Introduction
In tropical forests hunting and fishing are crucial to the livelihoods of Indigenous peoples as a source of protein and income (East et al., Reference East, Kümpel, Milner-Gulland and Rowcliffe2005). A growing number of studies suggest current harvests of a variety of species exceed sustainable levels, causing widespread population declines and local extinctions (Abernethy et al., Reference Abernethy, Coad, Taylor, Lee and Maisels2013; Castello et al., Reference Castello, Arantes, McGrath, Stewart and de Sousa2014; Morcatty & Valsecchi, Reference Morcatty and Valsecchi2015; Parry & Peres, Reference Parry and Peres2015). As a result there is a debate between those who view the presence of Indigenous peoples in protected areas as a direct threat to biodiversity and those who view such Indigenous peoples as conservation allies (da Silva et al., Reference da Silva, Shepard and Yu2005; Ohl-Schacherer et al., Reference Ohl-Schacherer, Shepard, Kapla and Yu2007). In-depth monitoring of hunting and fishing is a key prerequisite for promoting the sustainable use of natural resources, avoiding extinctions of important species while preserving the rights of Indigenous peoples to land, traditions and culture.
The decreasing sustainability of hunting and fishing practices has been attributed in part to the rapid growth in Indigenous populations and their integration into the market economy. These trends have triggered powerful socio-economic changes, leading to an increasing demand for wildlife products from both the rural and urban populations and a growing economic incentive to hunt and fish commercially (McSweeney & Jokisch, Reference McSweeney and Jokisch2007; Ohl-Schacherer et al., Reference Ohl-Schacherer, Shepard, Kapla and Yu2007; Suarez et al., Reference Suarez, Morales, Cueva, Bucheli, Zapata-Rios and Toral2009; Fa et al., Reference Fa, Olivero, Farfán, Márquez, Duarte and Nackoney2015). Simultaneously, improved technologies and transportation have enhanced the capacity of an increasing number of hunters and fishers to capture prey, including in previously inaccessible areas (Wilkie et al., Reference Wilkie, Shaw, Rotberg, Morelli and Auzel2000; Godoy et al., Reference Godoy, Undurraga, Wilkie, Reyes-García, Huanca and Leonard2010; Foerster et al., Reference Foerster, Wilkie, Morelli, Demmer, Starkey and Telfer2012). Yet, empirical studies have revealed mixed and even positive effects of socio-economic development on wildlife harvesting (Lu, Reference Lu2007). For example, opportunities for permanent and well-paid jobs combined with a preference among wealthier households for alternative protein sources such as store-purchased meat can lead to a reduction in wildlife harvesting (Wilkie & Godoy, Reference Wilkie and Godoy2001; Gray et al., Reference Gray, Bozigar and Bilsborrow2015; Vasco & Sirén, Reference Vasco and Sirén2016). Understanding the complex interactions between socio-economic factors and extractive activities in a variety of social, cultural and natural contexts remains imperative, especially given the need to alleviate poverty among Indigenous peoples.
In the Peruvian Amazon hunting and fishing constitute integral components of the Kukama-Kukamilla culture. This Indigenous group harvests a large variety of natural resources from surrounding areas, which include the Pacaya-Samiria National Reserve. In the past, a strict protectionist system in this Reserve provoked a backlash of rampant poaching and overexploitation by the local people (Bodmer et al., Reference Bodmer, Puertas, Fang, Manfredo, Vaske, Brown, Decker and Duke2008). In the late 1990s a new Reserve administration adopted a co-management approach, permitting low levels of hunting and fishing by the local people. Since then, populations of key species have been increasing in the Reserve, including threatened species such as the Vulnerable woolly monkey Lagothrix poeppigii, the Vulnerable lowland tapir Tapirus terrestris and the paiche Arapaima gigas (Bodmer & Puertas, Reference Bodmer, Puertas, Redford and Fearn2007). However, like many other Amazonian communities, the Kukama-Kukamilla are undergoing rapid socio-economic changes that could once again increase pressure on wildlife.
We aimed to explore how socio-economic factors influence the hunting and fishing patterns of the Kukama-Kukamilla people. Our findings provide insights into the factors that underpin sustainable resource use, specifically the threats of human population growth and market-driven hunting and fishing brought about by rural development. Previous studies have generally explored the effects of socio-economic conditions on wildlife harvesting between households. However, because households within a community harvest wildlife from a communal catchment area, we explored the combined impacts of wildlife harvesting by the community as a whole. Through the use of semi-structured interviews, we tested the hypothesis that larger communities with greater access to the economic market exert higher pressure on wildlife and target more commercially valuable species. These communities are expected to be affected by higher levels of wildlife depletion, with preferred species disappearing near villages, triggering shifts in the spectrum of species harvested.
Study area
This study was carried out in the 2,080,000 ha Pacaya-Samiria National Reserve in the Department of Loreto, in the north-eastern Peruvian Amazon. It is bordered by two tributaries of the Amazon River, the Ucayali and Marañón rivers, and encompasses the two major drainage basins of the Pacaya and Samiria rivers. The Reserve is characterized by massive hydrological fluctuations that occur between the high-water (October–May) and low-water (June–September) seasons (Kvist et al., Reference Kvist, Gram, Cácares and Orem2001).
The majority of inhabitants are descendants of the Tupi-Guarani speaking Kukama-Kukamilla people and more recent immigrants of Caucasian and Indigenous origin (Gow, Reference Gow, Fausto and Heckenberger2007). Their main livelihood activity is fishing, which is most productive during the low-water season, when fish become trapped in shrinking water bodies. Nonetheless, migrations of fish feeding on fallen fruit in the várzeas (white-water flooded forests) make some fisheries productive during the high-water season (Kvist et al., Reference Kvist, Gram, Cácares and Orem2001). The Kukama-Kukamilla also engage in opportunistic hunting, primarily during the high-water season, when the terrestrial fauna is concentrated on the non-inundated restingas (levees; Bodmer et al., Reference Bodmer, Puertas, Garcia, Diaz and Reyes1998).
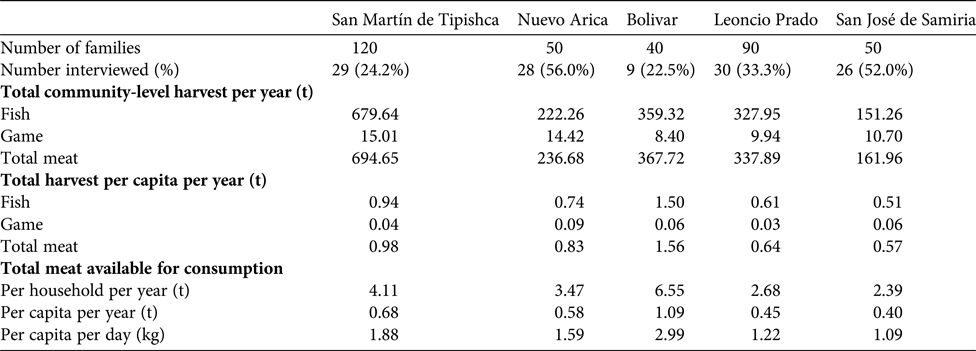
Approximately 100,000 people live in over 200 communities along the boundary of the Pacaya-Samiria National Reserve (INRENA, 2009). We selected five Kukama-Kukamilla villages located at the mouth of the Samiria River, which were divided into two distinct areas: (1) San Martín de Tipishca, Nuevo Arica and Bolivar lie on the shores of Tipishca Lake; and (2) San José de Samiria and Leoncio Prado are located along the Marañón River (Fig. 1). These villages contain 40–120 households (Table 1), and differ in their exposure to the market economy. The communities of the Marañón River supply produce to the urban markets of Loreto by selling to freezer vessels or directly to market vendors.
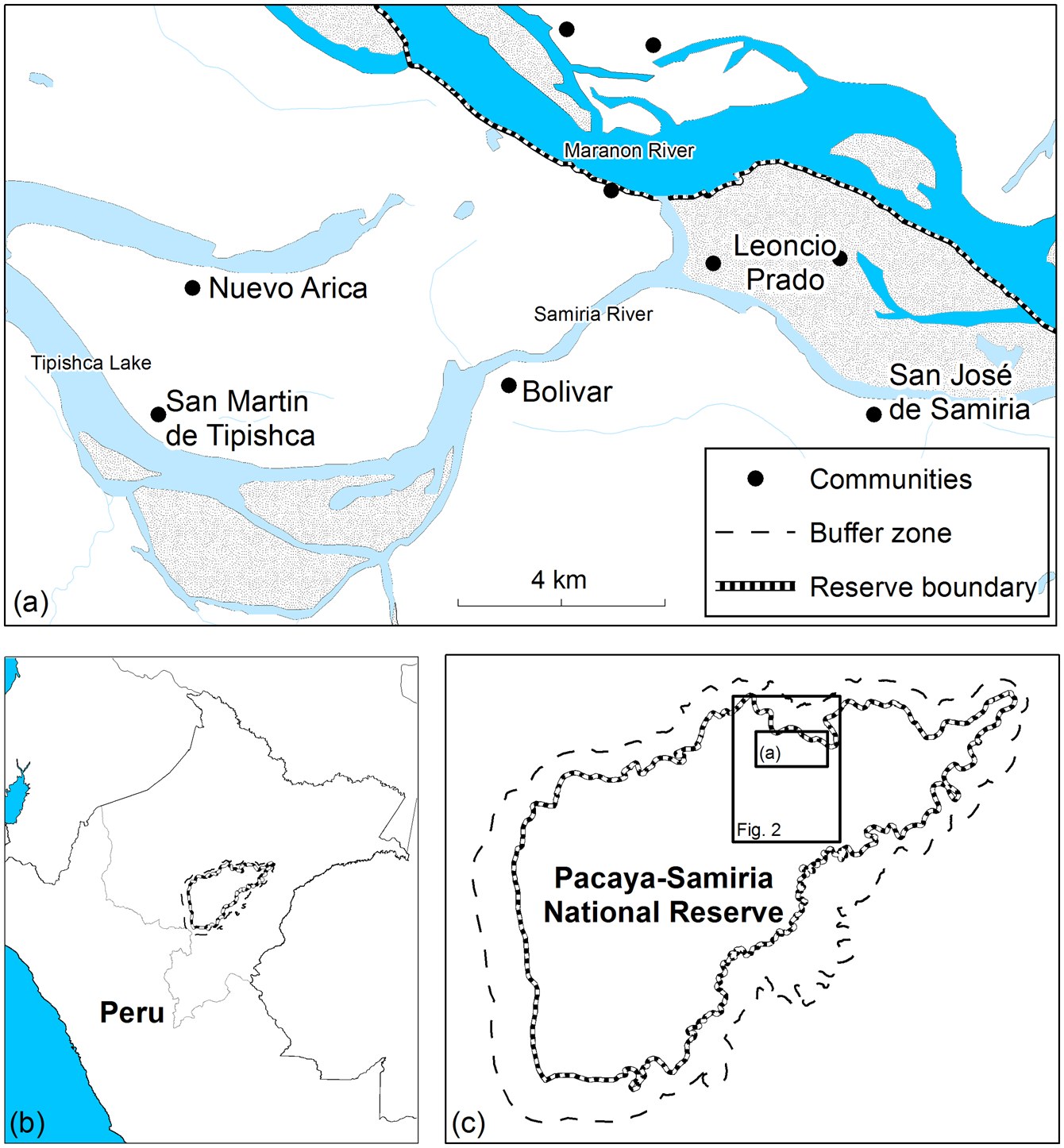
Fig. 1 The study area at the mouth of the Samiria River, showing (a) the five Kukama-Kukamilla settlements, (b) the location of the Reserve in Peru, and (c) the location of the study area in the Pacaya-Samiria National Reserve.
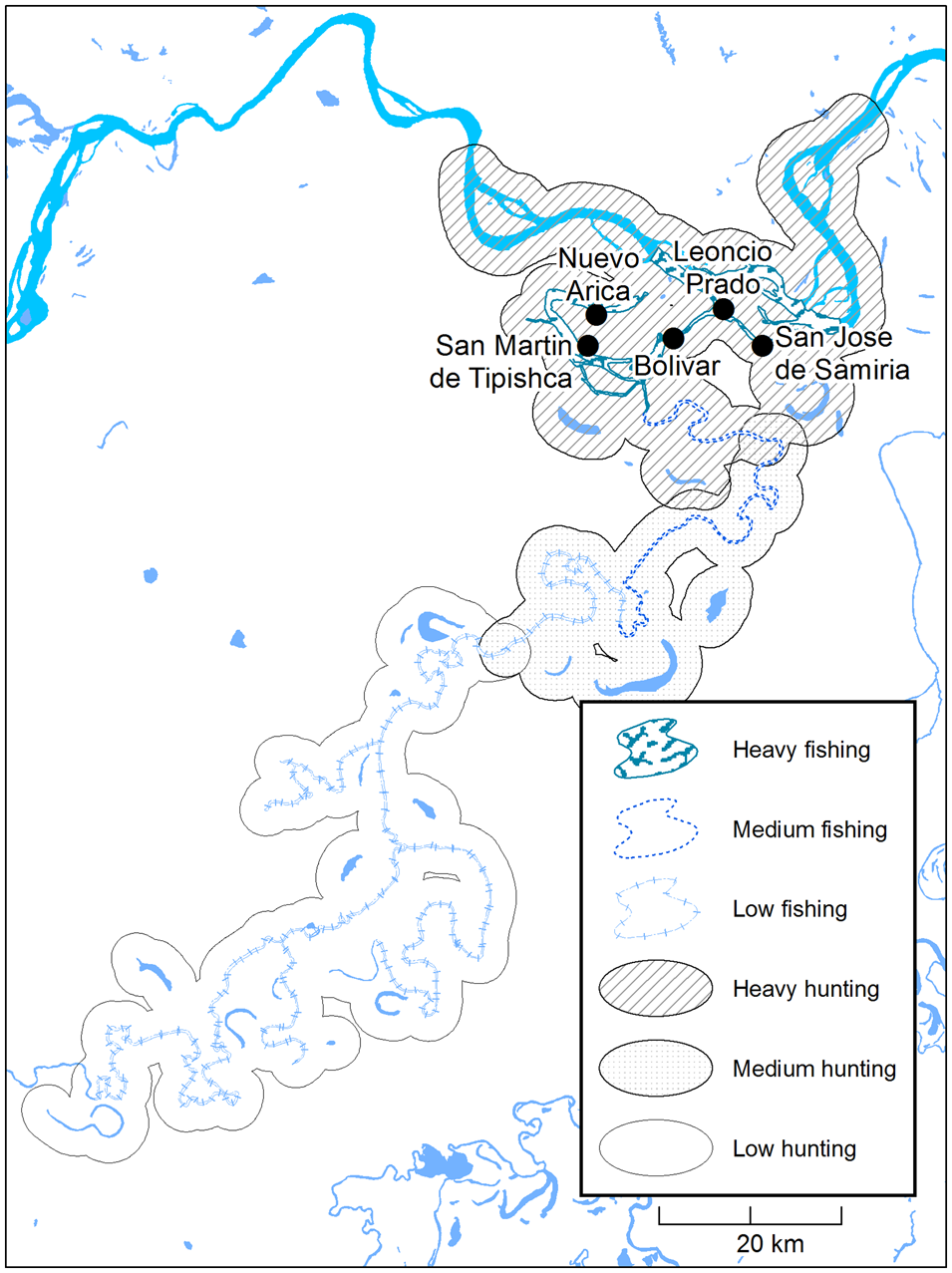
Fig. 2 The hunting and fishing catchment area of the Kukama-Kukamilla communities at the mouth of the Samiria River (Fig. 1), showing zones of heavy, medium and low exploitation based on travelling patterns provided by interviewees.
Table 1 Details of interviews and harvest rates in the five Kukama-Kukamilla communities located at the mouth of the Samiria River (Figs. 1 & 2). The amount of meat available for consumption refers to the edible portion of fish and game meat, which was calculated as 70% of biomass extracted (Hill et al., Reference Hill, Hawkes, Hurtado and Kaplan1984; Roos et al., Reference Roos, Wahab, Chamnan and Thilsted2007).
Methods
Data collection
We conducted 122 semi-structured interviews, which accounted for 34.9% of households within the study area, during June–August 2013 (Table 1). The use of semi-structured interviews was the preferred data collection method, as they allow emphasis on specific topics depending on the interviewees’ knowledge and experience (Rubin & Rubin, Reference Rubin and Rubin2005). Recall bias was expected to be minimal, as quantitative information requested was simple and activities are regular and highly seasonal (Golden et al., Reference Golden, Wrangham and Brashares2013). All households were found to be dependent on hunting and/or fishing, so we adopted a convenience sampling approach, selecting the most accessible households (Patton, Reference Patton2002). We targeted male heads of households for interviews, but in some cases interviewed women instead, either because they too participated in hunting or fishing, or more often because they had acquired detailed information about harvests through cooking. We obtained informed consent from participants before conducting interviews.
The social sensitivity of the topic being explored may have created some bias in the data, resulting in the under-representation of harvests. Where possible, we used participant observation to verify interview responses. We informed interviewees that no information gathered would be used against them and that survey information would be anonymized.
Data analysis
We obtained household harvest rates of fishing by asking fishers to state the mean total biomass of fish caught per day, during high- and low-water seasons separately. This was extrapolated to annual harvest rates by multiplying each estimate of mean daily yield for each season by 182.5 (6 months). A limitation of using interviews to collect harvest data was that fishers were unable to state the quantity of each species harvested, because they measure the weight of the entire catch. We therefore recorded the per cent of households that harvest each species, using these data as proxies for relative harvest rates. We obtained annual household harvest rates of game species by asking hunters to state the mean number of wild animals hunted per year for each species, as hunting is less frequent than fishing. This was converted to biomass using body weight data (Peres & Dolman, Reference Peres and Dolman2000; Ohl-Schacherer et al., Reference Ohl-Schacherer, Shepard, Kapla and Yu2007; Cardoso et al., Reference Cardoso, de Souza, Menezes, Pereira and Tortelly2012; Mayor et al., Reference Mayor, Pérez-Peña, Bowler, Puertas, Kirkland and Bodmer2015). We calculated per-capita harvest rates, assuming an average of six individuals per household. We determined total community-level harvest rates of fish by multiplying mean household harvest rates by the number of households in each community, and in the case of game species, by the per cent of households that engage in hunting.
We used household harvest rates to estimate catch-per-unit-effort (CPUE). The assumption of CPUE as an indicator of sustainability is that hunters and fishers must increase their efforts in areas with depleted populations to achieve the required meat and fish return rates. A difference in CPUE is assumed to reflect a difference in prey density or abundance (Rist et al., Reference Rist, Milner-Gulland, Cowlishaw and Rowcliffe2010). We calculated CPUE of fish as Y/H and CPUE of game species as B/D, where Y is the total daily yield of fish harvested, H is the number of hours per day fishers leave their nets in the water (the most common method), B is the total biomass of games species hunted annually, and D is the number of days per year hunters are active. We averaged across households to obtain community-level CPUE estimates.
We calculated the distance travelled on hunting and fishing trips using reports of average time travelled. Based on information given by a local informant, we estimated that 6 km were travelled in 1 hour in peque peque (motorized canoe) and 4 km on foot. As hunters use watercourses to navigate to hunting sites and limit their activities to within 2 km into the forest from the river's edge, distance travelled was multiplied by four to obtain the size of the total catchment area (Begazo & Bodmer, Reference Begazo and Bodmer1998). The corresponding catchment area was drawn around the channels and lakes of the Samiria and Marañón rivers and divided into zones of low, medium and heavy exploitation, using the maximum distances travelled by the top 25 and 50 percentiles as the thresholds (Fig. 2). Given our project's social science dimension and use of interviews, we determined that this measure of relative exploitation was appropriate (Brodizio & Chowdhury, Reference Brodizio, Chowdhury, Vaccaro, Smith and Aswani2010; Hawken & Munch, Reference Hawken and Munch2012). We used Welch's analysis of variance and the Kruskal–Wallis H test to compare distance travelled on hunting and fishing trips between communities. Pearson's rank correlation coefficient allowed us to examine the relationship between CPUE and distance travelled as an indication of local resource depletion (Fa et al., Reference Fa, Seymour, Dupain, Amin, Albrechtsen and Macdonald2006; Laurance et al., Reference Laurance, Croes, Tchignoumba, Lahm, Alonso and Lee2006).
We used multiple linear regressions to investigate the effects of socio-economic variables on CPUE and harvest rates. We included village size as a continuous variable and market exposure as a categorical variable in all models, using season as an additional categorical variable in the analyses of fishing data. The response variables were log-transformed to account for non-normal distributions. We estimated the significance of variables by dropping them from the full model and using likelihood ratio tests to compare nested models. We examined variations in the species compositions of harvests, termed the harvest profile, using Principal Components Analysis (PCA). Results were considered significant at P < 0.05. Statistical analyses were undertaken in R 3.3.1 (R Development Core Team, 2016).
Results
All households in the study area fished daily throughout the year. In 57% of households, fishing was supplemented with hunting. Seventy-seven per cent of hunters were active for < 10 days per year, and only one hunted as often as 18 days per year. The total biomass of wildlife harvested annually by the five communities was c. 1,807 t (Table 1). The majority of fishers (96%) reported travelling in peque peque for no more than 6 hours, whereas 39% of hunters undertook trips of several days, travelling over 6 hours to reach remote restingas inside the Reserve. The mean distance travelled by fishers and hunters was 11.2 ± SE 4.1 km and 44.0 ± SE 11.1 km, respectively. The distance travelled on hunting trips did not differ between communities (H (4) = 5.70, P = 0.22), but fishers from Nuevo Arica and San Martín de Tipishca travelled further than fishers from other villages (Welch's F (4,29.67) = 18.21, P < 0.001). The combined hunting and fishing catchment area for all communities covered c. 576 km2 (Fig. 2). There was a positive correlation between distance travelled into the Reserve and CPUE for fish during the low-water season (Pearson r s(120) = 0.22, P = 0.017), but not the high-water season (Pearson r s(120) = 0.17, P = 0.07). There was no significant correlation between distance travelled and CPUE of game species (Pearson r s(69) = 0.14, P = 0.24).
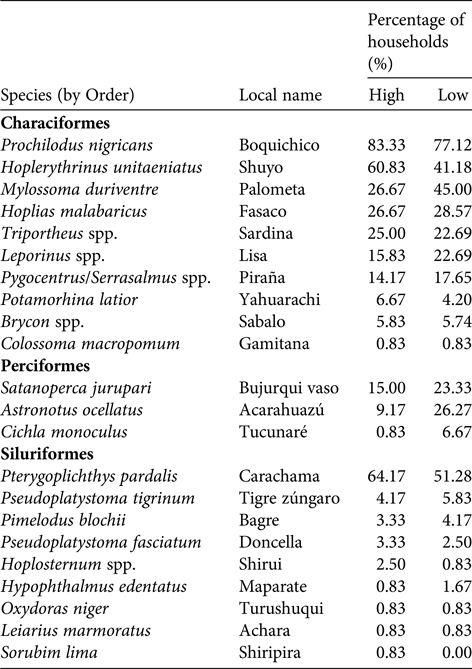
The communities of the Samiria basin collectively harvested c. 1,740 t of fish annually (96.3% of biomass extracted), comprising 23 fish species (Table 2). The most widely caught species was Prochilodus nigricans, a species of both commercial and subsistence importance. There was substantial variation in harvest profiles between communities (Fig. 3a). In San José de Samiria and Leoncio Prado fishers harvested a large proportion of small, commercial species such as Leporinus spp., as well as larger species such as Hoplias malabaricus. In San José de Samiria, smaller, less economically valuable species such as Oxydoras niger and Leiarius marmoratus also made up a significant proportion of the catch. The communities of Tipishca Lake depended on the most abundant species, including Pterygoplichthys pardalis, Pygocentrus spp. and Serrasalmus spp. We found evidence that the paiche, a species of conservation concern (INRENA, 2009), was also caught.
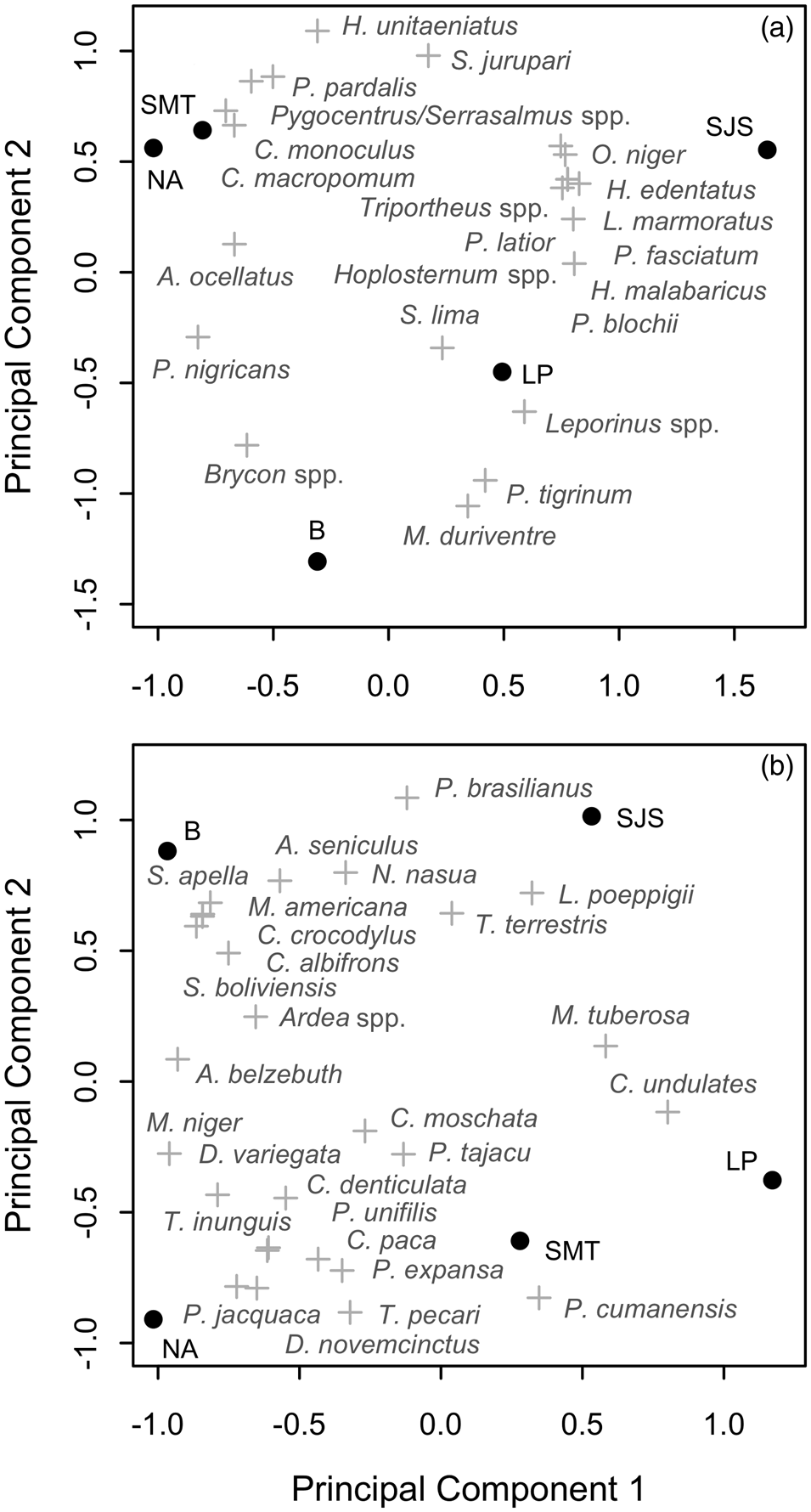
Fig. 3 Ordination axes based on species composition of (a) fish harvests and (b) game harvests, showing communities (●) and species (+). SMT, San Martín de Tipishca; NA, Nuevo Arica; B, Bolivar; LP, Leoncio Prado; SJS, San José de Samiria.
Table 2 Fish species harvested by the Kukama-Kukamilla people, showing the proportion of households harvesting each species during high- and low-water seasons. None of these species have yet been assessed for the IUCN Red List (2017).
The reported total annual harvest of game species in the study area was c. 4,275 individuals, equating to c. 67 t (3.7% of biomass extracted) and comprising 27 species (Table 3). Mammals were the most frequently extracted group, comprising 74.0% of hunted biomass and 56.0% of all hunted individuals, followed by reptiles (23.1, 19.1%) and birds (2.9, 24.9%). The majority of biomass harvested came from large-bodied animals, mainly the white-lipped peccary Tayassu pecari, lowland tapir, and black caiman Melanosuchus niger. The white-lipped peccary, paca Cuniculus paca and brown agouti Dasyprocta variegata were the most frequently hunted in terms of number of individuals. The Amazonian manatee Trichechus inunguis, which is strictly protected, was hunted occasionally. As with fish harvests, harvest profiles of game species varied substantially between communities (Fig. 3b). In San José de Samiria and San Martín de Tipishca, hunters harvested a larger proportion of large-bodied species, such as the lowland tapir, the South American river turtle Podocnemis expansa and the white-lipped peccary, whereas the other communities harvested a larger proportion of small primates and wetland birds.
Table 3 Annual per-capita harvest rates (biomass and number of individuals) of game species by the Kukama-Kukamilla people, with the IUCN Red List status (IUCN, 2017).
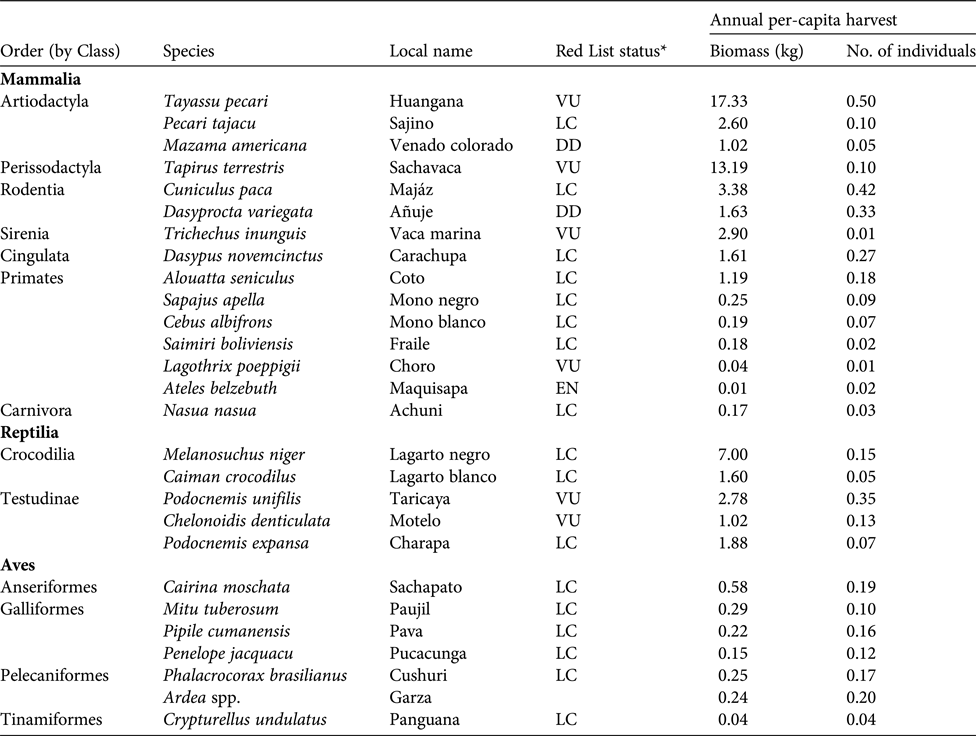
*LC, Least Concern; DD, Data Deficient; VU, Vulnerable; EN, Endangered.
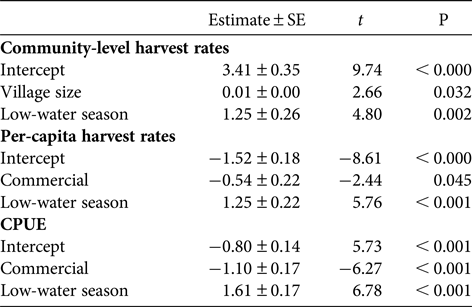
The multiple linear regressions revealed a significant positive relationship between annual community-level harvest rates of fish and village size (Table 4, Fig. 4). However, village size had no effect on mean per-capita harvest rates (F = 0.33, P = 0.59) or CPUE (F = 0.96, P = 0.37) of fish. There was no effect of market exposure on community-level harvest rates of fish (F = 4.60, P = 0.08), but commercial communities had significantly lower mean per-capita harvest rates and CPUE of fish (Table 4, Fig. 5). As expected, season had a significant effect on harvest rates and CPUE of fish, both of which were higher in the low-water season (Table 4). Neither market exposure nor village size had a significant effect on harvest rates or CPUE of game species (all P > 0.31).
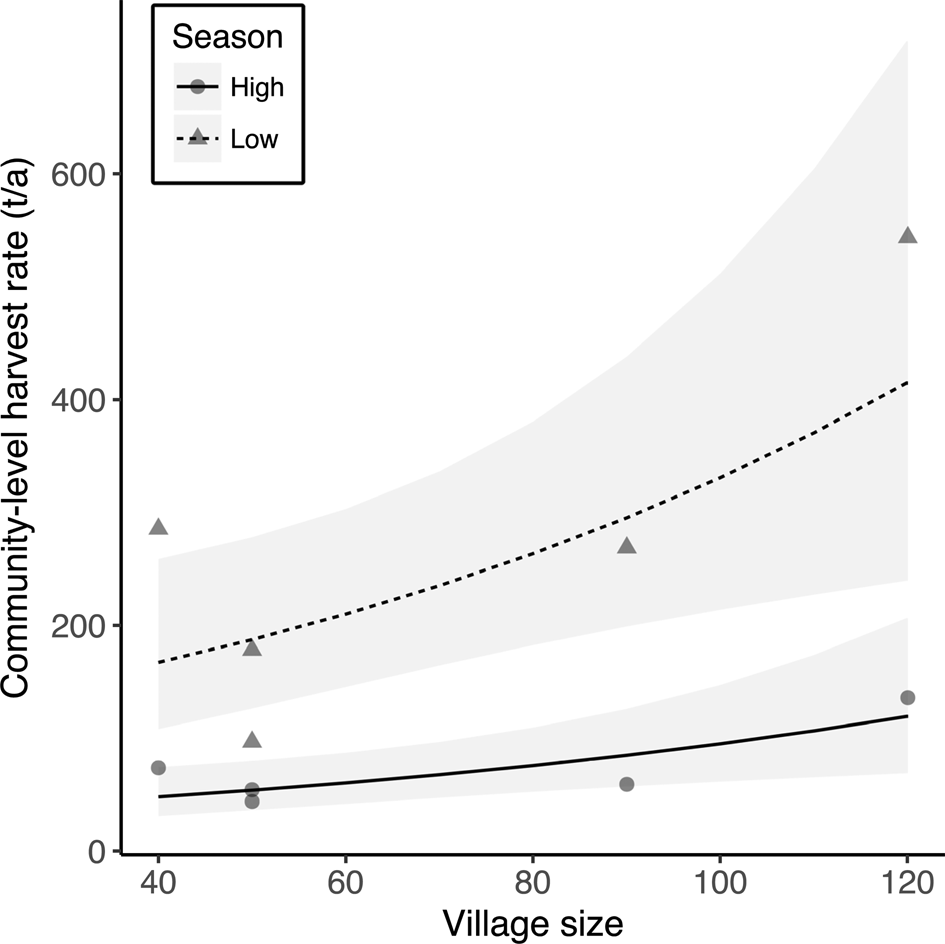
Fig. 4 The effect of village size (number of households) on annual community harvest rates of fish during the high- and low-water seasons in the five Kukama-Kukamilla communities at the mouth of the Samiria River (Figs 1 & 2). The grey symbols represent the raw data, the lines are the predicted slopes from the linear regression model, and the shaded areas are the 95% confidence intervals.
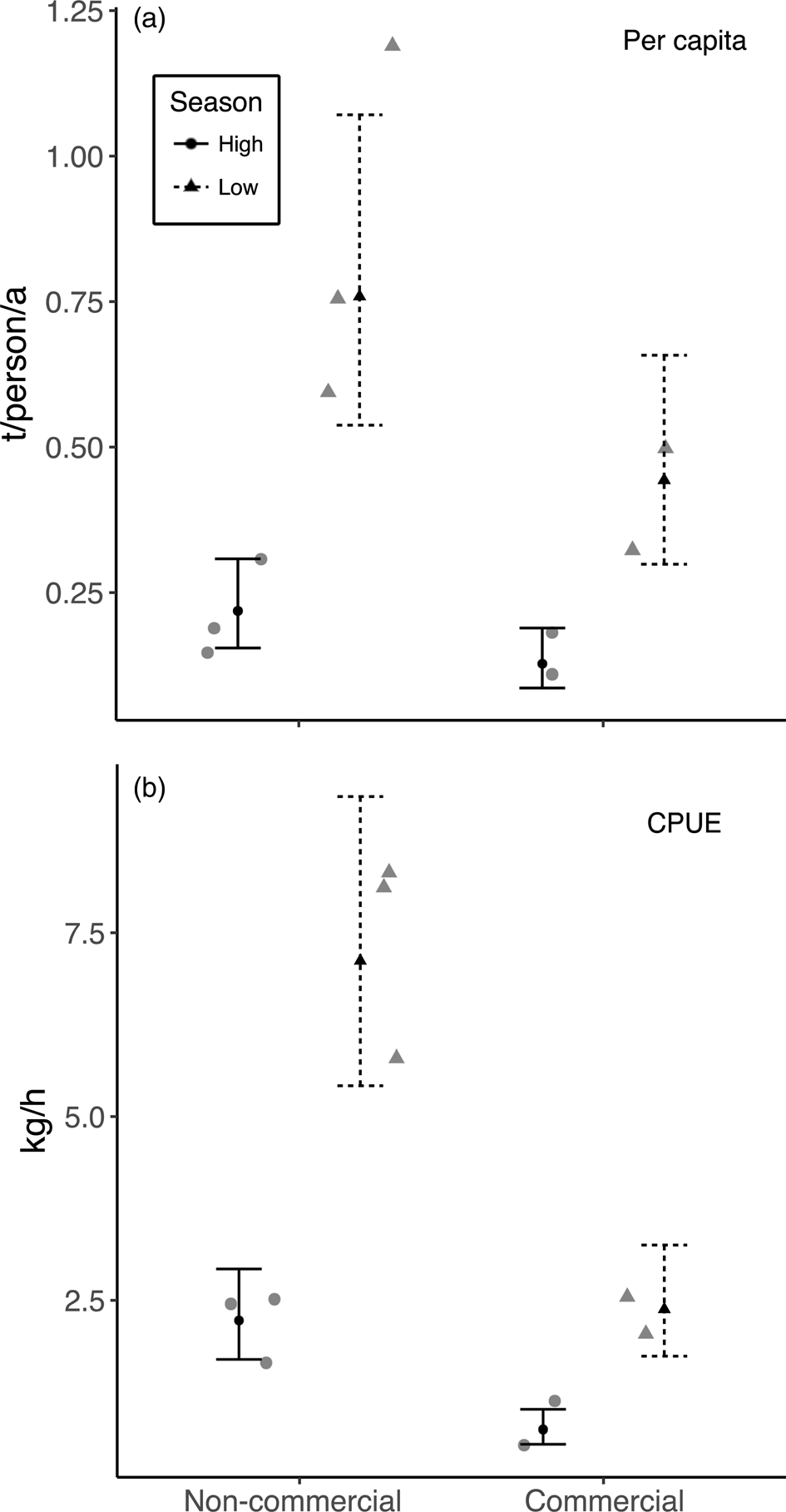
Fig. 5 The effect of market exposure on (a) annual mean per-capita harvest rates and (b) mean daily CPUE of fish (as kg per h of nets in the water) in the five Kukama-Kukamilla communities located at the mouth of the Samiria River (Figs 1 & 2), with the raw data (grey symbols), the predicted means (black symbols) from the linear regression model and 95% confidence intervals.
Table 4 Results of the multiple linear regression analyses showing the effect of village size, market exposure and season on log-transformed harvest rates and CPUE. Non-significant variables were excluded from each model.
Discussion
Our study adds to the growing body of research that suggests socio-economic factors influence wildlife harvesting by Indigenous peoples (Smith & Wishnie, Reference Smith and Wishnie2000; Lu, Reference Lu2007; Godoy et al., Reference Godoy, Undurraga, Wilkie, Reyes-García, Huanca and Leonard2010). Specifically, the patterns of hunting and fishing by the Kukama-Kukamilla people of the Pacaya-Samiria National Reserve reveal the potential threat of increased market integration and an increasing human population. The data presented in this study include a number of potential sources of variation that we did not control for, including environmental variables such as habitat quality, which may have limited the statistical power of the analyses. Furthermore, the small sample size of only five communities means the results of the significance tests should be interpreted cautiously. However, because data points are aggregates of household-level data, they reflect many more underlying observations, and we believe this allows us to make reliable inferences.
We uncovered evidence that increased market exposure leads to resource depletion, reflected in a lower CPUE of fish in commercial communities. A reduction in fish populations as a result of overfishing may have reduced the profitability of fishing and limited commercial fishing activity, which would explain why fishers in commercial communities had lower mean per-capita harvest rates (Vasco & Sirén, Reference Vasco and Sirén2016). Nevertheless, the net pressure that commercial fishing puts on depleted resources is probably greater than the pressure exerted by non-commercial communities on relatively undepleted fish stocks. In San José de Samiria and Leoncio Prado, fishers targeted small, economically valuable species, indicating possible overexploitation of larger species. This trend is observed in the nearby markets of Iquitos, where the sale of cheaper, smaller and faster-growing species has risen since the 1980s and the sale of larger species has declined (Garcia et al., Reference Garcia, Salvador, Vargas and Duponchelle2008; Atwood et al., Reference Atwood, Connolly, Ritchie, Lovelock, Heithaus and Hays2015). The large proportion of less economically valuable species in harvests from San José de Samiria could reflect an increasing reliance on these species for subsistence.
As expected, larger communities exerted greater pressure on fish resources through increased harvest rates, because there are both more people to feed and a greater number of fishers. We therefore expected to see similar signs of resource depletion in these communities. Nonetheless, community size had no significant effect on CPUE of fish. However, fishers from San Martín de Tipishca, the largest village, together with those from Nuevo Arica, travelled further on fishing trips than those from neighbouring communities, and during the low-water season CPUE was higher further into the Reserve. This is consistent with the paradigm that Neotropical people are central-place foragers, travelling greater distances in search of preferred prey species as wildlife populations become locally depleted (Levi et al., Reference Levi, Shepard, Ohl-Schacherer, Peres and Yu2009, Reference Levi, Lu, Yu and Mangel2011). Thus, fishing in previously unexploited sites inside the Pacaya-Samiria National Reserve could be masking resource depletion in Tipishca Lake. Fishers from San Martín de Tipishca also harvested small, abundant fish species, which may be able to sustain the larger human population.
We found no clear effect of village size or market exposure on harvest rates or CPUE of game species. This implies that people in larger, commercial villages have been able to shift to alternative sources of protein, such as fish or livestock, to meet subsistence and commercial needs. The strong presence of preferred species in harvest profiles suggests that wild meat harvests in the Pacaya-Samiria National Reserve are currently supplied by a relatively undepleted source. In San José de Samiria and San Martín de Tipishca, hunters harvested large-bodied prey species, including ungulates, large primates and reptiles. Encounter rates of these species in the forest are relatively low as a result of naturally low population densities, so hunters are probably targeting them for their greater meat harvests, as occurs in other Amazonian communities (Peres & Lake, Reference Peres and Lake2003; Zapata-Ríos et al., Reference Zapata-Ríos, Urgil and Suárez2009; Espinosa et al., Reference Espinosa, Branch and Cueva2014; Sirén & Wilkie, Reference Sirén and Wilkie2016). The current hunting patterns of the Kukama-Kukamilla people may be indicative of a source–sink dynamic, with immigration of game species from the unhunted core zone of the Reserve sustaining harvests in the catchment area (Navaro et al., Reference Navaro, Redford and Bodmer2000; Ohl-Schacherer et al., Reference Ohl-Schacherer, Shepard, Kapla and Yu2007).
Nevertheless, large-bodied game species are particularly vulnerable to overexploitation because of slow reproductive rates (Mayor et al., Reference Mayor, El Bizri, Bodmer and Bowler2017). The continued harvest of vulnerable species by larger, commercial communities will probably cause significant population declines in the Pacaya-Samiria National Reserve and a shift in prey selection towards a broader range of smaller, less-preferred species, following the general trend throughout the Amazon (Naranjo & Bodmer, Reference Naranjo and Bodmer2007; Peres & Palacios, Reference Peres and Palacios2007; Constantino, Reference Constantino2016). The region has also been experiencing more extreme droughts and seasonal flooding since 2009, which could exacerbate the impacts of unsustainable wildlife extraction by limiting resources for wildlife and causing direct mortality of animals (Bodmer et al., Reference Bodmer, Mayor, Antunez, Chota, Fang and Puertas2017). The recent sharp decline in populations of the white-lipped peccary throughout its range, for which non-anthropogenic impacts are suspected, will put further pressure on alternative and more vulnerable prey species (Fragoso, Reference Fragoso, Silvius, Bodmer and Fragoso2004; Richard-Hansen et al., Reference Richard-Hansen, Surugue, Khazraie, Le Noc and Grenand2013; Mayor et al., Reference Mayor, Pérez-Peña, Bowler, Puertas, Kirkland and Bodmer2015).
Overall, our results indicate that the forests of the Pacaya-Samiria National Reserve are able to provide important food supplements for the Kukama-Kukamilla people. However, hunting and fishing in some villages appears to be approaching critical thresholds, threatening the natural capital of the Reserve. Globally, the combination of human population growth and increased market integration of Indigenous peoples is linked to a downward spiral of local species extinctions and a diminishing supply of crucial protein and income. In this context, the sustainable management of natural resources represents a crucial opportunity for biodiversity conservation where protected areas and Indigenous territories overlap (Zimmerman et al., Reference Zimmerman, Peres, Malcolm and Turner2001). Development professionals, protected area managers and conservationists need to help maintain low hunting and fishing pressure by diversifying and enhancing existing livelihood strategies, thereby reducing poverty in rural communities and conserving vulnerable species (Bodmer & Lozano, Reference Bodmer and Lozano2001; Bassett, Reference Bassett2005; Gandiwa, Reference Gandiwa2011). Community-based management is needed to monitor the impacts of socio-economic and climatic change, and to ensure the long-term sustainable use of forest species, both inside and outside protected areas.
Acknowledgements
We are indebted to the communities of the Pacaya-Samiria National Reserve, who participated in data collection and without whom this project would not have been possible. We thank Teddy Urashima and Pool Erazo Arevalo for field assistance, Pablo Puertas for his help and advice, Hannah Kirkland for proof reading, Fund Amazonia, Wildlife Conservation Society, Earthwatch Institute and Operation Wallacea for assistance during fieldwork and financial support, and the Peruvian Protected Area Service (Servicio Nacional de Áreas Naturales Protegidas) for providing authorization for and coordination of fieldwork.
Author contributions
Design of the data collection methods: REB and MK; logistical support in the field: REB; data collection and analysis, writing of first draft: MK; preparation of the final text: JCA, MK, CE, REB and PM; production of the maps: AB.
Conflicts of interest
None.
Ethical standards
All authors abided by the Oryx Code of Conduct. The highest standards of research ethics were followed and the necessary approvals and permits were obtained to carry out field research in the protected area and to work with Indigenous communities.











