in our study by rinsing and soaking six times. This method allowed us to control the detoxification process and ensure that the volunteers in our study consumed no cyanide. However, this is not a typical method of preparing cassava in Africa. Popular detoxification methods in Africa include heap drying, soaking in running or still water and fermentation( Reference Padmaja 11 , 19 , Reference Hahn, Hahn, Reynolds and Egbunike 38 ). Some of these methods, especially garification, remove most of the cyanide from cassava; although the most common preparation methods leave cyanide residues, which can exceed the WHO guideline of 10 mg HCN equivalents/kg( 39 , Reference Cardoso, Mirione and Ernesto 40 ). Furthermore, even highly processed cassava-based foods can contain residual cyanide. For example, 77 % of the cassava chips tested in Australia exceeded the guideline, with a mean of 64·2 mg HCN equivalents/kg( Reference Miles, Jansson and Mai 41 , 42 ). This mean concentration is greater than our cyanide residues after soaking once (Table 2). Even so, individuals living in Congo who prepare cassava by methods that leave cyanide residues similar to our data from soaking cassava twice could be exceeding the RfD by thirty-two times (Table 3). These results suggest that cyanide levels in biofortified cassava and its preparations could be a health concern.
Certain limitations on the BC intake and cyanide exposure modelling must be addressed. Per capita data were used in this study and represent the average intake for an entire country, which does not account for inter- and intra-individual variation in eating habits. Currently, data on inter- and intra-individual variation of cassava intake for these countries nationwide are unavailable, and there is little information on the range of cassava consumption even at the local level. However, studies show a range of consumption from 0 to 75 % of energy, with means ranging from 15 to >50 % energy( Reference Stephenson, Amthor and Mallowa 43 , Reference Lancaster, Ingram and Lim 44 ). More studies are required in order to gather detailed food consumption data for African countries which will improve the accuracy of both the VA and the cyanide estimates.
A second limitation is that this study used ratios of VA conversion from ingested biofortified cassava for ten American women. These ratios will most probably differ from those of African women because of differences in the frequency of genetic polymorphisms( Reference Lietz, Oxley and Leung 35 – Reference Lietz, Lange and Rimbach 37 ), microflora, health history, VA status and diet( Reference De Pee and West 13 ). The techniques provided herein can be applied to studies of larger groups of people in Africa to increase the accuracy of these ratios.
Furthermore, the approach outlined in this study is not only suitable for the estimation of the risk–benefit from consumption of biofortified cassava, but can also be used to calculate the risk–benefit of other dietary interventions. Cassava crossbreeding and specific processing have been employed in this study to demonstrate a means of increasing nutrient intake, while minimising exposure to a food component of toxicological concern. The approach used in this study can be used for other foods with similar nutrient toxicological concerns.
Acknowledgements
This work was supported by Western Human Nutrition Research Center in-house funds, CRIS project no. 5306-51530-018. The human study upon which these results were based was supported by HarvestPlus grant no. 8227; and was made possible by grant no. 2UL1RR024146 from the National Center for Research Resources, a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research. The authors have no conflicts of interest. B. J. B., C. K. W. and J. M. K. designed the research; J. M. K. and M. R. L. F. conducted the research and analysed the data; J. M. K., M. R. L. F., and B. J. B. wrote the paper; B. J. B. and J. M. K. had primary responsibility for the final content. All authors read and approved the final manuscript. The United States Department of Agriculture is an equal opportunity employer and provider.






