The biological effects of diet on inflammation are complex. Very simply, oxidative stress, which can occur after the ingestion of an energetically dense (i.e. high in fat or high in simple carbohydrates) meal, results in the production and release of free radicals and reactive oxygen and nitrogen species into the tissues. This in turn can lead to damaged tissues and inflammation( Reference Esposito and Giugliano 1 , Reference Reuter, Gupta and Chaturvedi 2 ). Conversely, foods high in antioxidants and flavonoids, such as fruits and vegetables, reduce inflammation by scavenging free radicals, inhibiting pro-oxidant enzymes, binding free radicals and possibly modulating the expression of pro-inflammatory molecules( Reference Conner and Grisham 3 , Reference Garcia-Lafuente, Guillamon and Villares 4 ). Prolonged and unchecked inflammatory conditions create a microenvironment favourable for tumour growth and progression( Reference Coussens and Werb 5 ). Identifying dietary factors that promote a less favourable environment for inflammatory conditions, in light of the association between diet and inflammation, may be one way to minimize the incidence of adenomas and cancer.
The Dietary Inflammatory Index (DII)TM was developed to characterize the inflammatory nature of a person’s diet, with scores on a continuum from maximally inflammatory to maximally anti-inflammatory. This index has been shown to be associated with concentrations of several circulating inflammatory proteins, including C-reactive protein( Reference Shivappa, Steck and Hurley 6 ) and IL-6( Reference Wood, Shivappa and Berthon 7 ), in prospective and case–control studies. Previously published work has shown that a more inflammatory diet, as reflected by a higher DII score, is associated with a higher prevalence of asthma (an inflammatory condition)( Reference Wood, Shivappa and Berthon 7 ), pancreatic cancer( Reference Shivappa, Bosetti and Zucchetto 8 ) and prostate cancer( Reference Shivappa, Bosetti and Zucchetto 9 ) in hospital-based case–control studies. Most recently, higher scores on this index have been found to be associated with a higher incidence of colorectal cancer in the Women’s Health Initiative and the Iowa Women’s Health Study( Reference Tabung, Steck and Ma 10 , Reference Shivappa, Prizment and Blair 11 ). Another recent study showed an association between DII scores and polymorphisms in the gene for the anti-inflammatory cytokine IL-4 (rs2243250)( Reference Zamora-Ros, Shivappa and Steck 12 ). In that study, individuals with a more inflammatory diet and the IL-4 polymorphism had a higher risk of colorectal cancer than those with the polymorphism who consumed a less inflammatory diet. Less favourable DII scores are reflective of diets lower in antioxidants and higher in pro-oxidants. This imbalance could lead to oxidative stress and genotoxic damage, which may then lead to abnormal growths and cancers in the colon( Reference Stone, Krishnan and Campbell 13 ). Anti-inflammatory dietary factors exert their effects through the modulation and inhibition of inflammatory proteins and cytokines, such as transforming growth factor-β, cyclooxygenase-2, IFN-γ and NF-κB( Reference Terzić, Grivennikov and Karin 14 ).
While these studies have shown an association between the incidence of colorectal cancer and a more inflammatory diet, it is unknown whether colorectal adenomas (CRA), which are precursors for colorectal cancer, are associated with a more inflammatory diet. The purpose of the current study was to examine whether or not a more inflammatory diet, as indicated by a higher DII score, was associated with the prevalence of CRA in a large cohort of older adults.
Methods
Study population
Data were collected as part of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial and have been described previously in detail( Reference Gohagan 15 – Reference Weissfeld, Schoen and Pinsky 18 ). In short, over 148 000 men and women, aged 55–74 years, were recruited between 1993 and 2000 at one of ten screening centres across the USA (Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC). Each participant who was randomly assigned to the screening arm was asked to complete a detailed questionnaire at baseline with questions regarding sociodemographic characteristics, diet, physical activity, personal and family cancer history, smoking history and use of selected medications. Those with an abnormal finding on flexible sigmoidoscopy examination were referred for endoscopic follow-up. Results from diagnostic screening and treatment, including surgical procedures, were gathered by trained medical abstractors from each participant’s medical record. Institutional review board approval was obtained from the National Cancer Institute and the ten screening centres involved with the study. Informed consent was provided by all study participants.
Data from screening-arm participants who returned the baseline questionnaire, which had questions regarding sociodemographic information, health history, medications and physical activity, were used for the present secondary analyses (n 75 611). Participants were excluded in this order: if flexible sigmoidoscopy examination was not adequate (defined as insertion to at least 50 cm with >90 % of mucosa visible or suspect lesion found) or not done (n 18 148); had a positive flexible sigmoidoscopy examination but had either no follow-up or ambiguous follow-up (n 3717); had a personal history of any cancer (except melanoma) or did not know their personal history of cancer before the dietary questionnaire (n 2081); had ulcerative colitis, Crohn’s disease, Gardner’s syndrome or familial polyposis (n 652); did not complete the dietary questionnaire (n 4937); had eight or more missing responses on the dietary questionnaire (n 385); had extreme energy intake reported on the dietary questionnaire (top or bottom 1 % of sex-specific energy intake; n 796); or did not specify race (n 9). Participants were further excluded if they were missing data on key variables (BMI, education, physical activity or smoking status; n 23). The final sample size was 44 278.
Adenomas
Any prevalent adenoma, not including hyperplastic polyps, in the distal region (rectum to the splenic flexure) at baseline was the main outcome of interest. Advanced adenomas were those that were villous or tubulovillous in nature, large (≥1·0 cm), or displayed severe or high-grade dysplasia. Physician and non-physician examiners followed standardized procedures to determine visual size estimates at sigmoidoscopy.
Dietary data
Questionnaire
Dietary data were collected using the dietary questionnaire developed by the National Cancer Institute( Reference Subar, Ziegler and Thompson 19 ). The sixteen-page questionnaire asked about the usual frequency and portion size of 137 food items and ten dietary supplements over the year prior to enrolment. The dietary questionnaire has been shown to have good reliability and has been validated against both the Block and Willett FFQ( Reference Subar, Ziegler and Thompson 19 ). Values for daily nutrients and food groups were determined from the national dietary data and the Pyramid food group servings database from the 1994–1996 Continuing Survey of Food Intakes by Individuals with a method developed by Subar et al.( Reference Subar, Ziegler and Thompson 19 ).
Dietary Inflammatory Index
The DII is a tool used to score the inflammatory nature of an overall diet and was developed using data from individuals consuming diverse diets( Reference Cavicchia, Steck and Hurley 20 ). Forty-five food/nutrient parameters were identified in the original index as being associated with six cytokines important in determining inflammatory response. Design and development of the DII have been described in detail previously( Reference Cavicchia, Steck and Hurley 20 , Reference Shivappa, Steck and Hurley 21 ).
To calculate the DII score, dietary intake data from each participant in the PLCO cohort were linked to a previously developed global database that was created by calculating the global mean and global standard deviation for each of the forty-five foods/nutrients for eleven countries around the world( Reference Shivappa, Steck and Hurley 21 ). A Z-score for each dietary factor was created for each PLCO participant by subtracting the global standard mean from the individual’s reported amount of consumed food/nutrient, and dividing this value by its respective global standard deviation. This value was then converted to a percentile score to minimize the effect of ‘right skewing’ (fewer observations with higher intakes of dietary factors), which often occurs with dietary data.
The ‘inflammatory effect score’ for each dietary factor was calculated previously, based on results from experimental, prospective cohort, case–control, cross-sectional, animal experimental and cell-culture studies( Reference Shivappa, Steck and Hurley 21 ). The dietary factor percentile score for each participant in the PLCO cohort was multiplied by its respective ‘inflammatory effect score’ to derive a ‘food-specific dietary inflammatory score’. Each of the ‘food-specific dietary inflammatory scores’ were summed to derive an overall dietary inflammatory score, where negative scores are less inflammatory and positive scores are more inflammatory. Scores are based on both food and nutrient intakes. For these analyses, thirty-seven of the forty-five foods or nutrients from the original DII were available for use. Pro-inflammatory dietary factors included: vitamin B12, carbohydrate, cholesterol, energy, total fat, Fe, protein, SFA and trans-fat. Anti-inflammatory dietary factors included: vitamin B6, β-carotene, caffeine, fibre, folic acid, vitamins A, D, C and E, niacin, riboflavin, thiamin, Mg, Se, Zn, MUFA, n-3 fatty acids, n-6 fatty acids, PUFA, flavan-3-ols, flavones, flavonols, flavonones, anthocyanidins, isoflavones, green/black tea, alcohol and onion. DII scores were calculated per 4184 kJ consumed to account for inter-individual differences in energy intake, which is also termed the Energy-Density DII (E-DII). E-DII scores for the PLCO screening-arm population ranged between −5·87 (maximally anti-inflammatory) and 5·58 (maximally pro-inflammatory). For analytical purposes, the E-DII scores were then categorized into quartiles.
Covariate data
Potential covariates included: smoking (never, current, or former); sex (male or female); self-report of race (black, white, Asian or other); and non-steroidal anti-inflammatory use (regular use of aspirin/aspirin-containing or ibuprofen/ibuprofen-containing products or not). BMI (= [weight (kg)]/[height (m)]2) was categorized as underweight (<18·5 kg/m2), normal (18·5–24·9 kg/m2), overweight (25·0–30·0 kg/m2) or obese (>30·0 kg/m2), and was based on self-reported height and weight. Physical activity was categorized as vigorous activities for <2 h/week (low) v. ≥2 h/week (high) to stay consistent with current recommendations( Reference Haskell, Lee and Pate 22 ). Hormone status was categorized as never, current (ever taken or currently taking female hormones), former or unknown. Education was categorized into less than high school, high-school diploma, some college or post high-school training, and college or graduate degree. Age at randomization, alcohol intake (g/d), fibre intake (g/d), Ca intake (food and supplements; mg/d) and energy intake (kJ/d; kcal/d) were left as continuous variables.
Statistical analysis
Means and frequencies, with their respective standard deviations and percentages, were calculated for continuous and categorical characteristics, stratified by E-DII score quartiles. The χ 2 test and ANOVA were used to determine differences, if any, in descriptive characteristics between quartiles of E-DII score. Normal distribution was assessed with histograms (QQ plot or Shapiro–Wilk test) for each variable.
Multivariable logistic regression was used to calculate the odds of prevalent CRA for different quartiles of E-DII score( Reference Shivappa, Bosetti and Zucchetto 9 ). Separate models were created for adenoma type (all prevalent, advanced, non-advanced or multiple (>1) adenoma). Regression models were initially adjusted for sex, race, smoking, age, physical activity, education, hormone status, regular use of aspirin/aspirin-containing or ibuprofen/ibuprofen-containing products, and daily Ca, energy and alcohol intakes. Additionally, an interaction term for sex and E-DII score category was included. All potential covariates and interaction terms were included in the initial model and then were evaluated for variable selection; if they were not significant in the model (P<0·20), they were removed if their exclusion did not result in a lower Akaike information criterion statistic( Reference Akaike 23 ). The most parsimonious model, indicated by a lower Akaike information criterion value, was selected. Covariates used in calculating the overall adenoma odds were used for subgroup analyses. The models were stratified by sex if the interaction between sex and E-DII score was significant (P<0·20). Wald χ 2 was used to test for trends across E-DII categories. Models were also stratified by BMI status (overweight/obese v. normal/underweight) or smoking status (never v. former/current). All analyses were performed using the statistical software package SAS version 9.4 using a P value <0·05 to indicate significance, unless otherwise indicated.
Results
Descriptive characteristics for the quartiles of the E-DII are presented in Table 1. Compared with quartile 4, quartile 1 (least inflammatory) had a higher percentage of females (65·0 v. 26·0 %), Asians (7·4 v. 1·6 %), individuals with a college education (45·5 v. 27·7 %), individuals with a high amount of physical activity (68·2 v. 42·4 %), never smokers (52·4 v. 40·8 %) and individuals with a normal BMI (41·5 v. 22·4 %). Women in quartile 1 (least inflammatory) were more likely to be current hormone users than women in quartile 4 (most inflammatory; 56·6 v. 46·3 %). Additionally, compared with those in quartile 4, those in quartile 1 were older (62·9 v. 61·7 years), had a higher intake of Ca (1349·7 v. 1168·4 mg/d) and a lower energy intake (7684·3 v. 9939·9 kJ/d).
Table 1 Baseline characteristics of the screening-arm participants (n 44 278) by quartile of Energy-Density Dietary Inflammatory Index (E-DII) score; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, USA, 1993–2000
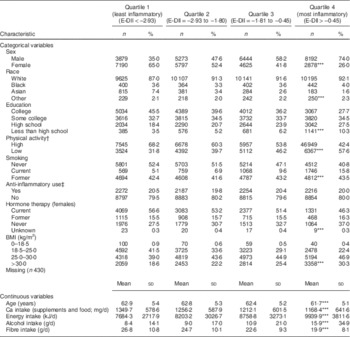
***P<0·0001; χ 2 test for categorical and ANOVA for continuous variables.
† Vigorous activities for <2 h/week (low) v. ≥2 h/week (high).
‡ Regular use of aspirin/aspirin-containing or ibuprofen/ibuprofen-containing products or not.
Prevalent distal adenoma
There was significant interaction between E-DII score and sex (P=0·02), so models for prevalent distal adenoma were stratified by sex. In fully adjusted models (adjusted for race, education, smoking status, BMI, age and Ca intake), compared with those with E-DII scores in quartile 1 (least inflammatory), males with E-DII scores in quartile 3 (adjusted odds ratio (aOR)=1·28; 95 % CI 1·12, 1·47) and quartile 4 (aOR=1·41; 95 % CI 1·23, 1·62; Table 2) were more likely to have prevalent distal CRA. Males with E-DII scores in quartile 3 (aOR=1·34; 95 % CI 1·12, 1·60) and quartile 4 (aOR=1·42; 95 % CI 1·19, 1·68) also were more likely to have a non-advanced adenoma, compared with those in the lowest quartile of E-DII scores. Males with E-DII scores in quartile 4 (aOR=1·39; 95 % CI 1·13, 1·71; Table 2) were more likely to have advanced CRA, compared with those with E-DII scores in quartile 1. Males with E-DII scores in quartile 3 (aOR=1·32; 95 % CI 1·01, 1·72) and quartile 4 (aOR=1·63; 95 % CI 1·26, 2·11) were more likely to have more than one distal CRA, compared with those with E-DII scores in quartile 1. In fully adjusted models, there were no differences in the odds of CRA (advanced, non-advanced or multiple (>1)) between E-DII quartile 1 and E-DII quartile 4 in females. However, there was a trend that higher E-DII scores were associated with higher odds of adenoma, overall.
Table 2 Associations between prevalent colorectal adenoma and quartile of Energy-Density Dietary Inflammatory Index (E-DII) scoreFootnote † in the screening-arm participants (n 44 255) by sexFootnote ‡; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, USA, 1993–2000

Significant (P<0·05) results are indicated in bold font.
† Quartile 1, E-DII<−2·93; quartile 2, E-DII=−2·93 to −1·80; quartile 3, E-DII=−1·81 to −0·45; quartile 4, E-DII>−0·45.
‡ Interaction P values for sex × E-DII score for adenoma (overall), non-advanced adenoma, advanced adenoma and multiple (>1) adenoma are, respectively, 0·03, 0·16, 0·20 and 0·29.
§ Adjusted for BMI, education, smoking status, race, Ca intake, alcohol intake and age.
║ Adjusted for BMI, smoking, race, hormone status, total daily energy intake, Ca intake, alcohol intake and age.
When results were stratified by smoking status or BMI classification, the odds of adenoma among overweight/obese men with the most inflammatory diet v. those with the least inflammatory diet (aOR=1·39; 95 % CI 1·18, 1·63) were similar to those of normal/underweight men (aOR=1·50; 95 % CI 1·14, 1·96; data not shown). However, the odds of adenoma among male smokers with the most inflammatory diet v. the least inflammatory diet (aOR=1·63; 95 % CI 1·38, 1·93) were higher than those of men who did not smoke (aOR=1·22; 95 % CI 0·97, 1·53; data not shown). Females showed similar patterns (smokers aOR=1·43; 95 % CI 1·12, 1·83; non-smokers aOR=0·97; 95 % CI 0·76, 1·25; data not shown).
Discussion
In this large cohort of men and women, enrolled as part of the PLCO screening arm, we sought to investigate the association between CRA and E-DII score and found that a more inflammatory diet was associated with distal CRA prevalence in men, and to a limited extent in women. Specifically, males who consumed a more inflammatory diet were more likely to have non-advanced adenomas, advanced adenomas and multiple (>1) adenomas than men who consumed a less inflammatory diet.
It is believed that inflammation promotes an environment that increases genetic mutations and disables the mechanisms that repair these errors( Reference Grivennikov, Greten and Karin 24 ). There also is evidence that inflammation may promote growth factors that enhance tumour growth, particularly through enhanced angiogenesis( Reference Haskell, Lee and Pate 22 ). Further, a vicious cycle is created in that tumour cells produce cytokines that attract leucocytes, which further promote inflammation( Reference Coussens and Werb 5 ). Higher systemic concentrations of inflammatory cytokines may then lead to the development of CRA( Reference Kim, Keku and Martin 25 ). Diet can affect systemic inflammation both positively and negatively. A high intake of energy and certain types of fat (e.g. trans-fats) may lead to pro-inflammatory states( Reference Lopez-Garcia, Schulze and Meigs 26 , Reference Hennig, Toborek and McClain 27 ), while fruits and vegetables contain antioxidants that counteract inflammation( Reference Walston, Xue and Semba 28 ). The DII has recently been developed as a way for researchers to characterize the overall inflammatory nature of diet( Reference Shivappa, Steck and Hurley 6 ). This index has been shown to be associated with inflammatory conditions, such as colon, prostate and pancreatic cancers, and asthma( Reference Shivappa, Bosetti and Zucchetto 8 , Reference Shivappa, Bosetti and Zucchetto 9 , Reference Shivappa, Prizment and Blair 11 , Reference Zamora-Ros, Shivappa and Steck 12 , Reference Dixon, Subar and Peters 29 ), as well as circulating inflammatory proteins( Reference Shivappa, Steck and Hurley 6 , Reference Wood, Shivappa and Berthon 7 ).
Findings of the present study are generally consistent with those from other studies that have found lower odds of prevalent CRA among those who consume a ‘healthy’ diet( Reference Dixon, Subar and Peters 29 , Reference Whalen, McCullough and Flanders 30 ). For example, men with higher scores on several dietary indices (Healthy Eating Index, Mediterranean diet, Dietary Approach to Stop Hypertension) were less likely to have a prevalent CRA compared with men consuming a less healthy diet( Reference Dixon, Subar and Peters 29 ). These results suggest that there may be a common element, such as an anti-inflammatory dimension, among the dietary indices which confers adenoma-protective effects, and that the specific type of diet may be less important than this common beneficial element (e.g. anti-inflammatory dimension). Indeed, for several of the dietary indices mentioned, better scores have been associated with lower concentrations of inflammatory markers( Reference Chrysohoou, Panagiotakos and Pitsavos 31 , Reference Ford, Mokdad and Liu 32 ).
It is interesting that lower E-DII scores, indicating a less-inflammatory diet, were not strongly associated with distal CRA prevalence in women, although there was a trend across quartiles of higher odds of CRA with higher E-DII scores. Some previous studies have shown that women with more inflammatory diets, as reflected by higher DII scores, were more likely to have developed colorectal cancer, compared with those with less inflammatory diets( Reference Tabung, Steck and Ma 10 , Reference Shivappa, Prizment and Blair 11 ). However, another larger study using individuals in the American Association of Retired Persons Diet and Health Study found that the association between a less inflammatory diet and lower risk of colorectal cancer was significant only in men( Reference Wirth, Shivappa and Steck 33 ). Since adenomas are precursors to cancer, we expected to find a positive association between E-DII scores and CRA prevalence in women. However, dietary predictors for adenomas may not be the same as dietary predictors for colon cancer in women. It has been estimated that only half of studies on the association between diet indices (e.g. Mediterranean diet and Healthy Eating Index) and colorectal cancers report sex-specific risks( Reference Kim, Paik and Yoon 34 ), suggesting that the current literature may not fully capture the effects of diet on adenoma prevalence or cancer incidence in males and females, individually. Of those studies that have reported on dietary factors for CRA in men and women separately, several did not find a protective effect of diet in women( Reference Dixon, Subar and Peters 29 , Reference Whalen, McCullough and Flanders 30 , Reference Reedy, Wirfält and Flood 35 ). Another explanation for the discrepancy in findings between the current study and previous studies may have to do with lower (less inflammatory) E-DII scores in the present study (−2·1 (sd 1·6) v. −0·9 (sd 2·0); P<0·0001( Reference Shivappa, Prizment and Blair 11 )). The generally lower scores in the present study may have limited the ability to see beneficial dietary effects because the diets were generally ‘adequate’ for adenoma prevention.
To further explore the association between E-DII scores and adenoma status in women, sensitivity analyses were performed combining quartiles 1 and 2 and comparing the odds of adenoma with those of quartile 3 or 4. Alternatively, cut points used in another study using the E-DII also were used( Reference Shivappa, Bosetti and Zucchetto 9 ). In both instances, results were similar to those presented in Table 2. The results appear to have a curvilinear response in women, which may suggest a differential effect of diet between sexes. A possible explanation is that these analyses were cross-sectional in nature and those who were consuming the most anti-inflammatory diet were doing so because they had concerns about a higher risk of adenoma. Another possible explanation is that women who have very anti-inflammatory diets also engage in other behaviours not fully accounted for in the analyses, which weaken the effect of a healthy diet, or because women misestimate their actual intake because of social desirability and under-report total fat and energy intakes( Reference Hebert, Ma and Clemow 36 ). Differences in actual v. reported intakes between men and women may also at least partly explain why the E-DII was associated with adenoma in men but not in women.
It is unknown to what extent the results of the current study can be applied to proximal adenomas. One study found that the risk adenoma and diet (fibre and fruit and vegetables) was lower for adenomas occurring in the proximal v. distal region( Reference Robertson, Sandler and Haile 37 ). However, results from other studies, including a literature review, are unclear as to whether or not there are anatomical site differences in the effects of diet on cancer or adenoma development or occurrence( Reference Wirth, Shivappa and Steck 33 , Reference Randi, Edefonti and Ferraroni 38 , Reference Reedy, Mitrou and Krebs-Smith 39 ). Future research would need to be done to more fully understand the association between diet and adenoma occurrence in site-specific areas.
One of the strengths of the present study is the large, diverse cohort of individuals with varied dietary habits, enabling the analysis of adenoma outcomes across a broad spectrum of food intakes. Another study strength is the novel way to characterize the inflammatory nature of diet. Inflammation is an important factor in disease occurrence and the E-DII is the first index to be developed specifically for measuring the inflammatory potential of an individual’s diet. Finally, participants in the PLCO study were screened uniformly, allowing for equal opportunity of adenoma detection.
A limitation of the study is that the E-DII was not able to fully determine the inflammatory nature of the diets due to some of the E-DII components not being included in the dietary questionnaire (e.g. eugenol, garlic, ginger, saffron, turmeric, pepper, rosemary and thyme/oregano). However, most items for the E-DII were included in the calculations and these represented the most commonly consumed foods/nutrients. Another limitation to these analyses is recall bias, most notably for the dietary recall. This may have resulted in biased estimates, particularly among women where the potential for misclassification was greater, thus resulting in estimates closer to the null.
Conclusion
A more inflammatory diet was associated with a higher risk of CRA, particularly in men. The results for women were less conclusive. Therefore, future research should use large prospective studies to replicate these findings with a focus on gender differences and the potential for recall bias in influencing these associations. Also, future work could be done to determine whether these results apply to incident or recurrent adenomas, as well as adenomas in the proximal region of the colon. Results from the present study support an inflammatory mechanism for the development of CRA. From a public health perspective, future work should focus on helping individuals understand and incorporate anti-inflammatory elements into their diet.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: M.D.W., N.S. and J.R.H. were supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant number R44DK103377). J.R.H. owns controlling interest in Connecting Health Innovations, LLC (CHI), a company planning to license the right to his invention of the DII from the University of South Carolina in order to develop computer and smartphone applications for patient counselling and dietary intervention in clinical settings. M.D.W. and N.S. are employees of CHI. Authorship: A.H., S.W.R. and M.H.E. were involved with all steps, including data acquisition, study design and interpretation, and manuscript preparation. H.H. and J.R.H. were involved with the data interpretation and manuscript preparation. Ethics of human subject participation: This work involved secondary analysis of data from the PLCO Cancer Screening Trial. Institutional review board approval for PLCO was obtained from the National Cancer Institute and the ten screening centres involved with the study. Informed consent was provided by all study participants.





