Adjustment for total energy intake is usually important in epidemiological analyses for the same reasons that isoenergetic diets are used in experiments to evaluate the effects of specific nutrients( Reference Willett 1 ). When total energy intake is associated with the outcome, it can operate as a confounding variable because intakes of most nutrients are associated with energy intake, often strongly. Even if not associated with the outcome, differences in total energy intake can result in extraneous variation in nutrient intake due to individual differences in physical activity, body size and metabolic efficiency. In this situation, failure to adjust for total energy intake can lead to misclassification of the biologically important variation in nutrient intake and result in attenuation of associations.
Variation in total energy intake can also arise from measurement error; indeed, total energy intake is usually measured less well than other nutrients because it can depend on details of serving sizes that are difficult to measure. For this reason, adjustment for reported energy intake has been criticized as being inadequate to control for energy intake in epidemiological studies( Reference Jakes, Day and Luben 2 , Reference Black, Prentice and Goldberg 3 ). Jakes et al. have suggested that adjusting for body weight and physical activity might be better than using energy intake estimates from FFQ or food diaries( Reference Jakes, Day and Luben 2 ). However, using body size and physical activity as independent surrogate measures for total energy does not yield the benefit of cancelling correlated errors. Because both nutrient and total energy intake measurements are computed from the same foods, their errors will be strongly correlated. Adjustment for energy intake using the nutrient density or residual method will result in these correlated errors cancelling each other out, thus improving the validity of the energy-adjusted nutrients. For this reason, in most studies using diet records( Reference Willett, Sampson and Stampfer 4 ), 24 h recalls( Reference Munger, Folsom and Kushi 5 ) or biomarkers( Reference Sun, Ma and Campos 6 ) as the standard, adjustment for self-reported energy intake has improved correlations with nutrient intakes measured by FFQ.
In a commentary on the Jakes et al. paper, Spiegelman notes that adjustment for physical activity and body weight will also be an imperfect method for energy adjustment because these are also measured with error( Reference Spiegelman 7 ). This is especially true for physical activity, even when measured by a heart-rate monitor, in part because this is a relative short-term measure and the reference period for dietary intake is usually longer; for example, a year in typical epidemiological studies. If long-term average total energy intake and total energy expenditure estimated by body weight and physical activity are equivalent conceptually, then a logical question to consider is which of these would be superior for adjusting nutrient intakes in epidemiological analyses. To our knowledge, there have been no other reports on direct comparison of these different energy-adjustment methods. Therefore, we calculated energy-adjusted nutrients using self-reported energy intake from FFQ as typically done in epidemiological analyses and also using physical activity and body weight, and determined the better of the two methods by examining correlations between energy-adjusted nutrients and their corresponding biomarkers.
Experimental methods
Study population
The Nurses’ Health Study (NHS) was established in 1976 and enrolled 121 700 registered female nurses aged 30–55 years( Reference Colditz and Hankinson 8 ). All participants in the current analysis were women in the NHS who were included in nested case–control studies of fatty acids (measured in erythrocytes and plasma) and CHD, or carotenoids and retinol (measured in plasma) and breast cancer. Both used blood drawn between 1989 and 1990 and stored in liquid nitrogen; the details of the studies have been published elsewhere( Reference Sun, Ma and Campos 9 , Reference Tamimi, Hankinson and Campos 10 ). Briefly, the first study consisted of 166 cases and 327 controls in which the cases of non-fatal myocardial infarction or CHD death were newly diagnosed between the time of blood draw and June 1996( Reference Sun, Ma and Campos 9 ). Controls were selected from the rest of the non-diseased participants and matched for age, smoking status and fasting status at blood draw. All study participants were free of cancers and CVD at the time their blood was drawn. The second study consisted of cases with incident invasive or in situ breast cancer, diagnosed by 1 June 1998, among those who returned a blood sample( Reference Tamimi, Hankinson and Campos 10 ). Women who had no prior cancer diagnosis except for non-melanoma skin cancer were randomly selected as controls matched to cases on birth year, menopausal status, postmenopausal hormone use, time of day and month, and fasting status at the time of blood draw, leaving 969 matched pairs with plasma carotenoids and retinol data available for analysis. The study was approved by the Institutional Review Board, the Brigham and Women's Hospital and/or the Harvard School of Public Health.
Data assessment
In the NHS, diet was first assessed in 1980 and has been updated every 2–4 years by self-administered semi-quantitative FFQ that have been evaluated for reproducibility and validity( Reference Willett, Sampson and Stampfer 4 , Reference Willett, Reynolds and Cottrell-Hoehner 11 ). Information on health and disease status, reproductive variables and other non-dietary covariates was collected at baseline and updated by follow-up questionnaires every 2 years. In the current analysis, we used the average dietary data from the 1986 and 1990 questionnaires due to their proximity in time to the blood draw. We carried forward values from the 1986 questionnaire to replace missing values in the 1990 questionnaire, and if data were missing for 1986, we only used the dietary data from 1990. We excluded participants with missing dietary data for both 1986 and 1990.
Dietary information was assessed using validated semi-quantitative FFQ. Participants were asked to specify, on average, how often they consumed each food as indicated by the unit or portion size on the questionnaire during the previous year. The frequencies of consumption were listed in a multiple-choice fashion as: ‘almost never’, ‘one to three times per month’, ‘once per week’, ‘two to four times per week’, ‘five to six times per week’, ‘once per day’, ‘two to three times per day’, ‘four to five times per day’ or ‘six or more times per day’. Total energy and nutrient intakes were then calculated by multiplying the frequency of consumption of the specified unit or portion size of food by its nutrient content, and summed across all foods. For margarines, breakfast cereals and cooking oils we collected information about specific types and brands; this information was used in the calculation of energy and other nutrients.
Participants were asked the amount of time spent on leisure-time physical activities such as walking and hiking, jogging, running, bicycling, swimming, tennis, squash, racquetball, and calisthenics and other aerobic exercise. From this information, the weekly energy expenditure in metabolic equivalent task hours (MET-h) was computed( Reference Ainsworth, Haskell and Leon 12 ). A MET is defined as the ratio of work metabolic rate to a standard RMR of 1·0. 1 MET is defined as RMR obtained during quiet sitting( Reference Ainsworth, Haskell and Leon 12 ). We used the average physical activity data from the 1988 and 1992 questionnaires, and carried forward values from the 1988 questionnaire to replace missing values in the 1992 questionnaire. If data were missing from the 1988 questionnaire, we only used the physical activity data from 1992. We excluded participants with missing physical activity data for both 1988 and 1992. For body weight, we used the average weight from the 1988 and 1990 questionnaires. The physical activity questionnaire has been tested for validity and reproducibility. In the NHS II cohort, another cohort study of nurses, the correlation between physical activity reported on 1-week recalls and that reported on the questionnaire was 0·79, whereas the correlation between moderate-to-vigorous activity recorded in physical activity diaries and that reported on the questionnaire was 0·62( Reference Wolf, Hunter and Colditz 13 ). Self-reported body weight has been reported to be highly correlated with average weight measured by two technicians (r = 0·96) in the NHS( Reference Willett, Stampfer and Bain 14 ).
In assessing correlations between dietary and plasma carotenoids and retinol, we limited the analysis to women who were not current smokers (n 1540) because an earlier study showed that the correlation between dietary and plasma carotene was lower in smokers compared with non-smokers despite only a slight difference in dietary intake of carotenoids( Reference Stryker, Kaplan and Stein 15 ).
Statistical analysis
Since both cases and controls were free of disease at the time of blood collection in the nested case–control studies, cases as well as controls from both studies were considered for analysis. After exclusions, 442 participants were included in the final analysis of fatty acids and 1254 in the carotenoids and retinol analyses. After examining the distribution of the data, all nutrient intake and biomarker variables were log-transformed to improve normality. We used the residual method to adjust dietary fatty acids and carotenoids for total energy by performing the regression of nutrient intakes v.: (i) self-reported total energy intake derived from FFQ; and (ii) body weight and physical activity.
We computed correlation coefficients between energy-adjusted fatty acid intakes and corresponding plasma and erythrocyte fatty acids and between energy-adjusted intakes of carotenoids and retinol and their plasma biomarkers. Plasma carotenoids and retinol were adjusted for serum cholesterol because they were positively associated with total cholesterol (P < 0·05; data not shown). Adjusting for total cholesterol would, thereby, reduce extraneous variation in plasma carotenoid and retinol levels and add precision to the analysis, so that plasma carotenoid and retinol levels could better represent dietary intake of these nutrients. We also computed correlation coefficients between unadjusted intakes of fatty acids, carotenoids and retinol and their corresponding biomarkers. A Wolfe's test was used to compare dependent correlation coefficients using a two-sided α level of 0·05( Reference Rosner 16 ). To conduct the Wolfe's test, the crude and energy-adjusted dietary variables as well as all biomarker variables were standardized by deriving Z-scores. The SAS statistical software package version 9·1 was used for all statistical analyses.
Results
General demographic characteristics, and distributions of dietary variables and their respective biomarkers, are presented in Table 1 for 442 women in the fatty acid analysis and 1254 women in the carotenoid analysis. The mean daily total energy intake was 7347 (sd 2092) kJ for women in the fatty acid data set and 7406 (sd 2916) kJ for women in the carotenoid data set. The mean body weight was 69·0 (sd 14·2) kg for women in the fatty acid data set and 68·0 (sd 12·8) kg for women in the carotenoid data set, and the mean physical activity was 17·1 (sd 16·0) MET-h/week for women in the fatty acid data set and 18·1 (sd 18·5) MET-h/week for women in the carotenoid data set. Nutrient intakes are presented as crude intakes. In the fatty acid data set, the mean intake of linoleic acid (18 : 2n-6) was the highest (10·2 (sd 3·9) g/d) and the mean intake of DHA (22 : 6n-3) was the lowest (0·16 (sd 0·12) g/d). In the carotenoid data set, the mean intake of lycopene was the highest (6·95 (sd 3·93) mg/d) and the mean intake of β-cryptoxanthin was the lowest (0·20 (sd 0·11) mg/d). The mean daily intake of retinol was 1868 (sd 1468) μg.
Table 1 Distributions of general demographic variables, crude dietary intakes of fatty acids, carotenoids and retinol, and their respective biomarkers; the Nurses’ Health Study, 1989–1990
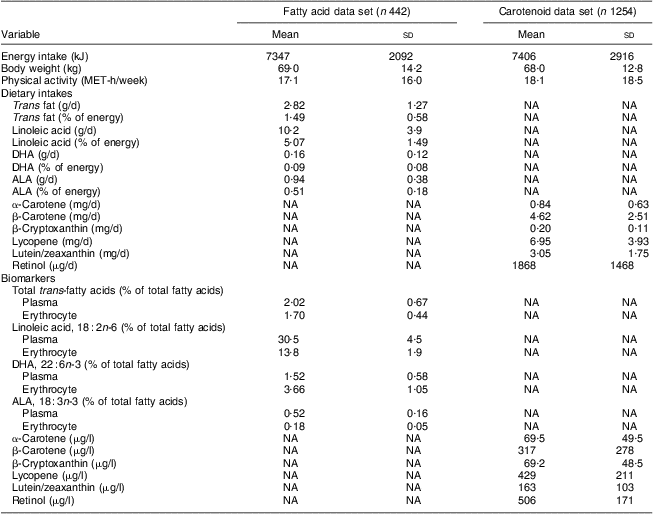
ALA, α-linolenic acid; NA, not available.
Correlations between dietary fatty acids, carotenoids, retinol and total energy intake as well as their respective blood biomarkers are presented in Table 2. Among the dietary fatty acids, trans-fatty acids, linoleic acid and α-linolenic acid (18 : 3n-3) were strongly correlated with total energy intake (r = 0·69, 0·76 and 0·66, respectively), whereas DHA was weakly associated with total energy (r = 0·23). Correlations of dietary carotenoids and retinol with total energy intake ranged from 0·23 to 0·44. Overall, adjustment for body weight and physical activity had little impact on the correlations between dietary fatty acid intakes and plasma or erythrocyte fatty acid levels. For trans-fatty acids and linoleic acid, energy-adjusted dietary intake was more strongly correlated with both plasma and erythrocyte biomarkers compared with intake adjusted for body weight and physical activity. The test for differences between correlation coefficients showed that correlations for energy-adjusted dietary trans-fatty acids with plasma and erythrocyte levels (r = 0·30 and 0·37, respectively) were significantly different from those for unadjusted dietary trans-fatty acids and the corresponding plasma and erythrocyte biomarkers (P for difference < 0·001 and P for difference = 0·001, respectively). The differences in the correlation coefficients for energy-adjusted dietary trans-fatty acids and plasma and erythrocyte trans-fatty acid levels, and the correlation coefficients for intakes adjusted for body weight and physical activity and the corresponding plasma and erythrocyte biomarkers, were also statistically significant (P for difference < 0·001 and P for difference = 0·001, respectively). As for linoleic acid, the difference between the correlation for energy-adjusted intake of linoleic acid with plasma level and that for body weight- and physical activity-adjusted intake with plasma level was not statistically significant (P for difference = 0·28).
Table 2 Correlations between intakes of specific fatty acids (n 442) and carotenoids (n 1254) and their respective biomarkers; the Nurses’ Health Study, 1989–1990
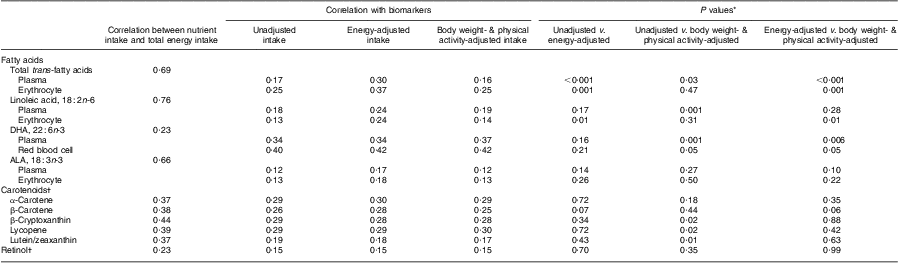
ALA, α-linolenic acid.
*Wolfe's test for comparison of dependent correlation coefficients, two-sided t test.
†Plasma carotenoids and retinol were adjusted for serum cholesterol.
The correlation between dietary DHA and plasma DHA levels was the strongest when dietary intake was adjusted for weight and activity (r = 0·37 v. 0·34 for unadjusted; P for difference = 0·001 and r = 0·37 v. 0·34 for energy-adjusted; P for difference = 0·006). For erythrocyte DHA, there were no significant differences in correlations across different energy-adjustment methods. Although energy adjustment yielded slightly stronger correlations between dietary α-linolenic acid and its plasma and erythrocyte biomarkers compared with weight and activity adjustment, tests for differences between correlation coefficients showed no statistical significance (P for difference > 0·05).
For most carotenoids and retinol, no major differences were observed across different energy-adjustment methods. Adjustment of intake for weight and activity slightly decreased correlations between dietary intake and the plasma biomarkers for β-cryptoxanthin and lutein/zeaxanthin, whereas adjusting for weight and activity significantly increased the correlation between dietary lycopene intake and its plasma biomarker. However, there were no significant differences between plasma carotenoid levels and intakes of these carotenoids adjusted for energy intake compared with intakes adjusted for weight and activity.
Discussion
In the present study, for nutrients that were strongly correlated with energy intake including trans-fatty acids and linoleic acid, energy-adjusted intake using total energy intake derived from the same FFQ used to calculate the nutrients yielded stronger correlations with their biomarkers than nutrients adjusted for body size and physical activity. In contrast, adjusting for weight and physical activity yielded stronger correlations for plasma DHA compared with adjusting for reported energy intake. This may have been due to chance because the difference in correlation coefficients was quite small and was seen only in the case of plasma DHA. Adjustment using either method had only small effects on correlations between DHA, dietary carotenoids and retinol and their corresponding biomarkers, probably due to their weak correlation with total energy intake.
For plasma linoleic acid, the difference between the correlation coefficient for unadjusted intake and that for weight- and physical activity-adjusted intake was statistically significant whereas the difference between the correlation coefficient for unadjusted intake and that for energy-adjusted intake was not statistically significant, even though the absolute difference in correlation coefficients was larger for unadjusted v. energy-adjusted intakes. This is because the power to detect a difference in correlations depends on the correlation between the two variables that are being compared with the ‘gold standard’, or in this case, the biomarker. If the two variables are highly correlated with each other, the variance will be small and the power to detect a difference in their relative correlations increases( Reference Dattalo 17 ). In the case of plasma linoleic acid, the correlation between unadjusted intake and weight- and physical activity-adjusted intake was high (r = 0·998; data not shown), whereas the correlation between unadjusted intake and energy-adjusted intake was 0·619 (data not shown). As such, we expect the power to detect a difference in correlations to be greater when comparing correlations of unadjusted dietary linoleic acid with its plasma biomarker v. weight- and physical activity-adjusted intake with the biomarker than when comparing correlations of unadjusted dietary linoleic acid with the biomarker v. energy-adjusted intake with the plasma biomarker.
The rationale behind adjusting for total energy intake in nutritional epidemiology is to control for confounding, remove extraneous variation resulting from factors like body weight, physical activity and metabolic efficiency, and simulate a dietary intervention in which the focus is on dietary composition rather than absolute intake of nutrients( Reference Willett, Howe and Kushi 18 ). Intakes of many nutrients are strongly correlated with total energy intake, so associations of absolute intakes with disease risk may simply be due to confounding by total energy intake. For example, in most prospective studies, total energy intake has been inversely associated with coronary artery disease risk( Reference Willett 1 ). It is not that an increase in overall food consumption reduces the risk of coronary disease per se, but this inverse association can largely be explained by the protective effect of physical activity. Furthermore, individuals with high total energy intake also consume greater amounts of specific nutrients, so absolute intakes of many nutrients also tend to be inversely associated with coronary disease risk( Reference Gordon, Kagan and Garcia-Palmieri 19 ). Since total energy intake stays relatively constant for an individual unless substantial changes are made in physical activity level or body size( Reference Willett, Howe and Kushi 18 ), intakes of specific nutrients are altered mainly by changing the composition of the diet rather than simply adding or subtracting an absolute amount of such nutrients( Reference Willett 1 , Reference Spiegelman 7 ). Therefore, the focus of epidemiological analyses should usually be on dietary composition rather than absolute intake of nutrients.
As such, epidemiological analyses should usually be performed to resemble controlled-feeding metabolic studies; that is, an isoenergetic substitution model should be used to evaluate the effects of specific nutrients independent of total energy intake. While the importance of adjusting for total energy intake is widely accepted, there has been some debate over which approach accomplishes this most effectively. The findings by Jakes et al.( Reference Jakes, Day and Luben 2 ) support adjustment for body weight and physical activity, but this method does not the render methodological advantages of adjusting for energy intake that are supported by the findings of our study. Compared with adjustment using body weight and physical activity, a major advantage of using total energy intake calculated from the same dietary data used to calculate the specific nutrient of interest is that it will ‘cancel’ correlated errors and thus improve the validity of energy-adjusted nutrients( Reference Spiegelman 7 , Reference Willett 20 , Reference Kipnis, Subar and Midthune 21 ). The correlation of errors for macronutrients can be very high because the nutrient and energy will be derived from many of the same foods, thus the impact of cancelling these errors can be significant. Also, physical activity is measured with substantial error because questionnaires cannot capture fine motor movements or details of intensity; whether objective measures like heart-rate monitoring are more valid is not at all clear and these are impractical for large epidemiological studies. Therefore, adjustment for total energy intake calculated from the same method as used to calculate nutrient intake, in this case an FFQ, not only provides the benefit of reducing extraneous variation and controlling for confounding, but also of improving the validity of energy-adjusted nutrients by partially adjusting for measurement error. This is also likely to apply to other methods of dietary assessment, but it would be desirable to evaluate this directly.
In the present study, we have investigated the study hypothesis using the residual method for energy adjustment. Other methods we could have used are the nutrient density method or the energy partition method. Nutrient densities are computed by dividing nutrients by total energy intake, so the nutrient density method can provide a simple and practical way of representing nutrient intake as a percentage of total energy intake for the general public( Reference Willett 1 ). However, in studying diet–disease relationships, placing the total energy intake variable in the denominator does not completely remove or adjust for the effect of total energy intake, and in some cases, may create unnecessary variation. If total energy intake is associated with disease, a nutrient density variable can also appear to be spuriously associated with disease in the direction inverse of that of total energy intake, even when the nutrient variable itself is not associated with disease outcome of interest( Reference Willett 1 ). Therefore, the nutrient density method cannot be considered appropriate for the purpose of energy adjustment. The energy partition method is also an inadequate energy-adjustment method because in the energy partition model, total energy intake is not held constant. In other words, a change in absolute intake of one macronutrient can lead to a proportional change in total energy intake, and thus, this model cannot be considered an isoenergetic substitution model that can be used to examine the effects of nutrients independent of total energy intake( Reference Willett 1 ). When we ran our main models using variables that had been adjusted for energy using the nutrient density model, we found correlations that were almost exactly the same as correlations obtained from the residual method for both fatty acids and carotenoids. Therefore, using the nutrient density model did not alter the main findings of the study (data not shown).
As seen in our data, the effects of adjusting for energy depend on the nutrient under investigation. As for DHA, carotenoids and retinol in the present study, energy adjustment may have little effect on nutrients that are not strongly correlated with total energy intake, and the benefits of energy adjustment may tend to be greater for nutrients that are more strongly correlated with total energy intake such as trans-fatty acids, linoleic acid and α-linolenic acid. However, the degree of correlation with energy intake can vary depending on the demographic variation in a population, dietary patterns and the dietary assessment method, so that energy adjustment is desirable in most analyses unless shown to have no benefit. Importantly, concentration biomarkers such as plasma or erythrocyte biomarkers should be used in these evaluations as they reflect the internal dose of nutrients( Reference Willett 1 ). Biomarkers such as 24 h urinary excretion measurements reflect absolute intakes and are therefore not appropriate for evaluating energy-adjusted intakes.
The increase in correlation coefficients for energy-adjusted nutrients observed in the present study is probably due to a combination of reduction in correlated measurement errors between intake of the nutrient and total energy and also reduction of extraneous variation in energy intake due to body size, physical activity and metabolic efficiency. The fact that adjustment for body weight and physical activity did not improve correlations suggests that reduction in measurement error was most important, although this conclusion must be tempered by the fact that physical activity itself is measured with considerable error and it is lean mass, not fat mass, that primarily determines energy expenditure. This may have been a limitation of our study as participants were of the same gender and similar ages. Also, it is likely that intakes assessed by FFQ are already partly energy-adjusted to the extent that individuals with higher total food intakes simply consume larger portion sizes and this may not be accounted for by the questionnaire completely.
Conclusion
Our findings document that though the effect of energy adjustment varies with the nutrient being examined, adjustment for total energy calculated from the same dietary questionnaire used to estimate nutrient intakes can significantly improve the correlation of some nutrients with their biomarkers. For the nutrients examined in the present study, adjustment using body weight and physical activity had only a small effect on these correlations. Therefore, while inclusion of body size and physical activity in statistical models may be appropriate if they are predictors of the outcome, this does not appear to be a sufficient method to adjust for total energy intake in epidemiological studies.
Acknowledgements
Sources of funding: This study was supported by the National Institutes of Health (grants AG00158 and CA87969). In addition, for activities related to the Nurses’ Health Studies, we have received modest additional resources from the Alcoholic Beverage Medical Research Foundation, the American Cancer Society, Amgen, the California Prune Board, the Centers for Disease Control and Prevention, the Ellison Medical Foundation, the Florida Citrus Growers, the Glaucoma Medical Research Foundation, Hoffmann-LaRoche, Kellogg's, Lederle, the Massachusetts Department of Public Health, Mission Pharmacal, the National Dairy Council, Rhone Poulenc Rorer, the Robert Wood Johnson Foundation, Sandoz, the US Department of Defense, the US Department of Agriculture, the Wallace Genetics Fund, Wyeth-Ayerst, and private contributions. Conflicts of interest: The authors do not have any conflicts of interest to declare. Author contributions: W.C.W. designed and conducted the research; J.J.R. performed the statistical analysis; J.J.R. and E.C. analysed the data; J.J.R., E.C. and W.C.W. wrote the manuscript; and W.C.W. had primary responsibility for the final content. All authors read and approved the final manuscript. Acknowledgements: The authors thank Rong Chen from the Channing Laboratory for technical assistance.




