There is uncertainty around the optimal dose of quetiapine in the treatment of schizophrenia. Clinicians in practice prescribe quetiapine at substantially higher dose than that established in clinical trials. Reference Citrome, Jaffe, Levine and Lindenmayer1 In a recent comprehensive review, Reference Sparshatt, Jones and Taylor2 the authors concluded that the balance of evidence does not support the belief that higher dosages are required for a full therapeutic response. The present meta-analysis is an attempt to answer this dilemma through combining data available from fixed-dose double-blind controlled studies, which are taken as the most robust evidence in such a dose–response relationship scenario. Reference Sparshatt, Jones and Taylor2 The aim was to look for any definitive and categorical significant differences in efficacy and effectiveness between low- and high-dose quetiapine in the acute treatment of schizophrenia.
Method
In August 2007, the following databases were searched: PubMed, EMBASE, PsycINFO, AMED (Allied and Complementry Medicine), CINHAL and SSCI (Social SciSearch), with the search terms ‘quetiapine AND schizophrenia’. For this meta-analysis, only fixed-dose, double-blind, randomised controlled trials in the acute treatment of schizophrenia were included. Cross-references of identified articles were checked manually and AstraZeneca in the UK was contacted to access any missing data. The search identified a total of seven fixed-dose published trials. Reference Arvanitis and Miller3–Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 Single fixed-dose trials Reference Emsley, Raniwalla, Bailey and Jones6,Reference Conley, Kelly, Nelson, Richardson, Feldman and Benham8 and the studies with clearly sub-therapeutic dosage of quetiapine (50 mg/day), Reference King, Link and Kowalcyk4,Reference Barzega, Bogetto, Maina and Ravizza5 were excluded from the analysis. A pilot study Reference Roy Chengappa, Parepally, Brar, Mullen, Shilling and Goldstein7 (n = 21) that included participants with schizoaffective disorder was also excluded. This ultimately led to the inclusion of only two studies. Reference Arvanitis and Miller3,Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 Quality analysis of the included studies was carried out as per the protocol of the Centre for Reviews and Dissemination (CRD). 10 Individual and pooled effects of studies were expressed in the form of odds ratio and standardised mean difference with 95% confidence intervals. A fixed or random effect model was chosen according to the level of heterogeneity within the studies, for which the chi-squared method was used.
Results
Tables 1 and 2 show the descriptive and pooled results.
Table 1 Details of studies included in meta-analysis
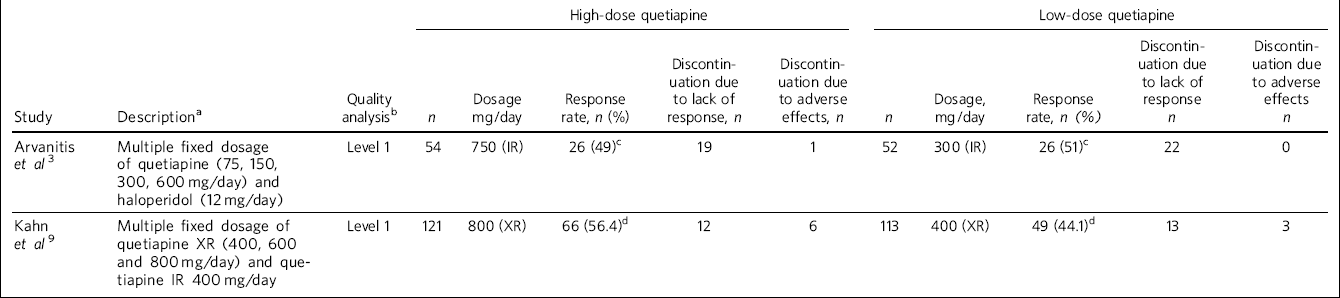
| High-dose quetiapine | Low-dose quetiapine | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Descriptiona | Quality analysisb | n | Dosage mg/day | Response rate, n (%) | Discontinuation due to lack of response, n | Discontinuation due to adverse effects, n | n | Dosage, mg/day | Response rate, n (%) | Discontinuation due to lack of response n | Discontinuation due to adverse effects n |
| Arvanitis et al Reference Arvanitis and Miller3 | Multiple fixed dosage of quetiapine (75, 150, 300, 600 mg/day) and haloperidol (12 mg/day) | Level 1 | 54 | 750 (IR) | 26 (49)c | 19 | 1 | 52 | 300 (IR) | 26 (51)c | 22 | 0 |
| Kahn et al Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 | Multiple fixed dosage of quetiapine XR (400, 600 and 800 mg/day) and quetiapine IR 400 mg/day | Level 1 | 121 | 800 (XR) | 66 (56.4)d | 12 | 6 | 113 | 400 (XR) | 49 (44.1)d | 13 | 3 |
Table 2 Pooled results for meta-analysis

| Results | Response rate | Discontinuation due to lack of response | Discontinuation due to adverse effects |
|---|---|---|---|
| Odds ratio (95% CI) | |||
| Arvanitis et al Reference Arvanitis and Miller3 | 0.93 (0.43-1.99) | 0.74 (0.34-1.62) | 7.12 (0.14-359.12) |
| Kahn et al Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 | 1.63 (0.97-2.74) | 0.85 (0.37-1.94) | 0.47 (0.12-1.77) |
| Test of heterogeneity,a Q (P) | 1.44 (0.22) | 0.05 (0.82) | 1.66 (0.19) |
| Pooled effect (95% CI)b | 1.36 (0.88-2.10) | 0.78 (0.44-1.39) | 0.64 (0.19-2.17) |
Publication bias
As only two studies were included, funnel chart statistics were not feasible.
Heterogeneity of studies
No significant heterogeneities were found between the studies with regard to the response rate, discontinuation as a result of lack of response or as a result of adverse effects, but heterogeneity existed for positive symptoms scores (P<0.05).
Pooled results
There was no statistically significant difference between high- and low-dose quetiapine in terms of the response rate and the discontinuation due to lack of response or due to adverse effects. An alternate analysis was done for the response rate after excluding those individuals who had dropped out from the total number of participants. Again, the odds ratio in favour of high-dose quetiapine was not statistically significant (OR = 1.40, 95% CI 0.80–2.44). There was no statistically significant difference between high- and low-dose quetiapine for improvement in positive symptoms score (Fig. 1).

Fig 1 Change in positive symptoms score (forest plot). IV, inverse variance.
Discussion
Findings of this meta-analysis are in a line with those of Sparshatt et al Reference Sparshatt, Jones and Taylor2 and Buckley. Reference Buckley11 Buckley Reference Buckley11 undertook a combined analysis of three randomised, placebo-controlled trials and divided participants into two groups – those receiving quetiapine <400 mg/day and those receiving >400 mg/day. Although differences in the Brief Psychiatric Rating Scale positive symptom cluster scores was numerically greater in the higher dosage group it was not statistically significant. The possibility of this difference becoming significant is raised if the study by Kahn et al Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 (which shows a statistically significant relationship between increasing dosage and therapeutic effect) is included. Reference Sparshatt, Jones and Taylor2 Present meta-analysis shows that this is not the case as standardised mean difference on positive symptoms score is not significantly different in both groups (Fig. 1). It is possible that high-dose quetiapine might prove to be superior in the long term as these trials were only 6 weeks long. Also, certain participants with treatment resistance or comorbid substance misuse, who are not represented in these trials, might respond only to the high-dose quetiapine. From the effectiveness prospective, high- and low-dose quetiapine do not show very different discontinuation rates, but the small number of participants included in the analysis and the very wide range of the confidence interval raises the question of the validity of these results.
The major limitation of this meta-analysis is that only two studies Reference Arvanitis and Miller3,Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 could be included in the meta-analysis, which not only adds a significant publication bias but also limits the power of the study to give any definitive answer. Regarding heterogeneity, both studies used different preparations of quetiapine and different scales for measuring outcome. Kahn et al Reference Kahn, Schulz, Palazov, Reyes, Brecher and Svensson9 excluded people with treatment resistance, substance misuse and a hospital stay >1 month; whereas in the study by Arvanitis et al Reference Arvanitis and Miller3 all the participants were in-patients. Also, it should be remembered that limitations inherent to individual studies are carried over in meta-analyses; and meta-analyses tend to neglect the specifications of the individual studies.
In conclusion, this meta-analysis does not prove the therapeutic superiority of high-dose quetiapine in acute treatment of schizophrenia; both in terms of efficacy and effectiveness. From a clinical practice point of view, in general, 300–400 mg/day seems to be the optimal dose of quetiapine and the common practice of targeting quetiapine dosage to 600 mg/day or above is not supported by the evidence from fixed-dose trials.
Acknowledgements
Sincere thanks to Dr Subodh Dave (Consultant Psychiatrist and Clinical Teaching Fellow, Derby City General Hospital) for his valuable suggestions in revision of the manuscript.






eLetters
No eLetters have been published for this article.