Introduction
Oxidative stress is the imbalance between the physiological production of reactive oxygen species, as a consequence of metabolic activities and signalling processes, and the ability of the biological system to neutralise the reactive intermediates or to repair the resulting damage( Reference Finkel and Holbrook 1 ). Free radicals operate at low but measurable concentrations in cells and the balance between their production and removal rates determines their ‘steady-state’ concentrations. Thus, each cell is characterised by a particular redox state, and this determines cellular signalling and functioning( Reference Valko, Leibfritz and Moncola 2 , Reference Schafer and Buettner 3 ).
When intracellular scavenging capacity is limited or, under certain situations of metabolic stress, the intracellular oxidant levels rise, normal redox homeostasis fails. Toxic effects may be caused by means of peroxides and free radical production that damage all cell components indiscriminately involving proteins, lipids and DNA, and triggering the activation of specific signalling pathways. Both these effects can influence numerous cellular processes linked to the development of pathological conditions, including CVD, cancers, neurological disorders, diabetes, ischaemia/reperfusion injury, as well as other diseases and physiological ageing( Reference Finkel and Holbrook 1 , Reference Valko, Leibfritz and Moncola 2 , Reference Dalle-Donne, Rossi and Colombo 4 – Reference Sayre, Smith and Perry 7 ).
This oxidative damage can be retarded by endogenous enzymic (catalase, superoxide dismutase (SOD) and glutathione peroxidise) defence systems, but exogenous antioxidants are required( Reference Parikh and Patel 8 ).
Vitamin D (calcitriol or 1,25-dihydroxyvitamin D3) is well known for its function as a steroid hormone on skeletal tissue. In particular, it is responsible for the increases in plasma Ca and phosphate levels required for bone mineralisation, as well as for neuromuscular junction activity, vasodilatation, nerve transmission and hormone secretion( 9 ). In the last decade, an extensive literature has been produced on vitamin D. The increasing interest of the scientific community may be explained by the potential enhanced roles of vitamin D in human health, due to the discovery that vitamin D acts through a nuclear receptor, and that this receptor is found in tissues not related to Ca and bone( Reference Plum and DeLuca 10 ). Several researchers have suggested relationships between vitamin D intake and health outcomes such as cancer prevention and increased immunity( Reference Plum and DeLuca 10 , Reference Hoeck and Pall 11 ). Others have proposed possible roles in preventing diabetes or pre-eclampsia during pregnancy( 9 ), as well as counteracting inflammation( Reference Hoeck and Pall 11 , Reference Holick 12 ), although the antioxidant effect of vitamin D is not uniformly proven( Reference Ticinesi, Meschi and Lauretani 13 , Reference Wang, Siu and Pang 14 ). At the same time, data suggest that a large part of the general population is vitamin D deficient, even in those countries where sun exposure is prolonged( Reference Palacios and Gonzalez 15 ). Considering human studies, several health outcomes have been associated with vitamin D plasma levels but little is known about its antioxidant effects.
Different markers have been proposed to assess antioxidant status, such as glutathione peroxidase, SOD, catalase, glutathione S-transferase, or by determining total antioxidant capacity (TAC) and nitrite levels. TAC is an analyte frequently used to assess the antioxidant status of biological samples: it measures the amount of free radicals scavenged by a test solution which evaluate the antioxidant response against free radicals( Reference Ghiselli, Serafini and Natella 16 ).
Oxidative stress may also be assessed by the detection of byproducts of lipid peroxidation; malondialdehyde (MDA) is commonly used as a marker for lipid oxidative damage( Reference Namdev, Bhat and Adhisivam 17 ). Given that increased oxidative stress has been implicated as a potential causal factor in the development of several diseases, and given that knowledge about the potential role of vitamin D as antioxidant is still limited, the aim of the present review was to explore the current literature regarding the potential scavenger capacity of vitamin D in high-evidence human studies. Since the trials were conducted in a range of heterogeneous populations, in order to clearly discuss our findings, we decided to present results in three thematic sections, divided as follows: studies conducted in healthy subjects, studies conducted in participants with diabetes, and studies conducted in those with other health conditions.
Methods
We carried out a thorough search for relevant papers on three databases (MEDLINE/PubMed, Scopus and Embase) using a combination of text and MeSH (medical subject headings) to enhance search strategies using specific key words.
We imposed restrictions for language (English) and species (human subjects). In order to reach reliable evidence, we included only randomised controlled trials investigating the effect of vitamin D on antioxidant state biomarkers. Reviews and meta-analyses were excluded from the search. Results are reported in the form of a narrative review.
Antioxidant effect of vitamin D supplementation in healthy subjects
Only three trials examined the antioxidant effect of vitamin D supplementation in healthy subjects (Table 1) and results have been heterogeneous. In the randomised, double-blind, placebo-controlled study of Asemi et al. ( Reference Asemi, Samimi and Tabassi 18 ), the authors evaluated the effects of 9 weeks of cholecalciferol supplementation on high-sensitivity C-reactive protein, metabolic profiles and biomarkers of oxidative stress (plasma total glutathione, GSH, and plasma TAC) in a population of healthy pregnant women aged 18–40 years old. In particular, the study considered forty-eight total participants, split into two groups: twenty-four women in the intervention group (400 IU (10 μg) cholecalciferol per d), and twenty-four in the placebo group. The authors observed a significant increase in plasma TAC (vitamin D v. placebo groups: +152 v. –20 mmol/l; P-interaction = 0·002) and GSH (vitamin D v. placebo groups: +205 v. –32 μmol/l; P-interaction = 0·02) concentrations in individuals supplemented with vitamin D when compared with the placebo group. Consistently, the research group of de Medeiros Cavalcante et al. ( Reference de Medeiros Cavalcante, Silva and Costa 19 ) conducted a double-blind, randomised, placebo-controlled trial in a population of forty elderly women diagnosed with vitamin D insufficiency. In this study, the authors evaluated the effect of 200 000 IU (5000 μg) cholecalciferol supplementation (by means of four capsules of 50 000 IU (1250 μg) each) for a 4-week period on TAC and MDA levels, finding that the megadose supplementation did not affect plasma MDA levels, but TAC appeared significantly increased (65·25 (sd 15·66) to 71·83 (sd 10·71); P=0·03). Scholten et al. ( Reference Scholten, Sergeev and Song 20 ) investigated the effect of vitamin D3 supplementation (4000 IU (100 μg)/d) alone or in combination with quercetin (1000 mg/d) for 8 weeks on physical performance and on markers of oxidative stress in a sampled population made by forty adult male subjects engaged in moderate-to-vigorous-intensity exercise training. Participants were randomly assigned in a double-blind manner in different groups including placebo. In contrast with the previously presented results, this research showed a significant decrease in TAC, while SOD activity, lipid and protein oxidation, MDA and protein carbonyl groups (PCO) did not show any significant variation.
Table 1 Summary randomised controlled trials exploring the association of vitamin D and oxidative stress markers
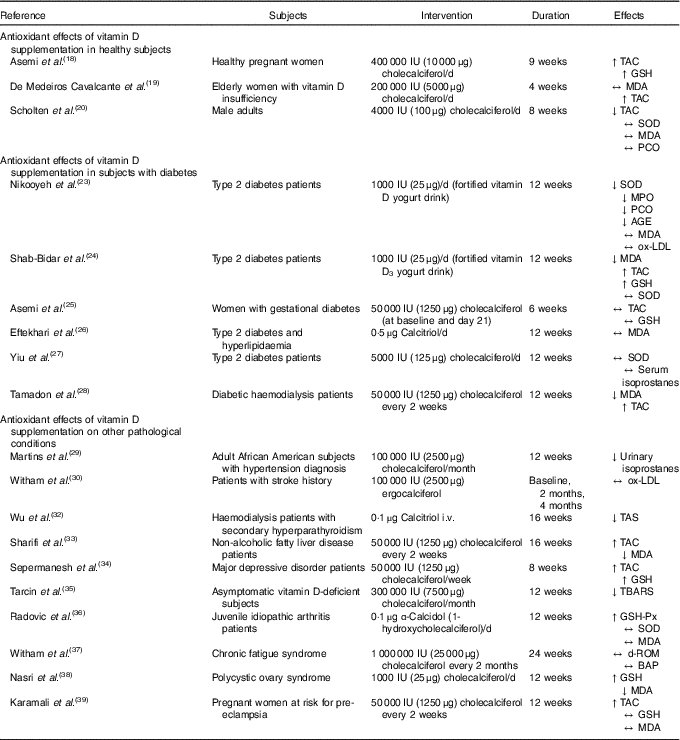
IU, international units; ↑, increased levels; TAC, total antioxidant capacity; GSH, glutathione; ↔, no effect; MDA, malondialdehyde; ↓, decreased levels; SOD, superoxide dismutase; PCO, protein carbonyl groups; MPO, myeloperoxidase; AGE, advanced glycation endproducts; ox-LDL, oxidised LDL; i.v., intravenous; TAS, total antioxidant status; TBARS, thiobarbituric acid reactive substances; GSH-Px, glutathione peroxidase; d-ROM, derivatives of reactive oxygen metabolites; BAP, biological antioxidant potential.
To summarise, data from healthy subjects appear controversial. In some cases, supplementation with a relatively low dose of vitamin D3 (400 IU (10 μg)/d) caused increases in TAC and GSH activity( Reference Asemi, Samimi and Tabassi 18 ). On the other hand, higher doses (4000 IU (100 μg)/d) resulted in a decrease of TAC and no significant effects on other biomarkers of oxidative stress, such as SOD activity and damage to biological macromolecules (lipid and MDA and carbonyl groups)( Reference Scholten, Sergeev and Song 20 ). The three studies considered pregnant women, elderly women and active male adults: these are healthy but heterogeneous populations, characterised by different vitamin D needs and by a different metabolic status; thus it is not possible to draw general conclusions. Furthermore, the baseline characteristics of subjects in terms of vitamin D status were different. In the trial of Scholten et al. ( Reference Scholten, Sergeev and Song 20 ), 88·6 % of participants were described as vitamin D sufficient (25-hydroxyvitamin D (25(OH)D)>50 nmol/l), while elderly women in the study by de Medeiros Cavalcante et al. ( Reference de Medeiros Cavalcante, Silva and Costa 19 ) were diagnosed as vitamin D deficient (24·7 (sd 3·1) ng/ml) and mean 25(OH)D concentrations at baseline in pregnant women( Reference Asemi, Samimi and Tabassi 18 ) were low (25(OH)D>18 µg/l). Currently, the definition of vitamin D deficiency, threshold levels of circulating vitamin D, as well as optimal levels of 25(OH)D, are debatable( Reference Holick, Binkley and Bischoff-Ferrari 21 ). The Endocrine Society Clinical Practice Guideline defined ‘vitamin D deficiency’ as 25(OH)D below 20 ng/ml (corresponding to 50 nmol/l), ‘vitamin D insufficiency’ as 25(OH)D of 21–29 ng/ml (corresponding to 525–725 nmol/l), and suggested as optimal a blood level of 30 ng 25(OH)D/ml (corresponding to 75 nmol/l)( Reference Holick, Binkley and Bischoff-Ferrari 21 , Reference Levy, McKinnon and Barker 22 ). In general, vitamin D replacement was improved by treatment in all the studies, with a significant effect in all groups considered, even if 25(OH)D levels in elderly women after treatment were still inadequate. Additionally, the duration and type of treatment (daily, intermittent or through a megadose) may lead to different effects. Thus, no strong conclusions can be drawn on vitamin D supplementation in healthy subjects.
Antioxidant effect of vitamin D supplementation in subjects with diabetes
A total of six studies investigated the effects of vitamin D supplementation in subjects with diabetes (Table 1). Nikooyeh et al. ( Reference Nikooyeh, Neyestani and Tayebinejad 23 ) enrolled ninety patients with type 2 diabetes (T2D) aged 30–50 years randomly allocated into three groups receiving two 250 ml yogurt drink bottles per d. The yogurt was either plain or fortified with 500 IU (12·5 μg) vitamin D per dose (equivalent to 1000 IU (25 μg) vitamin D/d total intake) or with 500 IU (12·5 μg) vitamin D plus 250 mg of Ca per dose (equivalent to 1000 IU (25 μg) vitamin D plus 500 mg Ca/d total intake) for 12 weeks. The authors found that 1000 IU (25 μg) vitamin D per d was able to significantly reduce the intra-group serum levels of protein carbonyl groups, cardiac myeloperoxidase and SOD. Furthermore, a significant decrease in advanced glycation endproducts was described in both groups receiving either the vitamin D or the vitamin D plus Ca fortified yogurt, when compared with the plain one. At the same time, the authors failed to describe significant changes in lipid peroxidation markers, such as MDA and oxidised LDL. Shab-Bidar et al. ( Reference Shab-Bidar, Neyestani and Djazayery 24 ) performed a 12-week randomised controlled clinical trial investigating the effect of two servings of a vitamin D3-fortified yogurt drink daily (containing 170 mg Ca and 500 IU (12·5 μg) vitamin D3/serving), compared with a plain yogurt drink (containing 170 mg Ca and no vitamin D3/serving). The authors considered 140 T2D patients, finding that improvement in vitamin D status was accompanied by significant changes in MDA levels (–0·54 (sd 0·82) μmol/l in a fortified drink v. +0·17 (sd 1) μmol/l in a plain drink; P<0·001), TAC (+0·14 (sd 0·43) mmol/l v. +0·02 (sd 0·45) mmol/l bovine serum albumin equivalents; P=0·03) and GSH (+8·4 (sd 40·1) ng/l v. –13·1 (sd 29·4) ng/l; P=0·002), but not for SOD activity. To ensure that changes in the biomarkers were due to the intervention, the authors analysed dietary intakes, finding no significant inter- or intra-group differences. The dietary assessment represents a strength of the study, and allowed us to exclude habitual dietary intake as a potential confounding factor. Asemi et al. ( Reference Asemi, Hashemi and Karamali 25 ) evaluated the effects of a double dose of 50 000 IU (1250 μg) vitamin D3 supplementation during a 6-week intervention (at baseline and on day 21 of the study) on glucose and lipid profiles, as well as inflammation and oxidative stress, in women with gestational diabetes. In particular, the study considered fifty-four total participants, split into two groups: twenty-seven in the intervention group and twenty-seven in the placebo group. This trial showed that cholecalciferol supplementation did not significantly affect plasma TAC and total GSH concentration post-intervention. Based on a 3 d dietary record, no significant differences were reported between the groups in terms of dietary intakes of macronutrients, micronutrients (including vitamin D) and vitamin D status before the supplementation period. In the study by Eftekhari et al. ( Reference Eftekhari, Akbarzadeh and Dabbaghmanesh 26 ), seventy patients with T2D and hyperlipidaemia aged 30–75 years were included in a double-blind randomised placebo-controlled trial and received 0·5 µg calcitriol/d for 12 weeks. The authors measured serum concentrations of MDA, finding that it decreased in both the treatment and placebo groups, after 6 and 12 weeks of supplementation. However, the two groups were not significantly different, and time–treatment interactions did not emerge. Still, in this study, the authors did not assess potential individual differences in basal vitamin D status, or dietary habitual intakes in the two groups. Thus, it is not possible to speculate about the effects of the supplementation on MDA levels in this case.
Yiu et al. ( Reference Yiu, Yiu and Siu 27 ) investigated the effects of 5000 IU (125 μg) vitamin D daily supplementation for a 12-week period, in a group of subjects with T2D, on vascular function and some oxidative stress biomarkers, such as serum isoprostanes and SOD levels. Results from this trial showed that despite the improvement in 25(OH)D status, vitamin D supplementation did not achieve an improvement in the oxidative end-points considered by the authors. All subjects enrolled in the trial had suboptimal 25(OH)D status (<30 ng/ml) at baseline, showing a significant increase in serum 25(OH)D concentration post-supplementation at follow-up (week 12) compared with the placebo group (treatment effect 34·7 (95 % CI 26·4, 42·9) ng/ml). The authors did not assess dietary intake. In a recent double-blind placebo-controlled trial, Tamadon et al. ( Reference Tamadon, Soleimani and Keneshlou 28 ) administered 50 000 IU (1250 μg) cholecalciferol to diabetic haemodialysis patients every week for 12 weeks. The vitamin D-treated group showed increased TAC (+33·8 (sd 56·7) v. –2·0 (sd 74·5) mmol/l; P=0·04) and reduced MDA levels (–0·1 (sd 0·2) v. +0·1 (sd 0·2) μmol/l; P=0·009) compared with the placebo group. However, these results, while showing statistical significance, fail to show clinical significance.
Considered all together, these data show that vitamin D supplementation trials in patients affected by T2D fail to reach solid conclusions. Trials were characterised by different durations of treatment, that ranged between 6 and 12 weeks of intervention; doses chosen by authors were also variable, ranging between a total of 12 000 and 300 000 IU (300 and 7500 μg) of vitamin D supplementation during the time of treatment. The heterogeneity of protocols may explain the controversial effects of vitamin D supplementation on biomarkers of oxidative stress in this subset of patients. However, it remains unclear why some oxidative stress outcomes were affected by the treatment and others were not, even in the same clinical study.
Effect of vitamin D supplementation in other pathological conditions
We identified ten other studies that investigated the antioxidant effects of vitamin D in different clinical conditions (Table 1), making it difficult to perform a summary review of the subject. For example, in a double-blind randomised placebo-controlled study, Martins et al. ( Reference Martins, Meng and Tareen 29 ) evaluated the effect of a 100 000 IU (2500 μg) monthly vitamin D3 supplementation in a 3-month period on inflammatory and oxidative mediators of arterial stiffness. The study enrolled 130 African American subjects aged 18–70 years with a diagnosis of hypertension, BMI> 30 kg/m2 and serum 25(OH)D ranging from 10 to 25 ng/ml. In particular, the authors observed a reduction in urinary isoprostane levels (14·4 v. 11 ng/mg creatinine; P=0·0173) in the vitamin D-treated group when compared with placebo. The monthly 100 000 IU (2500 μg) vitamin D supplementation was effective in raising the mean 25(OH)D serum levels from a 10–25 ng/ml range at baseline to 34·5 (sd 7·1) ng/ml post-supplementation, and suggested an association with the significant decrease in urinary isoprostane levels. In a subset of fifty-eight patients with a history of stroke and baseline 25(OH)D level <75 nmol/l, vascular health markers were investigated by Witham et al. ( Reference Witham, Dove and Sugden 30 ) after vitamin D2 supplementation orally. Patients received either a 100 000 IU (2500 μg) vitamin D2 supplementation or placebo at baseline. Analysis of oxidative stress by means of oxidised-LDL, after 2 and 4 months, did not show any significant change in the treated group when compared with placebo. At follow-up (week 8), the supplementation produced a significant increase in 25(OH)D levels from baseline (+ 40 %) in the treatment group v. placebo. However, 25(OH)D levels after the intervention did not reach the concentration proposed to represent the recommended level for optimum health equal to 75 nmol/l( Reference Bischoff-Ferrari, Giovannucci and Willett 31 ). This moderate effect may be explained by the fact that the authors performed the supplementation with vitamin D2 instead of the more bioavailable vitamin D3. The case–control study conducted by Wu et al. ( Reference Wu, Chang and Chen 32 ) investigated the influence of 16 weeks of vitamin D treatment on oxidative stress and inflammatory markers in haemodialysis patients with secondary hyperparathyroidism. In particular, twenty-five subjects (mean age 58 (sd 12) years; thirteen males and twelve females) were enrolled and calcitriol supplementation was administered. An initial low-dose intravenous calcitriol (1 µg) treatment was administered to achieve effective and safe suppression of individual PTH levels. Calcitriol dosage was adjusted according to changes in serum alkaline phosphatase and intact PTH levels (2 µg calcitriol was the higher dose), if serum levels of Ca, P and Ca-P product remained within the acceptable clinical range. The authors measured total antioxidant status, finding a significant decrease in oxidative stress from baseline. Furthermore, they described a significant, negative correlation between total antioxidant status and intact PTH levels, suggesting the beneficial effects beyond the PTH-lowering effect of calcitriol treatment for secondary hyperparathyroidism. The supplementation with calcitriol produced a significant rise in 25(OH)D levels, ranging from 13·4 pg/ml at baseline to 18·67 pg/ml at week 16. Baseline 25(OH)D levels were similar for the two groups.
Sharifi et al. ( Reference Sharifi, Amani and Hajiani 33 ) conducted a double-blind, placebo-controlled study including fifty-three patients with non-alcoholic fatty liver disease who received one oral dose of 50 000 IU (1250 μg) vitamin D3 every 14 d for 4 months. Among secondary outcomes, the authors considered oxidative stress biomarkers, finding that the serum TAC levels were significantly increased in both trial arms, while no significant change was reported between the groups. However, vitamin D supplementation significantly decreased serum MDA (–2·09 v. –1·23 ng/ml; P=0·03). In this study, the median 25(OH)D serum level of the patients who received vitamin D supplements compared with the controls significantly increased, reaching 16·2 v. 1·6 ng/ml (P<0·001), corresponding to approximately 40·5 nmol/l.
Sepehrmanesh et al. ( Reference Sepehrmanesh, Kolahdooz and Abedi 34 ) investigated the effects of a single capsule of 50 000 IU (1250 μg) vitamin D per week for 8 weeks in forty patients (aged 18–65 years) with a diagnosis of major depressive disorder. Biomarkers of oxidative stress were assessed as secondary outcomes and the results described a significant beneficial effect of vitamin D supplementation on TAC (+63·1 v. –23·4 mmol/l; P=0·04) and GSH levels (+170 v. –213 μmol/l; P=0·04) at the end of treatment. In this study, 25(OH)D baseline levels did not show any significant differences between the groups. After 8 weeks of intervention, serum 25(OH)D concentrations were significantly increased in the vitamin D group, with a +20·4 µg/l mean increase v. –0·9 µg/l in the placebo group. Furthermore, 3 d dietary records were provided during the study to monitor potential differences in subjects’ dietary habits. Tarcin et al. ( Reference Tarcin, Yavuz and Ozben 35 ) evaluated the effect of vitamin D replacement on endothelial function and oxidative stress in a group of twenty-three asymptomatic vitamin D-deficient subjects, with 25(OH)D levels below 25 nmol/l. After 3 months of supplementation with 300 000 IU (7500 μg) cholecalciferol (once per month), levels of thiobarbituric acid reactive substances (TBARS) (byproducts of lipid peroxidation) were significantly decreased, compared both with baseline and with the control group. The intervention performed by the authors appeared effective in 25(OH)D replacement. In fact, the baseline levels of those receiving the supplementation significantly increased after treatment (baseline median value: 20·4 (sd 6·8) v. 116·9 (sd 45·5) nmol/l; P<0·05). Radovic et al. ( Reference Radovic, Lazaravic and Nikolic 36 ) investigated the effect of α-calcidiol supplementation on reactive oxygen species production in fifteen subjects with juvenile idiopathic arthritis. The treatment protocol considered the continuation of previously applied therapy with the addition of α-calcidiol (1 µg/d) for 3 months. α-Calcidiol therapy induced an increase in GSH peroxidase activity in the treatment group but did not show significant effects on SOD activity and MDA levels, when compared with baseline values. From this study, no clear data were reported about baseline 25(OH)D serum levels; thus it is not possible to speculate about the effectiveness of the treatment. Witham et al. ( Reference Witham, Adams and McSwiggan 37 ) performed a randomised, double-blind, placebo-controlled trial including fifty subjects affected by chronic fatigue syndrome who received 100 000 IU (2500 μg) of vitamin D3 orally every 2 months for 6 months. The authors did not find significant variation in oxidative stress biomarkers, such as derivatives of reactive oxygen metabolites and biological antioxidant potential. One placebo-controlled study investigated the conjunct effect of vitamin D3 and evening primrose oil( Reference Nasri, Akrami and Rahimi 38 ). The authors enrolled sixty vitamin D-deficient women with polycystic ovary syndrome and co-supplemented them with 1000 IU (25 μg) cholecalciferol plus 1000 mg evening primrose oil for 12 weeks. Although the results need to be interpreted in the light of co-supplementation, at the end of the trial the treated group showed increased GSH (+62·7 (sd 58·0) v. –0·7 (sd 122·7) µmol/l; P=0·01) and decreased MDA (–0·4 (sd 0·4) v. +0·5 (sd 1·8) µmol/l; P=0·008) levels.
A randomised double-blind placebo-controlled clinical study involving sixty pregnant women at risk for pre-eclampsia was designed by Karamali et al. ( Reference Karamali, Beihaghi and Mohammadi 39 ) to assess the effect of high-dose cholecalciferol supplementation, by means of 50 000 IU (1250 μg) vitamin D3, every 2 weeks from 20 to 32 weeks of gestation on TAC, GSH and MDA levels. The authors described a significant increase in TAC (+79 (sd 136·69) v. –66·91 (sd 176·02) mmol/l; P=0·001), while GSH content and lipid peroxidation were not affected by vitamin D supplementation. Baseline vitamin D status did not show significant differences between the two groups, and pregnant women who received cholecalciferol supplements showed a significant increase in serum 25(OH)D concentration (+17·92 (sd 2·28) v. +0·27 (sd 3·19) ng/ml; P<0·001). The authors obtained the 3 d dietary records from patients during the intervention to assessed potential differences in habitual dietary intake between the two groups.
It is difficult to summarise the results of the trials conducted in these heterogeneous conditions. Most of the clinical trials described present controversial data about the antioxidant effect of vitamin D supplementation, even though in all the studies evaluating the oxidative stress damage to membrane lipids( Reference Sepehrmanesh, Kolahdooz and Abedi 34 , Reference Radovic, Lazaravic and Nikolic 36 ), improvements in MDA and thiobarbituric acid reactive substances (TBARS) levels were described. However, as previously mentioned for the T2D clinical trials, the intervention conditions were not homogeneous among the studies, by means of specific molecules, dose, length and type of vitamin D administration. Moreover, the patients considered were highly heterogeneous samples and presented dissimilar metabolic and oxidative stress features. Among the studies retrieved, the clinical trials dealing with kidney diseases have not been considered in the present paper in order to limit the variability of the molecules used. In those studies, the molecule most frequently administered was paricalcitol (an agonist of the vitamin D receptor), which appears more effective in controlling hypercalcaemia. Finally, authors have not always assessed, or otherwise disclosed, habitual dietary intakes, as well as other variables such as the exposure to sunlight through outdoor activities, latitude of residence, or ethnicity that may influence blood levels of 25(OH)D.
Concluding remarks
Data collected in the present review revealed that the potential role of vitamin D as an antioxidant could not be confirmed. Current literature showed controversial effects of the ability of vitamin D to prevent or ameliorate the imbalance between oxidant and antioxidants species, even when clinical supplementation allowed an effective vitamin D replacement. The heterogeneity of protocols like duration, type of treatment (daily, intermittent or through a megadose), the heterogeneity of populations and of markers of oxidative status considered may lead to different effects and explain the disputable results. Thus, no strong conclusions can be drawn on vitamin D supplementation in healthy subjects.
TAC, lipid oxidative damage and GSH levels have been frequently used to assess oxidative status among clinical trials considered in the present review. Overall, vitamin D supplementation improved TAC level in the majority of studies (two of three in groups of healthy subjects, two of three in groups of subjects with diabetes, and three of three in groups of subjects with other diseases, respectively). A trend of an antioxidant effect of vitamin D has been described for MDA or TBARS concentrations in non-healthy subjects (two of four studies of subjects with diabetes, and three of six studies including subjects with other diseases). In more than 50 % of studies considering glutathione levels, it was increased after treatment with vitamin D (one of one study of healthy participants, one of two studies of subjects with diabetes, and two of three studies of groups with miscellaneous pathological conditions).
To support this, we consider it noteworthy that the most recent studies( Reference Asemi, Hashemi and Karamali 25 , Reference Martins, Meng and Tareen 29 , Reference Karamali, Beihaghi and Mohammadi 39 ) all show a common trend, attributing to vitamin D a probable antioxidant effect. A consensus on the average requirements and population reference intakes for vitamin D that allow achieving an optimal status beyond musculoskeletal health does not exist at this time. Recently, the European Food Safety Authority (EFSA) defined a serum 25(OH)D concentration of 50 nmol/l as a suitable target value for all population groups, taking into account the overall evidence base (mainly related to bone health) and uncertainties (related to other pleiotropic effects)( 40 ). The first reason behind the lack of definitive conclusions on this specific topic is initially the huge variability and heterogeneity in types of supplementation. Several forms of vitamin D with different bioavailability were used in the studies discussed. Second, the choice of dose largely differed. This aspect may be related to the type of patients enrolled in the trial, mainly considering that vitamin D is a pleiotropic hormone with widespread effects. Third, the duration of intervention appeared very different among the clinical trials retrieved. Finally, the type of administration, such as daily, or intermittent (weekly, monthly), appears to be a critical aspect that should be taken into account. The relationship between the baseline 25(OH)D serum concentration and effective replacement level should be considered to evaluate potential association with specific outcomes. One hypothesis is that vitamin D supplementation is effective only in those patients with very low baseline 25(OH)D levels. Furthermore, not all the studies investigated the dietary intakes of energy, macro- and micronutrients, as well as the sun exposure, geographical location, and the period of the year that subjects enrolled and were treated. As each has an impact on circulating levels of vitamin D, they should be considered as variables in a proper analysis. In conclusion, there is the need for further clinical trials with a more complete standard of evidence and methodology to deepen and elucidate the antioxidant role of vitamin D supplementation.
Acknowledgements
None of the authors received funding for the present review. We thank Matthew Francis for the language editing of the manuscript.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
S. T. and H. C. conceived this review. M. M., S. T., D. P. and F. A. performed the database research, S. T., D. P., R. D. G. and M. M. drafted the manuscript. All authors contributed to the interpretation of data and critical revision. All authors agreed to the manuscript in its current form.
There are no conflicts of interest.



