INTRODUCTION
Pneumonia and meningitis are the leading causes of death in developing countries [1]. About 3 million cases of serious disease and 400 000–700 000 deaths occur worldwide annually due to Haemophilus influenzae type b (Hib) infection [2]. Hib vaccination has successfully reduced the disease burden in developed nations, e.g. North America [3–Reference Millar5]. However, the benefits of Hib vaccination have not yet reached developing countries [Reference Peltola6]. This is mainly because of the difficulties associated with the estimation of Hib disease burden in resource-poor countries. Nasopharyngeal carriage can indicate the dynamics of H. influenzae infection in these settings. H. influenzae has no reservoir host apart from humans; therefore, asymptomatic carriage has been recognized as the major source of infection [Reference Levine7, Reference Ritva8]. The study of nasopharyngeal flora serves as an epidemiological tool especially when the data related to invasive disease is scanty.
Prospective hospital studies from Bangladesh have found Hib diseases to be more common in children aged <3 years [Reference Saha9, Reference Watt, Levine and Santhosham10]. Hospital-based studies in India have reported H. influenzae infections and emphasized the need to initiate additional prospective studies to define incidence in the population [11]. The carriage of H. influenzae has been reported among school-aged children [Reference Jain, Kumar and Awasthi12], but carriage data among children aged <2 years has not been reported. Therefore, this study was undertaken to determine the carriage of H. influenzae and its risk factors in children aged <2 years.
MATERIAL AND METHODS
Study population
The study was carried out from August 2005 to July 2006 after obtaining the approval of the ethical committee of the Post Graduate Institute of Medical Education and Research, Chandigarh. The sample size of 1000 was estimated by assuming prevalence to be 5% at a 95% confidence interval and a standard error of 2%. Study communities were deliberately chosen from rural, urban slum and city areas which are representative of the population of northern India in terms of socioeconomic and environmental conditions. Households in the chosen communities were sampled using a systematic sampling approach. Briefly, from each of the selected communities, one house was selected randomly, then starting from this house, eligible children were enrolled from consecutive houses until the required sample size was achieved. A structured interview schedule was used to record sociodemographic, environmental, and health data. Acutely ill children, having respiratory tract infection and a temperature >38°C at the time of survey were excluded from the study. Specimen collection was performed continuously throughout the year.
A total of 1080 children were contacted for enrolment. From these, 37 children were excluded as they had received antibiotics during the month prior to the study. Parents of 19 children refused participation. A further 24 children were found to be ineligible as they were not usual residents of the study area.
Laboratory procedures
After obtaining informed consent from the parent, calcium alginate-tipped swabs were placed into the nasopharynx of each child at one half of the distance between the tip of the child's nose and anterior portion of the ear. The swab was immediately placed in STGG (skim milk, tryptone, glucose, and glycerol) medium for shipment to the laboratory on the same day within 24 h.
The nasopharyngeal specimens were vortexed and inoculated onto a chocolate agar plate with 300 μg/ml bacitracin. After overnight incubation at 37°C in 5% CO2, colonies typical of Haemophilus were identified. These were again plated on chocolate agar, and after overnight incubation colonies with a morphology typical of H. influenzae were identified and plated on trypticase soy agar (Becton Dickinson & Co., Franklin Lakes, NJ, USA) along with x and v factor discs (Difco TSA/XV; Difco Laboratories, Detroit, MI, USA). All specimen with x and v factor-dependent growth were frozen at −70°C in trypticase soy broth with 20% glycerol. The isolates were later confirmed by screening with antisera (Difco). Briefly, organisms that failed to agglutinate with polyvalent antisera to H. influenzae groups (a–f) were considered non-typable, and organisms that agglutinated with specific type sera were designated as that specific type. American Type Culture Collection (ATCC) reference strains Haemophilus influenzae (ATCC 49247), Haemophilus influenzae (ATCC 10211) were used as controls.
Statistical analysis
Statistical analysis was done using SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA). The prevalence of H. influenzae colonization was calculated in various age, sex, residence, and sociodemographic and environmental groups such as the type of house. A house was defined as katcha if the material used for construction of walls and roof included grass, leaves, reeds, bamboo, mud, un-burnt brick or wood, and was considered pucca if the material used for construction included burnt brick, metal sheets, corrugated iron, stone or cement concrete; a mixture of these two features is referred to as katcha-pucca. Logistic regression analysis was done with adjusted odds ratios and 95% confidence intervals to evaluate the ‘independent’ effects of various sociodemographic and environmental factors on H. influenzae carriage.
RESULTS
The prevalence of H. influenzae colonization in the nasopharynx was 14·5% in rural and 10·2% in slum areas while in the urban population it was merely 5%. In males the prevalence was 12·5% and in females it was 9·0%. The carriage rate was highest (20·3%) in the 18–21 months age group (Table 1). Of the isolates 69% (78/112) belonged to type b and the rest were non-typable.
Table 1. Prevalence of H. influenzae carriage according to age and sex, northern India, 2005–2006
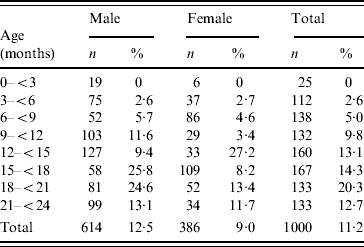
Large variation in carriage was seen in different seasons. Colonization was lowest during summer months and highest during winter months (Fig. 1). As shown in Table 2, in univariate analysis, the carriage rate was found to be highest (64%) for those belonging to a family numbering >8. In overcrowded households carriage was more than twofold higher. Carriage rate was also high among children living in katcha houses (32·5%), whose fathers were uneducated (15%), who lived with a smoker (12·2%), and among children belonging to households that used solid fuels (22·7%). However, in the multivariate logistic regression model, age >12 months, winter season, katcha house, >5 family members, and overcrowding were found to be significant risk factors for colonization with H. influenzae (Table 2).
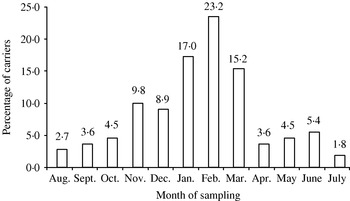
Fig. 1. H. influenzae nasopharyngeal carriage according to season, in northern India, during 2005–2006.
Table 2. Risk factors of nasopharyngeal carriage of H. influenzae, northern India, 2005–2006
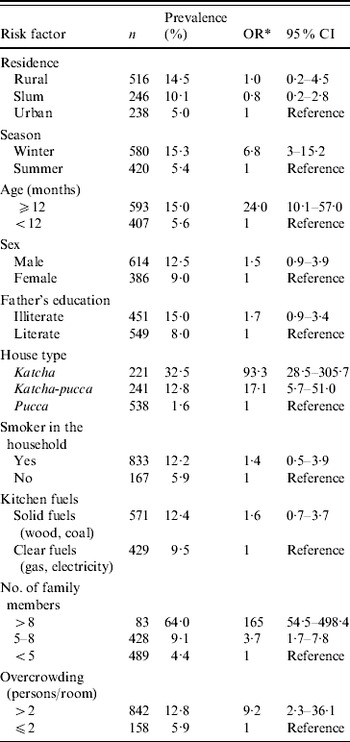
OR, Odds ratio; CI, confidence interval.
* Based on logistic regression model which included all variables.
DISCUSSION
H. influenzae type b isolates constituted the majority (69%) in our study indicating that Hib disease may also be high in the study population. This observation is of public health significance as Hib vaccination has not been widely practised in this part of the world. High carriage rate of type b is in accordance with a few other studies conducted in India among the 5–10 years age group [Reference Jain, Kumar and Awasthi12, Reference Das13].
In contrast to developed countries, where acquisition of Hib is rare before age 6 months, children from developing countries acquire Hib at an earlier age which was confirmed in the present study. During the first 6 months of infancy infants live in close contact with their mothers and are usually breast-fed resulting in passive antibody transfer. During this period pathogens start colonizing and attempt to evade host defences. H. influenzae colonization starts at the age of 3–5 months and reaches a maximum at 18–21 months. It can be inferred from the study that the <2 years age group should be considered as an epidemiological niche of infection in our target populations, and hence efforts should be made to prevent infection before the age of 3 months. The nasopharyngeal carriage rates are important indices for development of infection as they serve as surrogate markers of occurrences in the population and the risk with which these populations are living [Reference Timothy14, Reference Greenberg15].
Various hospital-based studies have observed higher infection rates in the male population [Reference Watt, Levine and Santhosham10], which was thought to be due to differential care seeking according to gender. This population-based study does not have a selection bias and prevalence in males was not found to be statistically different than females once confounding factors were controlled in multivariate analysis.
One of the factors that had a large influence on carriage was the type of housing. Inhabitants of katcha houses were more at risk of acquiring this organism than other types of housing. As expected, overcrowding was associated with acquisition of H. influenzae carriage in this study also. Carriage was also highly influenced by family size. This can be explained by the fact that in large families children live in close contact with a number of siblings, thereby increasing the chances of acquisition of H. influenzae. This is in complete accordance with other studies which have highlighted increased carriage in day-care centres [Reference Dabernat16, Reference Peerbooms17].
The carriage rate had a strong seasonal distribution. Carriage was highest in the winter months rather than summer. This can be attributed to the fact that during winter months people live in close proximity. Apart from this, winter weather conditions are more conducive for transmission of many pathogens. Various viral infections prevalent in these conditions also serve as accessory factors by predisposing the host immune system and thus aiding the infection of H. influenzae [Reference Miyamoto and Bakaletz18, Reference Hakansson19].
Passive smoking has been observed as a risk factor in a number of the studies [Reference Greenberg20, Reference Brook and Gober21]. The precise mechanism between cigarette smoke and risk of carriage of pathogens is not well understood. We observed the influence of smoking on the carriage rate in univariate analysis but when a multiple regression model was used passive smoking was found to be a mere confounder. A limitation of our study was that we completely relied upon the information obtained from the questionnaire administered to mothers and did not estimate cotinine levels to confirm the reports of smoking.
To conclude, the carriage rate of H. influenzae in the northern Indian population is associated with number of family members in the household, type of housing, overcrowding, season and age of the child. Since the rate of carriage in this population is similar to rates of carriage in other populations where high rates of Hib disease have been reported, there is a strong rationale for the introduction of Hib vaccination in the national immunization programme of India.
ACKNOWLEDGEMENTS
We thank the parents/guardians and children for participation in this study. We are also grateful to the Indian Council of Medical Research (ICMR), New Delhi for financial assistance.
DECLARATION OF INTEREST
None.





