Free radicals are implicated in degenerative human diseases such as cancer and cerebro-cardiovascular pathologies by multiple mechanisms(Reference Ames, Shigenaga and Hagen1). There is evidence that reactive oxygen species, and in particular oxidative DNA damage, play a role in aetiology of atherosclerosis(Reference Halliwell, Zhao and Whiteman2, Reference Olinskia, Gackowskia and Foksinskia3). Recently, a wide number of studies have shown that the consumption of chocolate may induce cardioprotective effects by favourable action on blood pressure(Reference Grassi, Lippi and Necozione4), platelet function(Reference Innes, Kennedy and McLaren5, Reference Murphy, Chronopoulos and Singh6), HDL cholesterol(Reference Mursu, Voutilainen and Nurmi7), endothelium function(Reference Engler, Engler and Chen8) and oxidative status(Reference Gu, House and Wu9). The majority of work that investigated the antioxidant properties of chocolate focused on the effect on LDL oxidation susceptibility(Reference Wan, Vinson and Etherton10), while fewer and more conflicting are the studies about the effect on plasma total antioxidant capacity(Reference Vlachopoulos, Aznaouridis and Alexopoulos11, Reference Wang, Shramm and Holt12) and, although it has been widely shown whether oxidative DNA damage contributes to atherogenesis, to our knowledge, there are no studies in vivo that have investigated the effect of chocolate intake on DNA protection from oxidative damage.
Beneficial effects attributed to chocolate consumption and in particular antioxidant properties depend on flavonoid content. Cocoa and chocolate products have a much higher flavonoid concentration and total antioxidant capacity per weight than other flavonoid food sources such as red wine, green tea and black tea(Reference Lee, Kim and Lee13). Among milk and dark chocolate (DC), the latter contains higher amounts of cacao and higher amounts of flavonoids (i.e. 951 mg polyphenols/40 g serving compared to 394 mg in milk chocolate)(Reference Lee, Kim and Lee13). In particular, the predominant flavonoids are catechin and epicatechin, mostly present in the form of procyanidins; epicatechin is absorbed from human intestine better than catechin(Reference Keen14).
In the present study, we evaluated, in apparently healthy human volunteers, the effects of regular consumption of a 45 g serving of DC on plasma epicatechin concentrations, mononuclear blood cells (MNBC) DNA resistance to oxidative stress and plasma total antioxidant activity (TAA), in comparison with a white chocolate (WC) containing negligible amounts of flavonoids. Since data in the literature suggest that epicatechin plasma levels increase markedly 2 h after DC ingestion(Reference Ritchelle, Tavazzi and Enslen15), we also investigated the effect on DNA resistance to oxidative stress and total plasma antioxidant activity 2 h after consumption of the first and last servings.
Materials and methods
Reagents
All the chemicals used were AnalaR grade. Acetonitrile, methanol, ethanol, n-hexane, water (HPLC grade) and metaphosphoric acid were purchased from Merck Sharp & Dohme Italia S.p.A. (Rome, Italy). 2,2-Azinibis-(3-ethylbenzothiazoline-6-sulphonic acid), ( ± )-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), potassium persulphate (K2S2O8), Folin-Ciocalteu, l-ascorbic acid, α-glucuronidase and sulphatase (G 7017), Trizma base, percloric acid, Na2HPO4 and Na2 EDTA were purchased from Sigma-Aldrich (St Louis, MO, USA). Authentic standards of gallic acid, α-tocopherol, all-trans-retinol and ( − )-epicatechin were obtained from Sigma-Aldrich.
Subjects
Twenty healthy individuals (ten men and ten women, mean age 24·2 (se 0·7) years, range 20–32 years) were recruited from an Italian University campus by a written questionnaire about food habits, health and lifestyle.
All subjects were non-smokers, normal weight (18·5 kg/m2 < BMI < 24·9 kg/m2) and in good general health. Exclusion criteria were: diseases or serious medical conditions; supplements or medications (oral contraceptives included); special diets (vegan, vegetarian or macrobiotic diet, gluten-free diet); non-habitual fruit and vegetable consumption; sport d'elite. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committee of University of Milan. Written informed consent was obtained from all subjects.
Materials
DC with 70 % cocoa and WC (cocoa butter) was supplied by Ferrero (Alba, Italy) in single servings of 45 g. The nutrient content of the two kinds of chocolates is presented in Table 1.
Table 1 Macronutrient composition of study chocolates
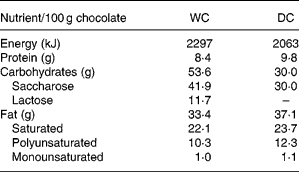
WC, white chocolate; DC, dark chocolate.
Experimental design
Study design was described in Fig. 1. The dietary intervention lasted for 4 weeks; it was characterised by an isocaloric diet balanced (55–60 % of energy from carbohydrates, 25–30 % from fat and 0·8–1 g protein/kg body weight) according to Recommended daily Assumption Levels of Nutrients(16).
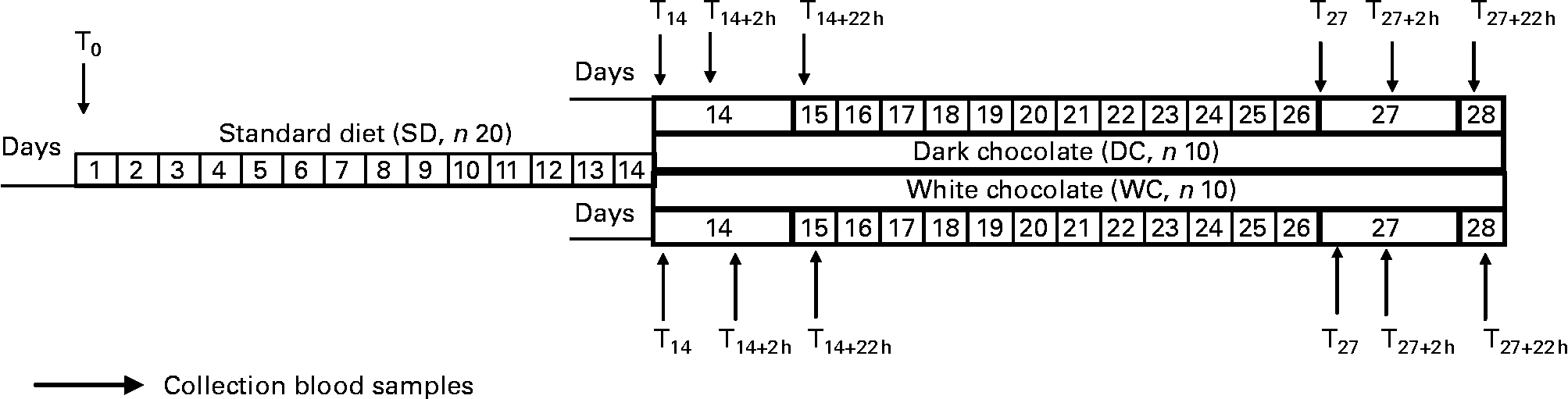
Fig. 1 Experimental protocol of dietary intervention. T0 (fasting), start experiment protocol; T14 (fasting), end standard diet and start chocolate diet; T14+2 h, 2 h after the first chocolate intake; T14+22 h (fasting), 22 h after first chocolate intake; T27 (fasting), last day of chocolate intake; T27+22 h, 22 h after last chocolate intake.
During the whole experimental period, intake of food antioxidants was limited to only two servings of fruit and vegetables each day, and not more than the amounts specified in Table 2 per week. Amount per serving was similarly restricted to 50 g salad, 200 g other kinds of vegetables, 200 g apple and pear, 150 g banana and 250 g citrus fruit.
Table 2 List of allowed and not allowed foods to standardise dietary antioxidant intake during the whole dietary intervention (frequencies of consumption for each week)

The volunteers were asked to complete a daily diary to verify, within the limitation of the technique, compliance with our dietary intervention.
The standardised diet (SD) was followed for 2 weeks before the intervention period to match the volunteers' short-term dietary habits and intake of antioxidant sources. In the final 2 weeks, the volunteers were given their preferred choice of either DC (45 g/serving) or WC (45 g/serving) chocolate to include. Of the twenty volunteers, five men and five women selected DC and the remainder WC. Volunteers were advised to consume chocolate as a snack with 40 g white bread. To avoid excess energy consumption while including the chocolate in their diet, the SD was adjusted; energy and in particular fat intake was reduced by replacing full-fat cows' milk with skimmed cows' milk. Crackers were eliminated as a snack, and the amounts of permitted sugar and pasta were also reduced.
Blood samples were collected at the beginning of the experimental period (T0), the end of the SD alone (T14), 2 and 22 h after the initial consumption (T14+2 h and T14+22 h) and again before and after the final chocolate serving (T27, T27+2 h and T27+22 h). With the exception of T14+2 h and T27+2 h, all samples were collected at 08·30 h after an overnight fast.
Epicatechin extraction
Chocolate
Chocolate samples (1 g) were extracted following the method described by Gu et al. (Reference Gu, House and Wu17). The analysis was carried out in duplicate using four samples of DC and four samples of WC. Each sample was from a different batch. Total polyphenols content was measured at 760 nm using the Folin-Ciocalteu reagent diluted tenfold before use, with gallic acid as the standard (5–10–25–50–100 μg/ml), and measurement after 5 min at 50°C(Reference Singleton and Rossi18).
Plasma
Plasma samples were extracted and analysed by the method described by Ritchelle et al. (Reference Ritchelle, Tavazzi and Enslen15) and Rein et al. (Reference Rein, Lotito and Holt19) with some modifications; samples (400 μl) were added to 40 μl vitamin C–EDTA solution (200 mg vitamin C and 1 mg EDTA in 1 ml water) and 40 μl β-glucuronidase and sulphatase. The mixture was incubated at 37°C for 45 min. Plasma was extracted with acetonitrile (1 ml) and centrifuged at 10 000 g for 5 min at 4°C. The supernatant was transferred to a tube containing 100 mg alumina and washed twice with 2 ml 2-amino-2-(hydroxymethyl)propane-1,3-diol buffer followed by a further wash with 2 ml methanol. Between each wash, the mixture was centrifuged at 4000 g for 5 min at 4°C and the supernatant discarded. After evaporation of the methanol, 250 μl percloric acid was added to the alumina, which was vortexed for 1 min and centrifuged at 4000 g also for 1 min at 4°C. The supernatant was filtered using a polyvinylidene fluoride syringe filter (Whatman, Clifton, NJ, USA) and 100 μl were injected on to the HPLC system.
Quantification of epicatechin
HPLC analysis
The HPLC system Waters (Waters Corporation, Milford MA, USA) consisted of a 996 W photodiode and array detector, 1525 W Binary pump, 717 W plus auto-sampler, array detector controlled by a data acquisition Empower software (Waters).
The HPLC analysis was performed on a reverse phase ODS HYPERSIL SB-C18 column (4·6 × 250 mm2, 5-μm pore size, Thermo, San Jose, CA, USA) using a guard column ODS HYPERSIL SB-C18 (4·6 × 125 mm2, 5-μm pore size, Thermo) at room temperature. Spectroscopy data from all peaks were captured in the range of 210–400 nm, and chromatograms were recorded at 279 nm. Separations were carried out at a flow rate of 1·5 ml/min with an isocratic mobile phase of 85 % Na2PO4 10 mol/l, pH 3 and 15 % acetonitrile. The identification of the epicatechin was made by comparison of retention times and spectra with those of commercially available standard compound (( − )-epicatechin, E-1753, Sigma). For the purpose of quantification, linear regression models were determined using standard dilution techniques.
Evaluation of mononuclear blood cell DNA resistance to oxidative damage
The resistance of MNBC DNA against oxidative stress (strand breaks after treatment with H2O2 – 500 μmol/l – for 5 min) was evaluated by means of the Comet assay as reported in detail by Riso et al. (Reference Riso, Pinder and Santangelo20). One hundred cells for each slide were electronically captured (400 × magnification) using an epifluorescence microscope (BX 60 OLYMPUS) equipped with an excitation filter BP520–550, dichroic beam-splitter DM565 and BA580-IF barrier filter (OLYMPUS, Olympus Italia S.R.L., Milan, Italy). The light source was a 100 W Hg lamp (Olympus Italia S.R.L.). The microscope was attached to a high-sensitivity charged coupled device video camera and to a computer provided with an image analysis system (Comet Program exploited on Image Pro-Plus; Immagini e Computer, Bareggio, Milan, Italy) and set to calculate the levels of strand breaks as percentage DNA in tail.
For each subject, the average percentage DNA in tail of control cells (cells that were not treated with H2O2) was subtracted from the average percentage DNA in tail of treated cells.
Analysis of total antioxidant activity
TAA of plasma samples was measured using the method of Re et al. (Reference Re, Pellegrini and Proteggente21). Results were expressed as μmol Trolox equivalent/ml.
Antioxidant vitamins in plasma
To evaluate the basal levels of the main vitamins with antioxidant power and the effect of the standardised intake of food antioxidants during the dietary intervention, we analysed ascorbic acid, α-tocopherol and retinol plasma levels at every experimental time.
Plasma ascorbic acid was determined by the method of Mannino & Cosio(Reference Mannino and Cosio22). Plasma α-tocopherol and retinol were detected following a previously published method by Vuilleumier et al. (Reference Vuilleumier, Keller and Gysel23).
Statistical analysis
All samples were prepared and analysed in duplicate. Statistical analysis was performed using STATISTICA (Statsoft Inc., Tulsa, OK, USA). A repeated-measures ANOVA design was used to investigate the effect of treatments. Differences between means were further evaluated by the least significant differences test and P values < 0·05 considered to be significant. Results are reported as the means with their standard errors.
The analysis of simple regression was used to evaluate the correlation between variables.
Results
All subjects finished the dietary intervention. Supplementation with DC or WC was well tolerated, and no subject reported untoward side effects. The collection of daily dietary diaries indicated that the volunteers were compliant with antioxidant standardisation.
Ascorbic acid, α-tocopherol and retinol plasma levels were within normal range at all times, and no difference was observed between the two groups. In both, the dietary standardisation caused a significant reduction in plasma ascorbic acid and α-tocopherol (T14v. T0, P < 0·05), which was maintained until T27+22 h (Fig. 2). Retinol levels detected at T0 were maintained similar for the whole experimental period.

Fig. 2 Plasma ascorbic acid, α-tocopherol and retinol levels during the dietary intervention. Results are expressed as means with their standard errors (n 10). *P < 0·05 T0v. all other experimental points. ●, dark chocolate (DC); ⋄, white chocolate (WC). Normal range: 6–20 mg/l (for ascorbic acid), 5·5–17·0 (for α-tocopherol), 0·4–1·2 μg/ml (for retinol).
Polyphenols in chocolate samples
Total polyphenol intake from DC (45 g) was 860 (se 19) mg (expressed as gallic acid equivalents), of which epicatechin intake was 58 (se 3·6) mg.
Ingestion of WC brought 5·1 (se 0·7) mg total polyphenols, and epicatechin was not detectable.
Plasma epicatechin concentrations
Fig. 3 shows epicatechin plasma levels detected at all time points.
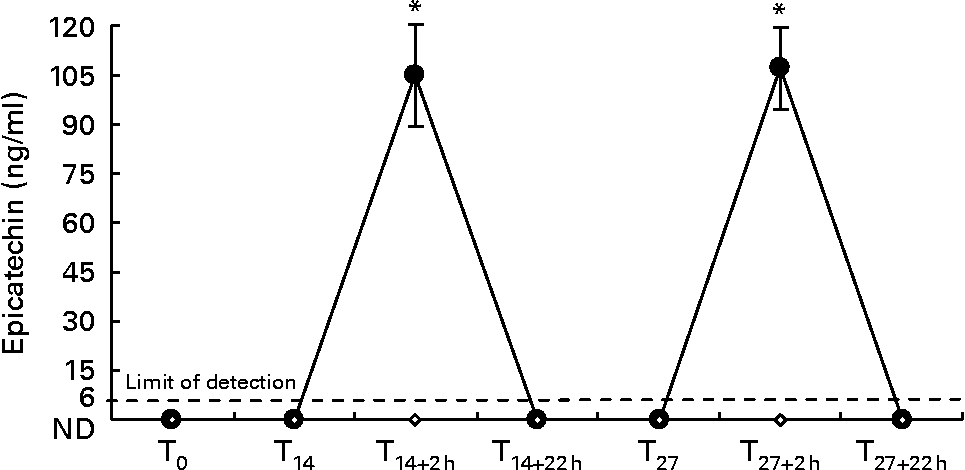
Fig. 3 Plasma epicatechin levels during the whole dietary intervention. Results are expressed as means with their standard errors (n 10). * P < 0·05 T0v. all other experimental points. ND, not detectable. ●, dark chocolate (DC); ⋄, white chocolate (WC).
Two hours after the first DC consumption (T14+2 h), plasma epicatechin rose to 105 (se 15) ng/ml (0·362 (se 0·052) μmol/l); maximum level detected was 155 ng/ml (0·534 μmol/l) and the minimum was 41 ng/ml (0·141 μmol/l). Similarly, 2 h after the last DC consumption (T27+2 h), plasma epicatechin rose to 107 (se 12·1) ng/ml (0·369 (se 0·041) μmol/l); maximum level detected was 163 ng/ml (0·562 μmol/l) and the minimum was 71 ng/ml (0·243 μmol/l).
Fasting plasma, collected before the dietary intervention (T0), after SD (T14), 22 h after the first DC intake (T14+22 h), at T27 before and 22 h after the last DC intake (T27+22 h), contained no detectable epicatechin in any subject (limit detection was 6 ng/ml). In the plasma of subjects in the WC group, epicatechin was undetectable at all time points during the investigation.
Mononuclear blood cells DNA damage
Fig. 4 shows MNBC DNA damage (as % of DNA in tail) evaluated after oxidative treatment at T0, T14, T14+2 h, T14+22 h, T27, T27+2 h and T27+22 h. SD consumption did not affect DNA resistance to oxidative stress at T0 in either group.

Fig. 4 Mononuclear blood cells DNA damage (%) evaluated by comet assay in white chocolate (WC, □; n 10) and dark chocolate (DC, ■) groups (n 10) during the experimental protocol. The results are expressed as means with their standard errors. * P < 0·05 T14+2 h, T27+2 hv. T14, T14+22 h, T27, T27+22 h.
Two hours after the first DC consumption (T14+2 h), MNBC DNA damage decreased significantly ( − 19·4 (se 3·4) % v. T14, P < 0·05). However, this effect was no longer evident after 22 h (T14+22 h = 62 (se 1·4) % v. T14 = 65 (se 2·8) %, P = 0·65).
Two hours after the last DC consumption (T27+2 h), we observed a significant reduction in MNBC DNA damage ( − 24 (se 7·4) % v. T27, P < 0·05), similar to that detected after the first DC consumption (T14+2 hv. T27+2 h, P = 0·7) and no longer evident 22 h after (T27+22 h = 67·9 (se 0·9) % v. T27 = 64 (se 2·8) %, P = 0·42).
An inverse correlation between epicatechin concentrations, detected at T14+2 h and T27+2 h, and DNA damage was observed (R 2 0·27, P < 0·05).
WC intake did not affect DNA resistance to oxidative damage at any time point.
Regular daily consumption of DC also did not affect DNA resistance to oxidative damage (fasting blood samples; T27+22 hv. T14, P = 0·38).
Total antioxidant activity
DC did not affect TAA after 2 h or following 14 d of daily intake (Table 3) as well as WC.
Table 3 Total antioxidant activity (TAA) in dark chocolate (DC) and white chocolate (WC) groups at all experimental times
(Mean values with their standard errors)

Trolox, ( ± )-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid.
DC intake did not significantly affect TAA at any time; no significative difference was observed between DC and WC groups.
No significant correlation was observed between epicatechin concentrations, detected 2 h after the first and the last DC intake, and TAA (R 2 0·009, P = 0·65).
SD did not affect TAA in any group detected at T0 (T14v. T0, P = 0·17).
Discussion
Recent studies suggest healthy benefits associated with DC(Reference Vlachopoulos, Alexopoulos and Stefanadis24–Reference Kay, Kris-Etherton and West26). In particular, great attention has been directed to the antioxidant potential of cocoa and chocolate flavonoids, epicatechin especially, and the potential protective effects against risk of CVD(Reference Wan, Vinson and Etherton10). Epicatechin from flavonoid-rich chocolate has the capacity to quench OH∙ (rather than approximately 100 times more effective than mannitol, a typical OH∙ scavenger(Reference Hanasaki, Ogawa and Fukui27), and it is rapidly absorbed in human subjects(Reference Holt, Lazarus and Sullards28).
Plasma epicatechin concentrations typically return to baseline values within 6–8 h after consumption of a flavonoid-rich chocolate meal(Reference Holt, Lazarus and Sullards28). Richelle et al. (Reference Ritchelle, Tavazzi and Enslen15), 2 h after consumption of DC (40 g) containing 892 mg polyphenols (82 mg were epicatechin), found that epicatechin plasma concentrations were comprised between 0·3 and 0·7 μmol/l with mean value of 0·383 μmol/l; Wang et al. (Reference Wang, Shramm and Holt29) administered DC (53 g) containing about 69 mg epicatechin to thirteen healthy volunteers, after 2 h, detected mean epicatechin plasma concentration of 0·258 (se 0·104) μmol/l. The present study confirmed that epicatechin plasma half-life is relatively short ( < 24 h), and showed that regular epicatechin intake over 2 weeks is not enough to increase fasting epicatechin plasma levels. Engler et al. (Reference Engler, Engler and Chen8) reported that plasma epicatechin concentrations were markedly increased at 2 weeks (0·204 (se 0·06) μmol/l, P < 0·001) in eleven healthy adult subjects that consumed daily 46 g DC containing 1 mg/g epicatechin. However, this result considered chocolate consumption not fasting levels. In contrast, in the present study, we considered fasting plasma levels. Moreover, similar epicatechin plasma levels at 2 h following consumption of DC on the first and last occasions are not associated with a long-term increase in epicatechin plasma concentrations, and suggest that flavonoid plasma levels are dependent upon intake from recent food sources.
The present study showed an increase of DNA resistance to oxidative stress in healthy subjects, although this effect was transient and limited. The significant reduction of DNA damage, induced by oxidative treatment, observed at 2 h from the DC consumption and no longer evident 22 h later, may be related to epicatechin and its plasma kinetics(Reference Hanasaki, Ogawa and Fukui27) or indeed other bioactive compounds in the chocolate.
Epicatechin concentrations achieved at 2 h from chocolate intake in the present study were in accordance with flavonoid concentrations resulted in protection against DNA oxidation in vitro (Reference Min and Ebeler30). Min & Ebeler(Reference Min and Ebeler30) showed that the treatment of calf thymus DNA with catechin at low concentration (0·1 μmol/l) inhibited 8-OH guanine formation by 92 %. Nair & Salvi(Reference Nair and Salvi31) showed that epicatechin offered protection to DNA against ionising radiation-induced damages; in particular, they reported that in mice administered intra peritonially with epicatechin (40 mg/kg body weight) 1 h before γ-irradiation, DNA strand breaks in peripheral blood leucocytes were 37 % less damage than those in mice administered with distilled water prior irradiation. The present experimental evidence and the present results support the hypothesis that decreased DNA damage depends on, at least in part, epicatechin levels. The shape of the molecule and the presence of OH∙ may enable epicatechin to interact with DNA to prevent damage as well as being a free radical scavenger. Planarity of the molecule and the presence of –OH groups might let epicatechin DNA intercalating activity and free radical scavenging action(Reference Min and Ebeler30).
We did not observe any significant effect of DC consumption on plasma TAA. Although there is a consensus about the effect of several cocoa products in increasing plasma flavonoids, evidence concerning the resultant changes in total antioxidant capacity is conflicting(Reference Wan, Vinson and Etherton10, Reference Wang, Shramm and Holt12, Reference Serafini, Bugianesi and Maiani32). The present results are in agreement with previous studies showing no increase(Reference Wang, Shramm and Holt12, Reference Fraga, Actis-Goretta and Ottaviani33) in antioxidant capacity after ingestion of a cocoa snack. Experimental studies have shown that when human plasma is subjected to an in vitro oxidation, epicatechin and related catechins, at concentrations between 0·01 and 1 mmol/l, can, in a dose-dependent manner, prevent depletion of endogenous lipid-soluble antioxidants(Reference Lotito and Fraga34). In the present in vivo study, epicatechin concentrations detected were at least tenfold lower than those tested in previous studies; this could be one reason we did not observe any positive correlation between epicatechin plasma concentration and TAA in plasma. Retinol and α-tocopherol levels unchanged after DC consumption. However, this was not as a result of depletion by epicatechin, but more probably continued consumption during the SD and homeostatic control. In fact, we detected similar levels of the two lipid-soluble vitamins at 2 h when epicatechin levels peaked rather than 22 h when it was undetectable. Moreover, no differences were observed between DC and WC groups either in terms of antioxidant vitamin plasma levels or TAA.
Rein et al. (Reference Rein, Lotito and Holt19) reported that plasma antioxidant capacity increased at the 6-h time point following 53 and 80 g DC, although epicatechin concentrations peaked at 2 h reaching concentrations similar to those we observed. In the present study, TAA was evaluated only at 2 h and again at 22 h after ingestion. Thus, we cannot determine the TAA response between 2 and 22 h.
Furthermore, antioxidant status evaluated as plasma antioxidant activity reflects modifications of the whole antioxidant system rather than of single compound(Reference Simonetti, Gardana and Pietta35). Thus, other studies are necessary in order to clarify the effect of DC consumption on TAA, considering epicatechin, other flavonoids and their metabolites as insertion into a physiological antioxidant network.
In conclusion, a DC snack within a balanced diet can improve DNA resistance to oxidative stress in healthy subjects. However, this effect is transient because of flavonoid kinetics. Regular DC intake was no more beneficial than a single occasional intake. These findings suggest that the DNA protection provided by DC against chronic degenerative disease is via an unknown mechanism. The oxidative stress in fact affects inflammatory process and atherogenesis(Reference Van Gaal, Mertens and De Block36), but has also mutagenic power to the human genome with important consequences in oncogene expression and cancer pathogenesis(Reference Matés and Sánchez-Jiménez37). The present results are clinically encouraging especially in the field of the diet therapy of obesity, pathology related to greater incidence of CVD and cancer(Reference Calle and Kaas38). In fact, DC, habitually excluded by hypoenergetic diets for its high-fat and energy content, is a sweet food that should be reconsidered: if included in controlled amounts, in a weight loss programme it could have healthy effects, and could improve the compliance of patients to diet therapy. However, further studies about bioavailability, kinetics of flavonoids from DC and protective effects against DNA oxidative damage are warranted. In fact, data about the dose, number of servings, times of administrations, dietary contexts and frequency of consumption could be important in order to give clear information to consumers about the benefits and risks of chocolate consumption.
Acknowledgements
There was no specific funding for the present work. The authors thank Ferrero (Soremartec, Alba, Italy) for supplying chocolate products. The idea of the present work was generated by A. S. and G. T. A. S. was involved in the planning of the work and she wrote the manuscript. C. M. C. contributed for writing the manuscript and carried out epicatechin analysis in plasma and chocolate samples. S. S. was involved with A. S. in comet assay, TAA and antioxidant vitamin analysis in plasma. G. T. was involved in funding providing. There are no conflicts of interest among members of the group.









