Introduction
The coronavirus disease 2019 (COVID-19) pandemic greatly impacted healthcare delivery. During the pandemic, virtual care became commonplace and management practices for infectious diseases shifted to accommodate new modalities of care, leading to downstream changes in treatment of infections. Reference Stephenson, Butt and Gronsbell1,Reference Khairat, Meng, Xu, Edson and Gianforcaro2 For instance, two studies characterizing physicians’ antibiotic prescribing trends and habits through the COVID-19 pandemic noted significant reductions in overall outpatient antibiotic prescribing. Reference Kitano, Brown and Daneman3,Reference King, Lovegrove and Shehab4 Declines in antibiotic prescriptions were largely attributed to fewer patients seeking care for respiratory illnesses resulting in fewer prescriptions being provided. There is a paucity of research into how between-physician antibiotic prescribing habits changed through the pandemic. Physician and practice characteristics shape antibiotic prescribing habits. Reference Fernandez-Lazaro, Brown, Langford, Daneman, Garber and Schwartz5 As such, it is important that changes in physicians’ antibiotic prescribing habits through the pandemic period be evaluated, including understanding predictors of differential antibiotic prescribing practices.
Our objective was to evaluate inter-physician variability in changes in prescribing behavior with respect to antibiotic initiation, selection, and duration of antibiotic prescriptions between before (2019) and during (2020/2021) the COVID-19 pandemic and to investigate prescriber characteristics associated with changes in antibiotic prescribing.
Methods
Setting, population, and data source
The study was conducted in Ontario, a Canadian province home to 14.9 million people and approximately 35,000 physicians, with 116 family physicians per 100,000 population. 6 Ontario went into lockdown on March 17, 2020. We conducted a retrospective cohort study of physicians prescribing oral antibiotics in the outpatient setting between January 2019 and December 2021 using the IQVIA Xponent data set. IQVIA is a global company that maintains several prescription drug databases available for purchase. IQVIA creates the Xponent database through dispensed medication data directly from a proportion of community pharmacies representing 65% of dispensed medications. Using a proprietary geospatial projection algorithm, the Xponent database represents 100% projection of oral physician prescribed antibiotic prescriptions. The methodology is proprietary but is routinely internally validated by IQVIA. Reference Hicks, Bartoces and Roberts7 This data set was the source of all data used in our analysis and has been previously externally validated by our study team to accurately identify high antibiotic prescribing physicians. Reference Schwartz, Chen and Langford8
The Xponent data set contains aggregated outpatient antibiotic prescription counts at the individual physician level, including some physician and practice characteristics through physician groupings of roughly equal sizes. Nonphysician prescribers are not included in this data set.
Physician inclusion and exclusion criteria
We included antibiotic prescribing physicians in Ontario whose data were available for all 3 years of the study. Physicians who prescribed fewer than 36 antibiotic courses in the prepandemic (2019) period were excluded to capture a study cohort of antibiotic prescribers who were actively prescribing before the COVID-19 pandemic. Additionally, physicians with illogical data points (antibiotic prescribing proportions of 0% or 100% of their total number of prescriptions) were also excluded. Resident physicians in training were also excluded from the study population. As the data set captures outpatient oral antibiotics only, inpatient, intravenous, topical, and parenteral antibiotics were not included. Ultimately, 17,288 physicians were included in the study (Figure 1).

Figure 1. Flow diagram of the study cohort creation.
Outcomes
The primary outcome for our study was the prepandemic to intrapandemic change for the initiation of antibiotic prescriptions. For this initiation parameter, the unit of analysis was the raw number of new antibiotic prescriptions at the physician level and we evaluated the difference in antibiotic initiation between the prepandemic and pandemic periods (antibiotic prescriptions from January 1, 2019 to December 31, 2019 minus the average antibiotic prescriptions from January 1, 2020 to December 31, 2021).
We also evaluated changes in selection and duration of antibiotic prescriptions as secondary outcomes between the prepandemic and intrapandemic periods, with the unit of analysis being percent of broad-spectrum and long-duration antibiotics, respectively. We used the World Health Organization’s (WHO) AWaRe classification, which classifies antibiotics under three groups (Access, Watch, and Reserve) based on the impact of antibiotics on antimicrobial resistance to define broad-spectrum. The AWaRe classification is a widely accepted stewardship tool and was chosen as it provides a standardized method for evaluating antibiotic use (Supplementary Table 1). Assignment of antibiotics to the WHO group was chosen based on the most frequently used drugs within each class. 9 In this study, we defined narrow-spectrum antibiotics as those classified under the Access group and broad-spectrum antibiotics as those classified under the Watch/Reserve group. We then evaluated the change in the percent of broad-spectrum antibiotics. To study the duration parameter, we classified prescriptions for 7 d or less as “short-duration” and those longer than 7 d as “long-duration.” Reference Grant and Saux10 We then evaluated the change in the percent of long-duration antibiotic prescriptions.
Exposures and covariates
We examined prescriber and practice characteristics to determine whether they are predictors of a change in any of the antibiotic prescribing parameters of initiation, selection, and duration.
Prescriber characteristics
We included physician sex, career stage, and specialty. Career stage was defined as years since graduation from medical school, split into early (<11 yr), mid (11–23 yr), or late (>23 yr). Physicians’ specialties were categorized as family medicine, internal medicine/pediatric medicine and subspecialties, emergency medicine, surgical specialties, and other (Supplementary Table 2). This categorization facilitated comparison of other specialties with family medicine, as outpatient prescribing of antibiotics occurs predominantly in the primary care setting. 11
Practice characteristics
We included practice geography using the forward sortation area (FSA) to dichotomize physicians’ practice locations as rural versus urban. The FSA is the first three digits of Canadian postal codes and is a way to designate a geographical unit in Canada. A zero as the second digit indicates a rural area. We included patient age (<18, 18–64, ≥65) and sex (male vs. female), defining them as the proportion of patients in these age and sex groupings who received an antibiotic prescription. We additionally included a practice-level patient comorbidity index using the Chronic Disease Score (CDS). Reference Von Korff, Wagner and Saunders12 The CDS is an aggregate comorbidity measure based on current medication use and pharmacy data and has been validated at the patient level. Reference Yurkovich, Avina-Zubieta, Thomas, Gorenchtein and Lacaille13,Reference Schneeweiss, Seeger, Maclure, Wang, Avorn and Glynn14 In the Xponent data set, the CDS is scaled up to the physician level to describe the average complexity of patients seen within a physician’s practice. We categorized physicians’ practices into low, medium, and high comorbidity levels for scores of <5, 5, and >5, respectively, based on approximate tertiles. Practices were also grouped by new patient volume—a metric that reports the proportion of prescriptions to new patients per antibiotic course prescribed—into low, medium, and high categories based on approximate tertiles. This variable is a surrogate for physicians who practice more in urgent care or walk-in clinic settings. We included antibiotic prescribing rate, defined as the number of antibiotics prescribed by each Ontario MD for every 100 new prescriptions, and split practices into tertiles (low, medium, and high categories for rates of ≤7%, 7%−14%, and ≥14%, respectively). Lastly, we included overall patient prescriptions, which accounts for all new prescriptions including antibiotics and split practices into categories of low, medium, high, and very high based on approximate quartiles.
Statistical analysis
The dependent variable used in the analysis for the initiation parameter was the difference in antibiotics prescribed between the pre and intrapandemic periods. The dependent variables analyzed for the selection and duration parameters were the differences in percent of broad-spectrum antibiotics and the percent of long-duration prescriptions between the pre- and intrapandemic periods, respectively. We used linear regression modeling to estimate the change (between the pre- and intrapandemic periods) in antibiotic prescribing parameters. Crude and adjusted multivariable linear regression models were used to estimate predictors (including prescriber and practice characteristics) of a change in antibiotic prescribing parameters, and results were reported as mean difference (MD) and adjusted mean difference (aMD), respectively. All covariates above were a priori included based on potential clinical significance on antibiotic prescribing patterns. All statistical tests were two-sided with a p < .05 significance level and done with R version 4.2.0. The Public Health Ontario Ethics Review Board approved the study.
Results
Prescriber and practice baseline characteristics
Across the 3-year study period, the cohort included 17,288 physicians who prescribed oral antibiotics in the outpatient setting. The studied physicians were 55.3% male, mostly in family medicine (69.2%), in later career stages (49.8%) with a median of 21 (interquartile range [IQR], 11–33) years since graduating medical school, and overwhelmingly located in urban settings (94.4%). Adult patients (aged 18–64) received most antibiotics (56.4%) compared to children (14.3%) and older adult (29.3%) patients, while female patients received more antibiotics (61.4%) compared to male patients (38.6%) (Table 1).
Table 1. Prescriber and practice characteristics of antibiotic prescribing physicians in Ontario in 2019 (n = 17,288)

SD, standard deviation.
a Years since graduating from medical school.
b See Supplementary Table 1 for specialties contained under specialty categories.
c Metric used to approximate average complexity of patients.
d The proportion of new patients’ prescriptions per antibiotic course prescribed.
e The number of antibiotics prescribed by each Ontario MD for every 100 new scripts provided.
f All antibiotic and nonantibiotic prescriptions to patients.
Overall changes in antibiotic prescribing pre and during pandemic
Overall, there were 103.2 (31.9%) fewer antibiotic prescriptions per physician on average from the prepandemic to pandemic period. In the unadjusted analysis, we observed substantial variability in this change (median change of −43.5 antibiotics per physician, IQR −136.5 to −5.0). Additionally, we observed a decrease in overall new prescriptions with a reduction of 324.7 (10.3%) new prescriptions per physician, suggesting a decrease in patient visits during the COVID-19 pandemic. A decrease of 51.3 (21.2%) antibiotics was seen in early-career physicians compared to a decrease of 131.7 (35.6%) in late-career physicians (p < .001). Internal medicine/pediatrics and family medicine had the largest decrease in antibiotic prescriptions of 66.3 (39.4%) and 127.7 (33.1%), respectively, compared to surgical specialties with a decrease of 24.8 (14.2%) (p < .001) (Table 2).
Table 2. Change in initiation of oral antibiotic prescriptions among Ontario physicians between the prepandemic and pandemic period

*Model adjusted for physician sex, physician career stage, physician specialty, practice geography, patient age, patient sex, patient comorbidity index, and prescriptions to new patients.
a Average antibiotic prescriptions per physician were 232.2 antibiotics in 2020 and 206.9 antibiotics in 2021.
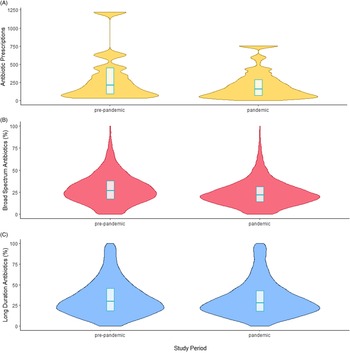
We also observed small reductions in antibiotic selection and duration, represented by the percentage of broad-spectrum and long-duration antibiotic prescriptions, respectively (Figure 2).

Figure 2. Changes in antibiotic prescribing across the initiation (A), selection (B), and duration (C) parameters. Box represents IQR with the median represented by a horizontal line.
Predictors of change in antibiotic prescribing
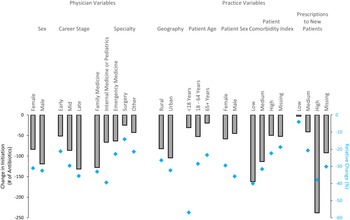
Antibiotic course initiation decreased for physicians across all prescriber and practice characteristics (Figure 3). In the multivariable analysis, late-career stage (decrease of 132 prescriptions, aMD −45.3, 95% confidence interval [CI] −52.9 to −37.8, p < .001 compared to early-career physicians) and male physician sex (decrease of 119 prescriptions, aMD −14.3, 95% CI −19.6 to −8.9, p<.001 compared to female physicians) were associated with a significantly greater reduction in antibiotic prescribing (Table 2). Family physicians had the greatest reduction in antibiotic prescriptions (decrease of 128 prescriptions, p < .001) compared to all other specialties except internal medicine/pediatrics. Practice characteristics of relatively high volume of prescriptions to new patients (decrease of 238 prescriptions; aMD −216.5, 95% CI −223.5 to −209.5, p < .001 compared to low volume of prescriptions to new patients) and urban setting (decrease of 104 prescriptions; aMD −33.3, 95% CI −43.9 to −22.7, p < .001 compared to rural setting) were strongly associated with decreases in antibiotic prescribing during the pandemic. Other practice characteristics associated with a decrease in prescribing were patient age <18 yr, male patient sex, and lower patient comorbidity scores (Table 2).

Figure 3. Absolute (gray bars) and relative (blue diamonds) difference in antibiotic initiation between the prepandemic and pandemic periods across prescriber and practice variables.
We observed a decrease in the percent selection of broad-spectrum antibiotics for physicians across all prescriber and practice characteristics (Supplementary Table 3) and a small decrease in prescriptions for long-duration antibiotics (>7 d) across most prescriber and practice characteristics; however, some exhibited increases (Supplementary Table 4).
Discussion
Our analysis of 17,288 physicians in Ontario, Canada demonstrated a 32% decrease in antibiotic prescribing and on average 103 less antibiotics prescribed per physician per year in the pandemic period compared to prepandemic. There was substantial inter-physician variability in changes in prescribing behavior with respect to initiation, selection, and duration of antibiotics, and some characteristics were more likely to be predictors of a change in prescribing. We observed variability in this decrease by physician and practice characteristics such as specialty, years in practice, urban versus rural location, and patient age and sex. Lower patient comorbidity scores and high volume of new patients were both strongly associated with larger decreases in antibiotic initiation. Family medicine specialty and a high new patient volume were additionally associated with decreases in prescription of broad-spectrum and long-duration antibiotics.
The finding that late-career stage was associated with a decrease in antibiotic initiation and in prescribing of broad-spectrum antibiotics compared to early-career physicians may be supported by a previous finding that the attrition rate for family physicians in Ontario (who represent 69.2% of all physicians in our study) was higher than anticipated during pandemic years compared to prepandemic years, with older physicians being more likely to substantially reduce their practice, or retire, and therefore prescribe fewer antibiotics. Reference Kiran, Green and Wu15 Similarly, physicians with practices in urban settings were also more likely to reduce antibiotic prescribing and decrease prescribing of broad-spectrum antibiotics compared to rural physicians. One possible explanation is that rural physicians, who may have relied more on telehealth/virtual care modalities, were less likely to over-prescribe antibiotics as a precaution in the absence of physical clinical examinations. Reference Armitage and Nellums16,Reference Mehrotra, Paone, Martich, Albert and Shevchik17 However, some research suggests that virtual care was adopted at a greater rate by urban prescribers in Ontario and more generally in Canada, and the reason for urban physicians being more likely to decrease prescribing remains unclear. Reference Glazier, Green, Wu, Frymire, Kopp and Kiran18,Reference Chu, Cram, Pang, Stamenova, Tadrous and Bhatia19
Emergency medicine physicians, though exhibiting an absolute decrease in antibiotic prescription initiation, were among the least likely to decrease number of antibiotic prescriptions. One possible explanation could be the increased reliance on emergency departments for patient care during the COVID-19 pandemic. Reference Tsay, Bartoces, Gouin, Kabbani and Hicks20,Reference Pulia, Wolf, Schulz, Pop-Vicas, Schwei and Lindenauer21 Patients with COVID-19 who presented in emergency departments were often prescribed antibiotics prior to having a SARS-CoV-2 test result. Reference Tsay, Bartoces, Gouin, Kabbani and Hicks20–Reference Langford, So and Raybardhan22 However, patients may have been more reliant on emergency departments for care for non-COVID infections due to decreased availability of family physicians for in-person assessments during parts of the pandemic. Reference Kiran, Green and Wu15,Reference Glazier, Green, Wu, Frymire, Kopp and Kiran18
Prescribers with lower patient comorbidity indices were more likely to reduce antibiotic prescribing and select fewer broad-spectrum antibiotics. This result suggests that physicians with more comorbid patients continued to provide care and prescribe antibiotics during the pandemic compared to physicians with less complex patients. Patients with multi-morbidities often require more antibiotic prescriptions as they either are more vulnerable to acquiring bacterial infection, use antibiotics prophylactically, require antibiotics to control infections/complications associated with their illnesses, or are more exposed to healthcare settings where drug-resistant infections persist. Reference Rockenschaub, Hayward and Shallcross23 Similarly, patients with multi-morbidities are more often treated with broad-spectrum antibiotics, many of which fall under the Watch category of antibiotics. Reference Fernández-Urrusuno, Meseguer Barros and Anaya-Ordóñez24 This would support our finding that prescribers with more comorbid patients were less likely to decrease in their prescription of broad-spectrum antibiotics.
Practices with high prescribing to new patients (such as walk-in clinics and urgent care centers) were significantly more likely to decrease antibiotic prescribing. There is substantial overprescribing of antibiotics in the urgent care context before the COVID-19 pandemic. An American cohort study found that antibiotic prescribing for antibiotic inappropriate respiratory diagnoses was highest in the urgent care setting, relative to other traditional ambulatory care settings and the emergency department. Reference Palms, Hicks and Bartoces25
Our team has previously described the dramatic decrease in antibiotic use in Ontario, Canada during the first year of the pandemic, primarily driven by a reduction in physician visits for respiratory tract infections. Reference Kitano, Brown and Daneman3,Reference Zhang, Surette and Schwartz26 In this study, we investigated which physicians specifically changed their prescribing behavior. The IQVIA Xponent data set is a large population/province wide validated data set and provides prescribing data representative of all oral outpatient antibiotic prescribing in outpatient settings in Ontario. Reference Schwartz, Chen and Langford8 The findings from our study are in line with other studies from Canada, USA, and England suggesting that the absolute number of antibiotic prescriptions significantly decreased during the pandemic. Reference Kitano, Brown and Daneman3,Reference King, Lovegrove and Shehab4,Reference Buehrle, Wagener, Nguyen and Clancy27,Reference Silva, Estrela and Gomes28 Additionally, our finding that prescription of broad-spectrum antibiotics decreased during the pandemic was also seen in other studies. Reference Silva, Estrela and Gomes28,Reference Zhu, Aylin, Rawson, Gilchrist, Majeed and Holmes29 A study from Portugal found a significant decrease in the prescription of third-generation cephalosporins, fluoroquinolones, and clarithromycin immediately after the onset of the COVID-19 pandemic. Reference Silva, Estrela and Gomes28 However, our findings contrast those of a study from Wales, which found that a decrease in antibiotic prescribing was driven by decreases in narrow-spectrum antibiotics rather than broad-spectrum. Reference Wasag, Cannings-John, Hughes and Ahmed30
Our study has some notable limitations. We approximated physicians’ prescribing by using their grouping provided in the Xponent data set—relative to their colleagues—and subsequently examined changes in their prescribing over the three study years. Furthermore, the Xponent data set utilized did not include clinical information and we were unable to link prescribing to billing or clinical data, evaluate changes in indications for antibiotic prescriptions, nor analyze changes in rates of patient visits. A limitation of our study is the lack of patient-specific outcomes such as repeat clinic visits or other relevant metrics that could help assess the impact of reduced antibiotic use on patient health. Reference Barlam, Cosgrove and Abbo31 We did not differentiate predictors of high antibiotic prescribing in general, and there is likely substantial overlap in predictors of high antibiotic use and a decrease in antibiotic prescribing during the COVID-19 pandemic. Our previous work indicates that high antibiotic prescribing is strongly correlated with unnecessary antibiotic prescribing. Reference Kitano, Langford and Brown32 We adjusted for a number of physician and practice variables, but other potential confounders are possible. This study was limited to outpatient antibiotics and does not include antibiotics used in hospitals.
The results from our study provide insights on how antibiotic stewardship efforts could be further tailored to specific groups that demonstrated significant reductions in antibiotic prescribing during the pandemic and therefore may be potentially more amenable to behavior change and/or have greater opportunity to reduce antibiotic over-prescribing (e.g., late-career physicians located in urban centers, those practicing in walk-in/urgent care settings). Several antimicrobial stewardship interventions have previously been successfully implemented in primary care settings and have led to reductions in antibiotic overprescribing, including peer comparison audit and feedback. Reference Schwartz, Ivers and Langford33–Reference Meeker, Knight and Friedberg36 Clinician and patient educational interventions have also been deployed in urgent care settings with decreases in antibiotic prescribing without compromising patient satisfaction and outcomes. Reference Lee, Rico, Muench, Yost and Hall Zimmerman37,Reference Patel, Ng and Madani38 Tailoring such interventions along with other evidence-based stewardship strategies (e.g., communication training and point of care diagnostics) toward the groups identified in our study may represent an important stewardship opportunity. Reference King, Fleming-Dutra and Hicks39 This is particularly relevant as antibiotic prescribing is anticipated to revert back to prepandemic levels with relaxation of public health measures and increase in other non-COVID-19 viral respiratory infections, which will result in more opportunity for unnecessary antibiotic use. Reference Schwartz, Langford and Daneman40 Additionally, further research is needed to understand how a decrease in initiation of antibiotics, reduction in the use of broad-spectrum antibiotics, and a decrease in prolonged antibiotic prescribing during the COVID-19 pandemic have impacted antimicrobial resistance.
Overall, our data indicate that oral antibiotic prescribing patterns changed in Ontario, Canada since the COVID-19 pandemic with decreases in antibiotic initiation, decreases in the proportion of broad-spectrum agents, and a modest decrease in the proportion that were prolonged duration. The observed inter-physician variability in changed prescribing habits offers insights for antimicrobial stewardship efforts designed to reduce inappropriate antibiotic prescribing in the community. Enhancing these efforts is critical as antibiotic use is anticipated to increase with the return of seasonal respiratory infections.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2023.433.
Data availability statement
The data for this study are not publicly available. The IQVIA Xponent data set is owned by and proprietary to IQVIA. The license agreement between PHO and IQVIA does not permit us to share the data publicly. The authors had no special access privileges and other researchers may license the data from IQVIA directly (www.iqvia.com).
Author contributions
All authors participated in the conceptualization and design of the study. PT performed the data analysis and drafted the manuscript. KLS provided supervision. All authors contributed to data interpretation and provided critical edits to the manuscript.
Financial support
This study was funded by the Physicians Services Incorporated Foundation.
Competing interests
The authors report no conflicts of interest.
Disclaimer
The statements, findings, conclusions, views, and opinions expressed in this report are based in part on data obtained under license from IQVIA Solutions Canada Inc. concerning the following information service(s): Xponent, from January 1st, 2019 to December 31st, 2021. All Rights Reserved. The statements, findings, conclusions, views, and opinions expressed herein are not necessarily those of IQVIA Solutions Canada Inc. or any of its affiliated or subsidiary entities.