Introduction
Alcohol use is common among middle-aged and older adults, and is increasing disproportionately among older adults (Grant et al., Reference Grant, Chou, Saha, Pickering, Kerridge, Ruan, Huang, Jung, Zhang, Fan and Hasin2017). A large body of literature shows that relative to non-drinkers, light, or moderate alcohol drinkers demonstrate better cognitive performance and show reduced risk of dementia (Beydoun et al., Reference Beydoun, Beydoun, Gamaldo, Teel, Zonderman and Wang2014; Neafsey & Collins, Reference Neafsey and Collins2011; Rehm et al., Reference Rehm, Hasan, Black, Shield and Schwarzinger2019). However, most studies of alcohol use and cognitive function examine cross-sectional associations of current drinking with current cognitive function, making it difficult to tease apart cause and effect. Prospective studies in which alcohol use at baseline is associated with cognitive status years later are also problematic due to the likelihood of non-random drop out, and the absence of information on changes in alcohol use over time. Fewer studies have looked at rate of change in cognitive performance as a function of alcohol use, which may be a more informative approach for assessing associations of alcohol intake with cognitive aging.
Studies that have examined longitudinal change in cognitive performance as a function of alcohol intake have reported varying results. For example, consistent minimal to moderate drinking over a 7-year follow-up period was associated with a reduced rate of concurrent cognitive decline relative to nondrinkers among a community sample of older adults in Pennsylvania (Ganguli et al., Reference Ganguli, Vander Bilt, Saxton, Shen and Dodge2005). Similarly, women who consumed less than 1 alcoholic drink per day showed a lower rate of cognitive decline over the subsequent 10 years than women who abstained from alcohol use, in the Whitehall II study of middle-aged civil servants (Sabia et al., Reference Sabia, Elbaz, Britton, Bell, Dugravot, Shipley, Kivimaki and Singh-Manoux2014). Men in this cohort showed no evidence of a protective association of alcohol intake on cognitive decline, and consumption of >3 alcoholic drinks/day was associated with an increased rate of decline relative to drinking <2 drinks/day (Sabia et al., Reference Sabia, Elbaz, Britton, Bell, Dugravot, Shipley, Kivimaki and Singh-Manoux2014). In contrast, men in the British 1946 birth cohort who consumed alcohol in any amount showed less memory decline from age 43 to 56 than those who did not drink (Richards et al., Reference Richards, Hardy and Wadsworth2005). In the U.S. Health and Retirement Study, all drinking groups, including former drinkers, showed less decrease in overall cognitive ability with advancing age than never drinkers (Zhang et al., Reference Zhang, Shen, Miles, Shen, Cordero, Qi, Liang and Li2020). In the Northern Manhattan Study, current drinkers, including those who consumed more than 2 drinks/day, showed less decline on the modified Telephone Interview for Cognitive Status than never drinkers (Wright et al., Reference Wright, Elkind, Luo, Paik and Sacco2006).
Several factors may contribute to these discrepancies, including differences in sample size, age, culture, race/ethnicity, drinking patterns, type of alcohol consumed, control for potential confounding variables, and definition of the reference group. Use of a nondrinker reference group comprising abstainers and former drinkers may bias results because former drinkers may have quit drinking due to poor health (Rehm et al., Reference Rehm, Irving, Ye, Kerr, Bond and Greenfield2008). However, lifetime abstainers may not be an ideal reference group either, since abstainers tend not to be representative of the wider population. They differ from alcohol consumers in several ways that may affect cognitive function, including low childhood socioeconomic status, early life health problems, and close relatives with alcohol use disorders (Kerr et al., Reference Kerr, Lui, Williams, Ye, Greenfield and Lown2017). Thus light or occasional drinkers have been proposed as a preferred reference group (Rehm et al., Reference Rehm, Irving, Ye, Kerr, Bond and Greenfield2008).
Studies are also often limited by a small number of cognitive tests that do not sensitively assess different cognitive domains, and by lack of control for effects of repeated testing on cognitive performance over time. Few studies are able to adjust for earlier life cognitive ability, which may confound associations (Corley et al., Reference Corley, Jia, Brett, Gow, Starr, Kyle, Mcneill and Deary2011; Krahn et al., Reference Krahn, Freese, Hauser, Barry and Goodman2003). Studies also differ in their statistical approaches for characterizing associations of alcohol use with cognitive aging, limiting comparison of results across studies. For example, some studies examine associations of alcohol use with rate of change in performance over time while others compare differences by age (Hoffman, Reference Hoffman2012). In the latter case, between-participant differences contribute to slopes of cognitive performance by age, since different individuals contribute to different portions of the age slope, depending on their age at entry, making this approach subject to some of the same interpretational difficulties associated with cross-sectional studies. This is of less concern when time is the temporal variable, as slopes reflect within-participant change over time.
An additional consideration is the role of genetic risk. Conflicting results have been reported on whether the APOE ϵ4 risk factor for Alzheimer’s disease modifies associations between alcohol use and cognitive function. Some studies report no effect modification by APOE ϵ4 status (Herring & Paulson, Reference Herring and Paulson2018; Stampfer et al., Reference Stampfer, Kang, Chen, Cherry and Grodstein2005; Wright et al., Reference Wright, Elkind, Luo, Paik and Sacco2006), whereas others have reported apparently contradictory effects. For example, in a cross-sectional analysis of middle-aged men in the Vietnam Era Twin Study of Aging (VETSA), worse cognitive performance was observed among heavy drinkers with an APOE ϵ4 allele than among heavy drinkers without an ϵ4 allele (Slayday et al., Reference Slayday, Gustavson, Elman, Beck, McEvoy, Tu, Fang, Hauger, Lyons, Mckenzie, Sanderson-Cimino, Xian, Kremen and Franz2020). In the Rancho Bernardo Study of Healthy Aging (RBS), nondrinkers with an APOE ϵ4 allele showed greater memory decline with age than nondrinkers without an ϵ4 allele, and than drinkers regardless of APOE ϵ4 status (Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2019). However, the RBS cohort is older and contains few heavy drinkers. Thus whether and how APOE ϵ4 affects associations of alcohol use with cognitive aging requires further study.
Here, we take advantage of detailed longitudinal data collected as part of the VETSA to examine associations between alcohol use and cognitive function among a well-characterized cohort of middle-aged men followed for 12 years (Kremen et al., Reference Kremen, Franz and Lyons2013; Kremen et al., Reference Kremen, Thompson-Brenner, Leung, Grant, Franz, Eisen, Jacobson, Boake and Lyons2006). We used two analytic approaches to explore the association of alcohol intake with cognitive aging. We first examined change in cognitive performance over time as a function of baseline alcohol intake. This enables assessment of within-participant cognitive change as a function of baseline alcohol use, but makes the implicit assumption that alcohol use does not change over time, or that change in alcohol use over time does not affect rate of cognitive change. Thus in our second approach, we examined differences in alcohol intake by age, including alcohol use as a time-varying exposure. The limitation with this approach is that the observed slopes of cognitive function by age reflect both within-person change and between participant differences. We reasoned that comparison of results across methods would aid in interpretation of any observed alcohol-use cognitive performance associations. Our hypotheses were that, relative to very light drinking, light or moderate alcohol intake would show protective associations with cognitive aging whereas at-risk drinking (>28 drinks in 14 days) would show harmful associations, after adjustment for potential confounding sociodemographic and health-related measures.
Methods
Participants
The VETSA sample comprises 1,608 men who were recruited from the Vietnam Era Twin Registry, a national registry of male twin pairs inducted into the military during the Vietnam war era (1965–1975). VETSA participants are a random sample of twins who participated in the Harvard Twin Study of Substance Abuse, (the “Harvard Drug Study”; HDS), in 1991–1993 (Tsuang et al., Reference Tsuang, Bar, Harley and Lyons2001). VETSA inclusion criteria included willingness of both members of the twin pair to participate in the initial assessment, and age within the targeted range. Most of the sample was enrolled at wave 1 (n = 1,237, age range 51–61 years). To account for attrition and to allow for estimation of practice effects in cognitive tests, additional age-matched participants meeting the same inclusion criteria were recruited from the HDS sample and enrolled in VETSA at wave 2 (n = 247; age range 55–67 years) or wave 3 (n = 124; age range 63–71 years). Figure S1 contains a flow chart showing enrollment and attrition across waves.
Characteristics of the sample have been described (Kremen et al., Reference Kremen, Thompson-Brenner, Leung, Grant, Franz, Eisen, Jacobson, Boake and Lyons2006). Briefly, the sample comprises predominantly non-Hispanic white men (91%) with similar health and lifestyle characteristics as other U.S. men in their age range at the time of enrollment (Schoenborn & Heyman, Reference Schoenborn and Heyman2009). Although all participants served in the military, the majority (∼80%) did not experience combat (Franz et al., Reference Franz, Lyons, O’Brien, Panizzon, Kim, Bhat, Grant, Toomey, Eisen, Xian and Kremen2011).
This research was conducted in accordance with the Helsinki Declaration, and in compliance with institutional standards for human research. All participants provided written informed consent.
Alcohol use assessment
Alcohol use was assessed with a structured medical interview at each wave. Participants were asked whether they had consumed more than 20 drinks in their life. Those who responded “Yes” were asked to indicate on how many days during the past 2 weeks they drank beer, and on days on which they drank beer, how many beers they drank. These questions were repeated for wine and hard liquor. The number of drinks of each beverage type was summed to obtain the total number of alcoholic beverages consumed over the past 14 days.
Alcohol use was treated as a categorical variable with six categories defined at each wave. Lifetime Abstainers were defined as those who reported not having consumed more than 20 drinks in their life; who reported no alcohol intake in the current or prior waves, and whose responses on the Diagnostic Interview Schedule for the DSM-III-R, administered during the HDS when participants were an average of 44 years old, indicated no or minimal earlier life alcohol consumption. Former drinkers were defined as those who reported drinking more than 20 drinks in their lives but reported no alcohol consumption in the past 14 days. Very light drinkers were defined as those who consumed 1–4 drinks in the past 14 days. This cut-off was chosen to exclude anyone who may occasionally engage in binge drinking, defined as the consumption of 5 or more drinks in a day. Light drinkers were those who consumed 5–14 drinks, moderate drinkers consumed 15–28 drinks and at-risk drinkers consumed >28 drinks in the past 14 days.
Cognitive performance
Participants completed a detailed neurocognitive battery at each wave that contained multiple tests of several domains (see Table 1) (Kremen, Jak, et al., Reference Kremen, Jak, Panizzon, Spoon, Franz, Jacobson, Vasilopoulos, Vuoksimaa, Xian, Toomey and Lyons2014). In prior studies, confirmatory factor analyses were applied in the context of VETSA’s twin design to obtain domain-specific factor scores that are used as the cognitive outcome measures here (Gustavson, Panizzon, Elman, et al., Reference Gustavson, Panizzon, Elman, Franz, Beck, Reynolds, Xian, Toomey, Lyons and Kremen2018; Gustavson, Panizzon, Franz, et al., Reference Gustavson, Panizzon, Franz, Friedman, Reynolds, Jacobson, Xian, Lyons and Kremen2018; Kremen, Panizzon, et al., Reference Kremen, Panizzon, Franz, Spoon, Vuoksimaa, Jacobson, Vasilopoulos, Xian, Mccaffery, Rana, Toomey, Mckenzie and Lyons2014; Sanderson-Cimino et al., Reference Sanderson-Cimino, Panizzon, Elman, Gustavson, Franz, Reynolds, Toomey, Lyons and Kremen2019). Table 1 shows the neuropsychological tests used to derive each of six cognitive domain factor scores, including processing speed, episodic memory, general verbal fluency and semantic fluency, executive function and working memory. These factor scores were derived for each participant at each wave. Factor scores were standardized based on all VETSA participants at wave 1. Waves 2 and 3 data were standardized with respect to wave 1 data.
Table 1. Neuropsychological tests used to derive the six cognitive domain scores
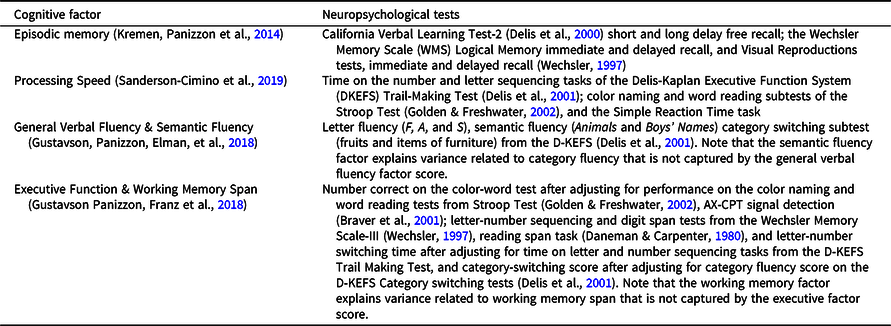
Longitudinal cognitive testing is subject to practice effects, which can mask subtle differences in cognitive performance with aging, and to bias from selective attrition. The VETSA design includes age-matched attrition-replacement participants at each wave. Comparison of replacements and returnees allows for more precise estimation and removal of practice effects than is possible with statistical approaches that adjust for repeated assessment, and minimizes attrition bias. Cognitive factor scores at waves 2 and 3 were corrected for task practice, taking into account the influence of selective attrition, as previously described (Elman et al., Reference Elman, Jak, Panizzon, Tu, Chen, Reynolds, Gustavson, Franz, Hatton, Jacobson, Toomey, Mckenzie, Xian, Lyons and Kremen2018).
Covariates
Young adult cognitive ability was defined as the scaled, normalized score on the Armed Forces Qualification Test (AFQT) administered at time of military induction (average age of 20 years) (Bayroff, Reference Bayroff1963; Uhlaner, Reference Uhlaner1952). The AFQT is a 100-item general cognitive ability test that is highly correlated (r = 0.84) with standard IQ (Lyons et al., Reference Lyons, Panizzon, Liu, McKenzie, Bluestone, Grant, Franz, Vuoksimaa, Toomey, Jacobson, Reynolds, Kremen and Xian2017).
Childhood SES (cSES) was determined based on participant report of parents’ highest levels of occupation and education during their childhood using the Hollingshead-Redlich occupational score (Hollingshead, Reference Hollingshead1975) as previously described (Beck et al., Reference Beck, Franz, Xian, Vuoksimaa, Tu, Reynolds, Panizzon, Mckenzie, Lyons, Toomey, Jacobson, Hauger, Hatton and Kremen2018). Participant education was categorized as ≤12 years of education (all participants achieved at least a high school diploma or equivalent), 13–14 years, 15–16 years, or greater than 16 years of education; race/ethnicity was dichotomized as non-Hispanic white and all others. Annual family income was categorized as <$40,000, $40,000–$89,999 and ≥$90,000. Smoking was classified as never, former, or current smoker. Participants were classified as physically active if they reported engaging in physical activity several times per week.
Height, weight, and waist diameter were measured at each visit. Systolic and diastolic blood pressure (SBP and DBP) were measured twice in the morning and twice in the afternoon of the same day from seated participants. Hypertension status was determined based on average DBP ≥ 90, average SBP ≥ 140, or self-reported physician diagnosis of hypertension and use of antihypertensive medication. Diabetes status was derived from self-reported physician diagnosis or diabetes medication use.
Total comorbidities were categorized as none, 1, 2, or ≥ 3 of the following chronic conditions: hypertension or diabetes (as defined above), self-report of heart disease (angina, heart attack, or heart failure), stroke, peripheral vascular disease, osteoarthritis, rheumatoid arthritis, peptic ulcer (stomach or duodenal ulcer), pulmonary disease (asthma, chronic bronchitis, or emphysema), liver disease (hepatitis, cirrhosis, or liver damage due to alcohol), thyroid disease, and inflammatory bowel disease (Crohn’s disease or ulcerative colitis). History of head injury that resulted in loss of consciousness or confusion (yes/no) was derived from self-report. Depression (yes/no) was assessed with the Center for Epidemiologic Studies Depression (CES-D) scale (Franz et al., Reference Franz, Lyons, O’Brien, Panizzon, Kim, Bhat, Grant, Toomey, Eisen, Xian and Kremen2011; Radloff, Reference Radloff1977). A CES-D score greater than or equal to 16 indicates risk of clinical depression.
Statistical analyses
Group differences in sociodemographic and health-related measures at baseline (study entry) were assessed using linear mixed-effects models (Proc Mixed SAS, version 9.4) for continuous variables and multinomial ordinal regression (Proc Genmod) for categorical variables. Family identifier was included as a random effect to adjust for correlated measures between twins.
Linear mixed-effects models were used to evaluate differences in cognitive performance across waves by alcohol group, with separate models for each cognitive domain. Mixed-effects models account for correlations between repeated measures within participants through inclusion of individual-level random effects; and for correlations between twins through inclusion of individual family-level random effects (Frees, Reference Frees2004). Mixed effect models also accommodate inconsistent measurement intervals and missing data, permitting use of all observed data to provide valid inference under the missing at random mechanism (Little & Rubin, Reference Little and Rubin2002).
Cognitive trajectories over time by alcohol group at baseline
To examine the association of baseline alcohol use with cognitive performance over time, mixed-effects models included time as the temporal variable. Fixed effect terms, which model the mean trajectory of participants as a function of alcohol group and covariates, included baseline age (centered at 55 years, the mean age at wave 1), education, race/ethnicity, and time (years since baseline). Models also included random effect terms that allow individual baseline levels (intercept) and rates of decline (slope) to vary randomly about the mean trajectory described by the fixed effect terms. A family identifier was included as a random effect to adjust for relatedness between twins. A time-by-alcohol-group interaction term was included to assess the influence of alcohol group on cognitive change over time. We assessed the 3-way interaction between age, time, and alcohol group; this term was not significant for any cognitive domain and was excluded from all models.
Base models adjusted for age, education level, and race/ethnicity. Fully adjusted models additionally included young adult cognitive ability, cSES, and time-varying covariates including current family income, smoking status, and physical activity. There was minimal missing data (<3%). Missing observations were imputed using all observed variables across waves within a participant. When the omnibus F test indicated a significant difference among alcohol groups, pairwise comparisons between drinking groups were performed with the very light drinking group as the reference, to avoid potential biases associated with using former drinkers or lifetime abstainers as the reference group (Rehm et al., Reference Rehm, Irving, Ye, Kerr, Bond and Greenfield2008).
In sensitivity analyses, we additionally adjusted for health-related variables that may lie in the causal pathway between alcohol and cognition, including BMI, DBP, number of comorbidities, depression, diabetes status, and head injury. DBP, which showed the strongest difference across alcohol groups, was chosen as the single measure of hypertension to avoid collinearity issues with other hypertension variables.
To examine potential effect modification by APOE ϵ4 status, analyses were repeated including an interaction term of APOE ϵ4 status (present/absent) by alcohol group by time.
Age-related cognitive trajectories by alcohol group
To examine associations of alcohol use by age, all analyses described above were repeated, using time-varying alcohol group rather than alcohol group at baseline as the exposure, and using age as the temporal variable in the mixed effects models.
For all analyses, statistical tests were two-sided; p-values are reported as continuous measures and are not corrected for multiple comparisons. All analyses were run using SAS Enterprise Guide version 7.15 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Participants were 57.8 (± 4.6) years, on average, at baseline. They had a median income of $60,000; 60% reported at least 12 years of education. Most participants were overweight to obese, with an average BMI of 29.7 (± 5.09) kg/m2 and had at least one comorbidity (75%). At baseline, most participants (64%) reported some alcohol consumption in the past 14 days; only 6% were abstainers. Beer was the most frequently consumed alcoholic beverage; 80% of current drinkers reported beer consumption, 43% reported consumption of hard liquor and 38% reported consumption of wine. Most participants reported consuming more than one type of beverage. Correlation of the number of alcoholic drinks in the past 14 days was r = 0.71 between waves 1 and 2, and r = 0.73 between waves 2 and 3. Linear mixed-effect models of alcohol use over time showed no systematic change in amount consumed (β = −.061 drinks per year; p = 0.28).
Table 2 presents baseline characteristics according to alcohol group. Groups were similar with respect to age, cSES, and APOE ϵ4 status, but differed in sociodemographic measures (family income), behaviors (smoking, physical activity) and health measures (BMI, diabetes, SBP, DBP, number of comorbidities, and depression).
Table 2. Characteristics of VETSA participants at study entry by alcohol consumption group (total n = 1608)
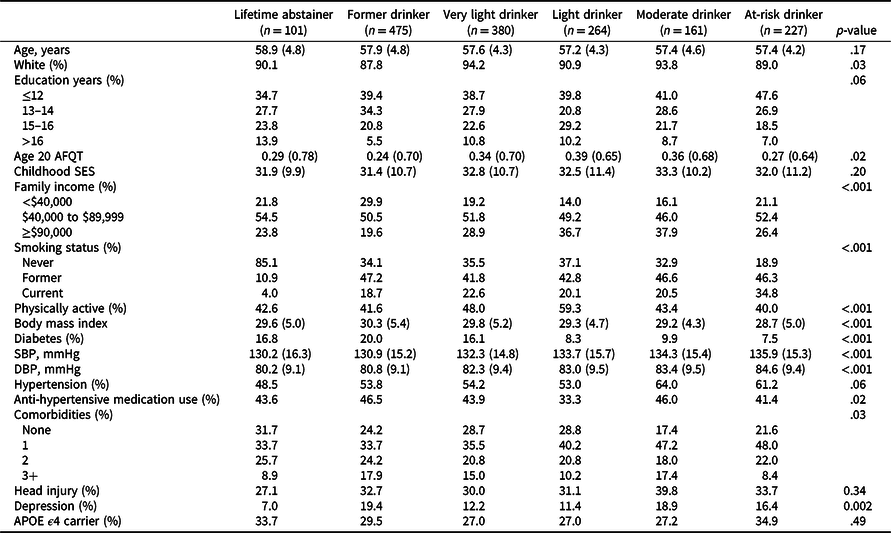
AFQT = Armed Forces Qualification Test (normalized values are shown); SES = socioeconomic status; SBP = systolic blood pressure; DBP = diastolic blood pressure; APOE = Apolipoprotein E.
Values are mean (standard deviation) unless otherwise indicated.
p-values are based on comparisons that included a family-relatedness variable as a random effect to adjust for correlated measures between twins.
Race categorized as non-Hispanic white or other.
Cognitive trajectories over time by alcohol group at baseline
Results of the minimally adjusted mixed-effects models examining within-participant change in cognitive performance over time by alcohol use at baseline are shown in Supplement Table 1; results from fully adjusted models are shown in Table 3. Plots of trajectories for each cognitive domain are shown in Figure 1. For all domains, and across all alcohol groups, performance declined over time (β = –0.03 to –0.1, standard deviation unit, SD, change per year, p’s < 0.001; for minimally and fully adjusted models). In minimally adjusted models, a main effect of alcohol group was observed for general verbal fluency, semantic fluency, working memory and processing speed (Table S1). Pairwise comparisons indicated that only former drinkers differed from the very light drinker reference group, with lower performance by ∼–0.2 SD (p’s ≤ .04) for each domain. Adjustment for potentially confounding sociodemographic measures and health-related behaviors attenuated the main effect of alcohol group for all domains except general verbal fluency (Table 3), for which former drinkers continued to show worse performance than the very light drinking group, by –0.21 SD (95% CI –0.35, –0.07; p = .004).
Table 3. Parameters from linear mixed effects models examining cognitive performance over time as a function of alcohol group at study entry for each cognitive domain, adjusting for potentially confounding sociodemographic factors and health behaviors
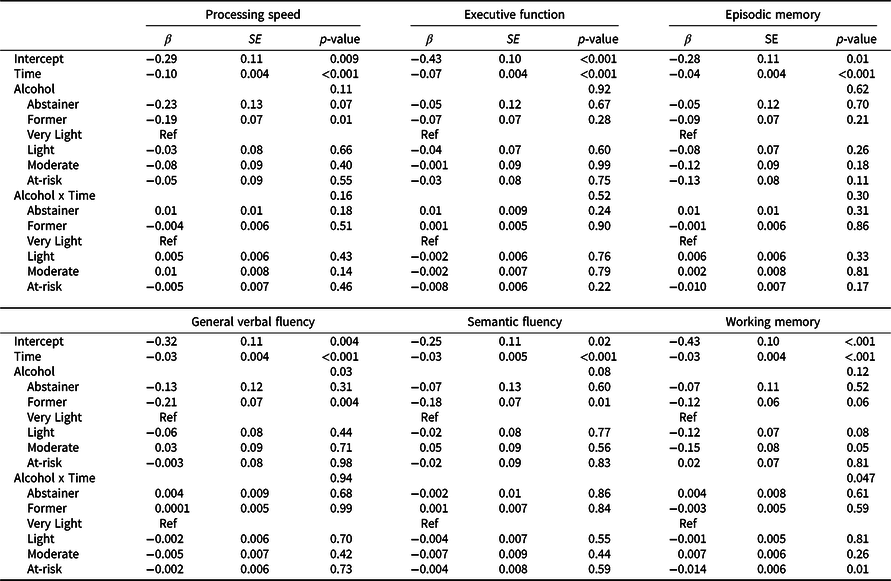
Beta values, standard error of the means (SE) and p values from the time-based linear mixed effect models adjusting for age, education, race/ethnicity, young adult cognitive ability, family income, childhood socioeconomic status (cSES), physical activity, and smoking, with family-relatedness included as a random effect.
The very light drinking group is the reference group for alcohol group comparisons. For interpretation of the intercept, continuous covariates (cSES, young adult cognitive ability, age) were modeled at their mean values, for age this was mean age at wave 1, 55 years; for categorical variables, reference groups were ≤12 years of education; non-Hispanic white, ≤$39,999 family income, never smoker, and physically inactive.
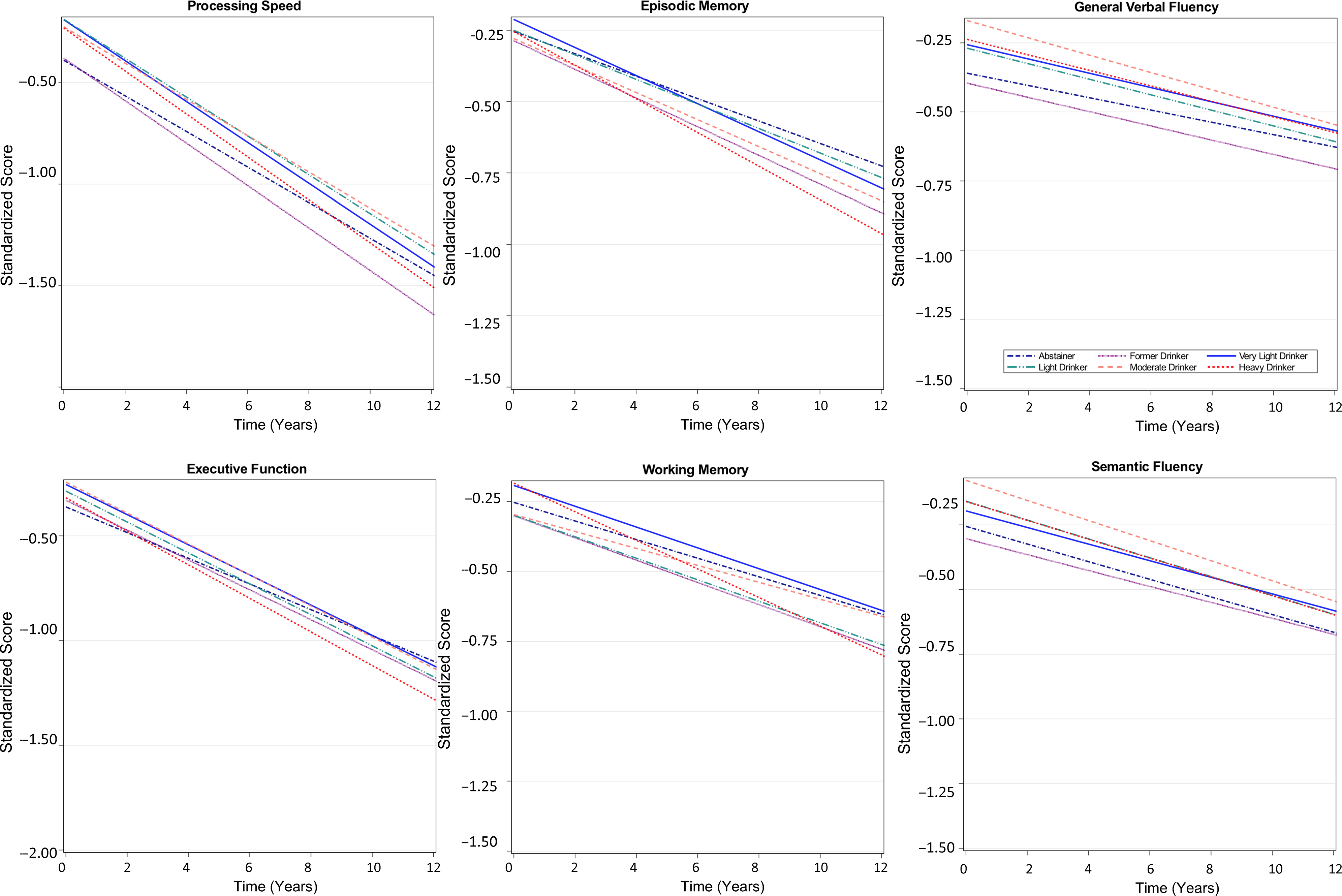
Figure 1. Trajectories of cognitive function over time by baseline alcohol intake group among participants of the Vietnam Era Twin Study of Aging. Modeled trajectories of cognitive performance over the 12-year follow-up are shown for the six categories of alcohol consumption for each cognitive domain. Plots are based on all model coefficients using the group median for age, ≤12 years of education; and non-Hispanic white race/ethnicity.
A time-by-alcohol-group interaction was observed for the working memory domain only in base and fully adjusted models. At-risk drinkers showed steeper working memory decline over time relative to the very light drinking group (Figure 1), with performance declining by an additional 0.14 SD (95% CI 0.02, 0.20; p = .01) per 10 years relative to the rate of decline among very light drinkers. Slopes of other alcohol groups did not differ from that of the very light drinking group.
Further adjustment for health-related covariates that may be potential mediators or confounders of the associations did not materially affect the results (Table S2). We did not detect evidence of effect modification by APOE ϵ4 status for any cognitive domain (three-way interaction term p’s > 0.17).
Age-related cognitive trajectories by alcohol group
Results from minimally adjusted mixed-effects models examining age-related differences in cognitive function by time-varying alcohol use are shown in Table S3; results of fully adjusted models are shown in Table 4. Plots of cognitive performance by age for each domain are shown in Figure 2. Across all groups and cognitive domains, scores were lower with advancing age (β = –0.03 to –0.1 SD per year, p’s < 0.001) for minimally and fully adjusted models. There were no main effects of alcohol group for any cognitive domain. Age interacted with alcohol group for episodic memory performance: light drinkers showed better performance at advanced ages than very light drinkers, with a difference in scores at 10 years older age of 0.14 SD (95% CI 0.02, 0.20; p = 0.03; Table 4 and Figure 2).
Table 4. Parameters from linear mixed effects models examining cognitive performance by age as a function of time-varying alcohol group for each cognitive domain, adjusting for potentially confounding sociodemographic factors and health behaviors
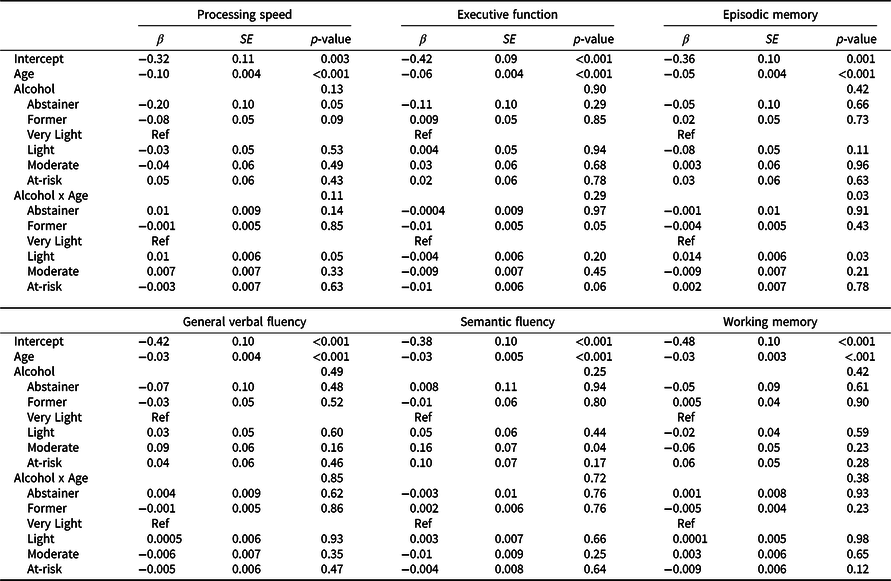
Beta values, standard error of the means (SE) and p values from age-based linear mixed effect models, which included alcohol group as a time-varying exposure, and adjusted for education, race/ethnicity, young adult cognitive ability, family income, childhood socioeconomic status, physical activity, and smoking, with family-relatedness included as a random effect.
For other details see footnote to Table 3.
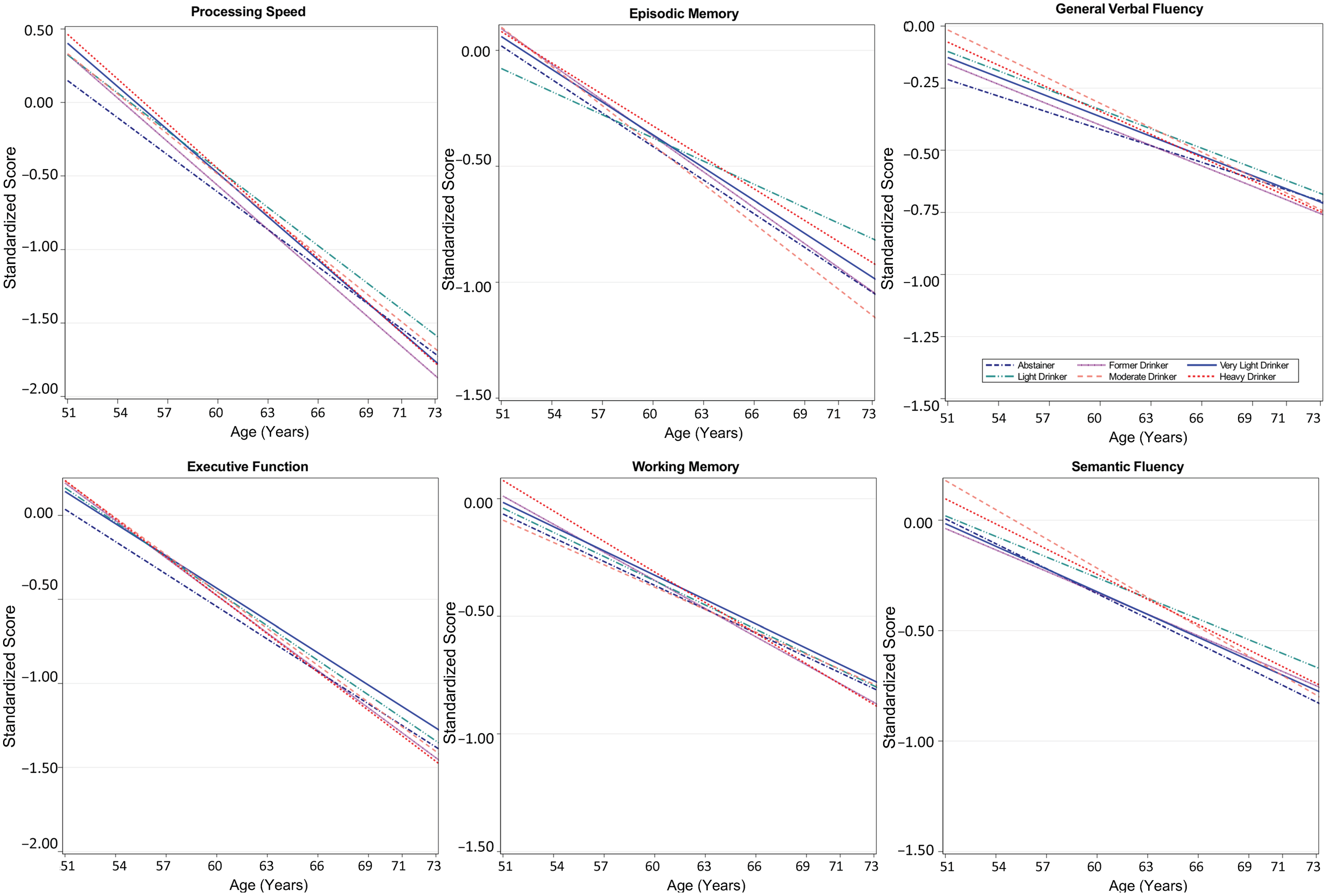
Figure 2. Age-related differences in cognitive performance by time-varying alcohol intake group among participants of the Vietnam Era Twin Study of Aging. Modeled differences are shown for each of the six cognitive domains by age for six categories of alcohol consumption. Plots are based on all model coefficients using ≤12 years of education, and non-Hispanic white race/ethnicity. The x-axis shows the full age range of the study sample.
Adjustment for health-related covariates that may lie in the causal pathway did not materially affect the results (Table S4). Nor was there evidence for effect modification by APOE ϵ4 status (three-way interaction p’s > 0.21).
Discussion
In this well-characterized sample of middle-aged men followed for 12 years, few robust associations of alcohol use with cognitive aging were observed, and where differences were observed, effect sizes were small. When examining change in cognitive function over time as a function of alcohol use at baseline, former drinkers showed consistently worse general verbal fluency performance over time, by 0.2 SD, than very light drinkers. At-risk drinkers showed steeper working memory decline than very light drinkers, by 0.14 SD per decade; an effect magnitude that is unlikely to be clinically meaningful. A similarly small effect size was observed when examining cognitive performance by age as a function of current drinking: light drinkers showed better episodic memory performance with advancing age, by 0.14 SD per decade increase in age, relative to very light drinkers. We found no evidence of effect moderation by APOE ϵ4 status in either time- or age-based models.
Our finding that former drinkers showed worse general verbal fluency performance than very light drinkers may be consistent with the sick-quitter hypothesis (Rehm et al., Reference Rehm, Irving, Ye, Kerr, Bond and Greenfield2008). Former drinkers were less healthy than other groups: they had the highest BMI and were more likely to have diabetes and multiple comorbidities than other groups, as we previously reported (Slayday et al., Reference Slayday, Gustavson, Elman, Beck, McEvoy, Tu, Fang, Hauger, Lyons, Mckenzie, Sanderson-Cimino, Xian, Kremen and Franz2020).
Although the magnitude of the association was small, our finding that at-risk drinkers showed steeper working memory decline than very light drinkers is partially consistent with results from the Whitehall II study (Sabia et al., Reference Sabia, Elbaz, Britton, Bell, Dugravot, Shipley, Kivimaki and Singh-Manoux2014), which reported steeper decline over time among men who drank above guidelines than men who drank less. However, steeper decline with at-risk drinking was observed across multiple cognitive domains in the Whitehall II study, whereas it was limited to working memory in our study. This discrepancy may be due to differences in definition of at-risk drinking, with at-risk drinking defined as >36 g/day (approximately equivalent to 3 drinks per day) in the Whitehall II report, whereas we used a lower threshold (equivalent to >2 drinks/day). Differences in type of beverage consumed may also contribute. Accelerated cognitive decline in the Whitehall II participants was stronger among men who consumed hard liquor than among those who consumed beer or wine; a higher percentage of men in the Whitehall II study consumed hard liquor (approximately 70%) than in our sample (approximately 43%).
The minimal associations of at-risk drinking with rate of cognitive decline observed here were somewhat unexpected, given prior findings among VETSA participants that at-risk drinking was associated with evidence of increased brain aging: relative to nondrinkers and lighter drinkers, at-risk drinking at wave 1 was associated with larger difference in predicted brain age relative to chronological age at VETSA 2 (by an average of 3 additional years) (Slayday et al., Reference Slayday, Franz, Hatton, McEvoy, Lyons and Kremen2019). This suggests that changes in the brain with at-risk drinking may precede changes in cognitive function. The VETSA sample is younger than the age at which cognitive function shows steepest age-related decline (Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2017); thus larger differences in rates of cognitive decline with at-risk drinking may become more apparent with continued follow-up of this cohort.
We found no evidence of a protective association of light or moderate alcohol intake at baseline on rate of cognitive decline relative to very light intake. However, in our age-based analyses, in which alcohol group was included as a time-varying exposure, light drinkers showed better episodic memory performance at advanced ages than very light drinkers, by 0.14 SD per 10-year increase in age. As mentioned previously, slopes in age-based models are influenced by between-participant differences as well as by within-person change with age (Hoffman, Reference Hoffman2012). By allowing alcohol group membership to vary over time (to take into account current drinking at each wave), between-participant effects are likely to dominate. Thus, the discrepancy between the within-participant analysis indicating no protective associations of alcohol use on rate of cognitive decline over time, and the finding of a protective association on episodic memory at advanced ages, suggests that the apparent protective association may arise from residual confounding or from a reverse association in which individuals with better memory performance at older ages choose a light drinking lifestyle.
The lack of protective association of light or moderate alcohol intake with cognitive aging in our study is consistent with findings in the ADAMS study (Herring & Paulson, Reference Herring and Paulson2018) and with findings among men in the Whitehall II study (Sabia et al., Reference Sabia, Elbaz, Britton, Bell, Dugravot, Shipley, Kivimaki and Singh-Manoux2014). Prior studies that have reported protective associations of light or moderate drinking with cognitive aging have used nondrinking groups as the reference (Ganguli et al., Reference Ganguli, Vander Bilt, Saxton, Shen and Dodge2005; Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2019; Richards et al., Reference Richards, Hardy and Wadsworth2005), included older adults (Bond et al., Reference Bond, Burr, McCurry, Rice, Borenstein and Larson2005; Ganguli et al., Reference Ganguli, Vander Bilt, Saxton, Shen and Dodge2005; Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2019; Zhang et al., Reference Zhang, Shen, Miles, Shen, Cordero, Qi, Liang and Li2020) or examined differences in cognitive function by age rather than examining change in cognitive function over time (Bond et al., Reference Bond, Burr, McCurry, Rice, Borenstein and Larson2005; Ganguli et al., Reference Ganguli, Vander Bilt, Saxton, Shen and Dodge2005; Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2019; Zhang et al., Reference Zhang, Shen, Miles, Shen, Cordero, Qi, Liang and Li2020).
We found no evidence that APOE ϵ4 modifies the relationship between alcohol use and cognitive decline. This contrasts with findings from the RBS, in which alcohol intake had a protective association against memory decline associated with APOE ϵ4 carriage (Reas et al., Reference Reas, Laughlin, Bergstrom, Kritz-Silverstein, Barrett-Connor and McEvoy2019). RBS participants were older and followed for a longer period (up to 27 years) than VETSA participants. It is possible that APOE ϵ4 may modify associations of alcohol with cognitive decline only at older ages, when more rapid rates of cognitive decline are typically observed.
Few studies have been able to examine whether earlier life cognitive ability confounds associations of alcohol use with later life cognitive function. In the Lothian Birth Cohort (Corley et al., Reference Corley, Jia, Brett, Gow, Starr, Kyle, Mcneill and Deary2011) adjustment for childhood general cognitive ability attenuated associations of alcohol intake with cognitive function at age 70 years. Adjustment for adolescent general cognitive ability also attenuated the beneficial association of light drinking on cognitive ability assessed at age 53 in the Wisconsin Longitudinal Study but did not completely account for the adverse association of heavy drinking with cognitive function among men (Krahn et al., Reference Krahn, Freese, Hauser, Barry and Goodman2003). In our study, higher young adult cognitive ability among light/moderate drinkers did not translate into less steep cognitive decline, and adjustment for young adult cognitive ability did not materially affect alcohol-cognitive function associations.
Strengths of our study include repeated assessment of alcohol use, time-varying covariates, and availability of information on earlier life alcohol use and cognitive ability. They also include robust estimation of distinct cognitive domains enabled by the comprehensive neuropsychological battery; and correction for practice effects (Elman et al., Reference Elman, Jak, Panizzon, Tu, Chen, Reynolds, Gustavson, Franz, Hatton, Jacobson, Toomey, Mckenzie, Xian, Lyons and Kremen2018). Limitations include the method of alcohol use assessment, in which participants were queried about the typical number of specific beverage types consumed on days during the past 2 weeks when they consumed that beverage type. Such self-report measures typically underestimate actual amounts consumed. We did not use the Time-Line Follow-back method (Sobell & Sobell, Reference Sobell, Sobell, Litten and Allen1992) or daily diaries, which may have improved accuracy. Additionally, our analyses examined amount of alcohol use over the past 14 days but did not take into account how that use was spread across the 14 days, which may affect associations. Prior studies have shown that consuming alcohol on several days per week, rather than restricting alcohol intake to 1 or 2 days, is associated with better health outcomes (Jani et al., Reference Jani, McQueenie, Nicholl, Field, Hanlon, Gallacher, Mair and Lewsey2021), including cognitive outcomes (Richard et al., Reference Richard, Kritz-Silverstein, Laughlin, Fung, Barrett-Connor and McEvoy2017). Our findings, based upon primarily beer drinkers, may not generalize to individuals who primarily consume other types of alcohol. The all-male sample with little racial or ethnic diversity are also important limitations to the generalizability of our results because both amount of alcohol intake and associations of alcohol intake with health-related outcomes differ between men and women and by race/ethnicity (Chartier & Caetano, Reference Chartier and Caetano2010; Bryant & Kim, Reference Bryant and Kim2019; Witbrodt et al., Reference Witbrodt, Mulia, Zemore and Kerr2014). Finally, given the number of cognitive domains assessed (6), and alcohol groups (6), observed associations could be attributable to type I error.
Conclusion
Alcohol intake showed minimal beneficial or adverse associations with cognitive aging among middle-aged men followed for 12 years with a comprehensive neuropsychological task battery. Observed associations were small and, in some cases, the pattern of associations differed according to whether the modeling strategy was age-based or time-based. A protective association of light alcohol use on slope of cognitive performance was apparent only in the age-based model, in which between-participant differences contribute to estimated age effects, and not in the time-based model that evaluates within-participant change over time. Adverse associations of at-risk drinking with cognitive performance were stronger in the time-based than in the age-based models, but associations were in the same direction, reflecting greater performance decrements with advancing age among at-risk drinkers. Careful consideration of analytic strategy is important when examining factors that influence cognitive aging because interpretation of results will vary dependent upon the model chosen. Employing more than one strategy can aid in interpreting findings. Stronger associations of alcohol use with cognitive aging, whether adverse or beneficial, may be revealed by longer follow up of this cohort.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1355617722000169
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University provided invaluable assistance in the creation of the VET Registry. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
Author contributions
Study Conception: LM; VETSA Study Design WK, ML, CF; Data Curation: AG; Formal Analysis: AG, XT, JB, KC, MP; Data Interpretation: AG, LM, GL, JB, XT, KC, CF, JE, ML, CR, MN, NG, HX, RK, RT, WK, MP Writing: Original Draft: AG, LM; Writing: Review and Editing: GL, JB, XT, KC, CF, JE, ML, CR, MN, NG, HX, RK, RT, WK, MP; Funding Acquisition: LM, WK, ML, CF.
Funding statement
This work was supported by the National Institutes of Health (LM: grant number R01 AG062483) (WK, CF, ML grant number: R01 AG050595) (WK grant numbers: R01 AG022381, R01AG037985) (WK CF grant number: P01 AG055367) (AG: T32MH122376).
Conflicts of interest
None.








