Introduction
During the last decade, research into the gut microbiota has gained traction due to its bidirectional associations with the health and disease of the host (Cryan et al., Reference Cryan, O’Riordan, Cowan, Sandhu, Bastiaanssen, Boehme, Codagnone, Cussotto, Fulling, Golubeva, Guzzetta, Jaggar, Long-Smith, Lyte, Martin, Molinero-Perez, Moloney, Morelli, Morillas and Dinan2019; Hooks et al., Reference Hooks, Konsman and O’Malley2019). Human studies have indicated associations between the gut microbiota, brain development and behavioral and emotional outcomes (Morais et al., Reference Morais, Schreiber and Mazmanian2021), such as autism spectrum disorder (Li et al., Reference Li, Han, Dy and Hagerman2017), major depression and bipolar disorder (Huang et al., Reference Huang, Lai, Du, Xu, Ruan and Hu2019). Increased risk for a range of psychiatric conditions has been linked to early neurodevelopment (Monk et al., Reference Monk, Lugo-Candelas and Trumpff2019), and consequently, early childhood has received increasing interest as a focus of studies on so-called gut-brain associations. This is especially relevant given the gut microbiota composition in early childhood differs substantially from adult gut microbiota and continues to develop throughout childhood and adolescence (Derrien et al., Reference Derrien, Alvarez and De Vos2019). Correspondingly, the brain undergoes rapid development, using genetic and environmental signals across the same period of time and reaches 90% of adult brain volume in size by 6 years of child age (Stiles & Jernigan, Reference Stiles and Jernigan2010). The overlapping maturation of the gut microbiota and brain underscores the need to understand their influences and interactions in children’s behavioral development and later neurodevelopmental disorders.
The bidirectional associations between the gut and the brain involve neural, metabolic, and immune pathways (Cryan et al., Reference Cryan, O’Riordan, Cowan, Sandhu, Bastiaanssen, Boehme, Codagnone, Cussotto, Fulling, Golubeva, Guzzetta, Jaggar, Long-Smith, Lyte, Martin, Molinero-Perez, Moloney, Morelli, Morillas and Dinan2019), which are influenced by gut microbes and their metabolites, including short-chain fatty acids (Dalile et al., Reference Dalile, Van Oudenhove, Vervliet and Verbeke2019). Recent studies indicate that disruption of the gut microbiota composition and their metabolite production during these first years of life can have a negative impact on neurodevelopment (Pronovost & Hsiao, Reference Pronovost and Hsiao2019; Warner, Reference Warner2019). Gut microbiota composition with negative loadings of Bacteroides in infancy seems to be associated with communication, personal and social, and fine motor skills at age 3 years and increasing the likelihood of possible developmental delays (Sordillo et al., Reference Sordillo, Korrick, Laranjo, Carey, Weinstock, Gold, O’Connor, Sandel, Bacharier, Beigelman, Zeiger, Litonjua and Weiss2019). Similarly, gut microbiota composition with high levels of Bacteroides at 1 year of age predicted the highest level of cognitive performance at 2 years of age (Carlson et al., Reference Carlson, Xia, Azcarate-Peril, Goldman, Ahn, Styner, Thompson, Geng, Gilmore and Knickmeyer2018). These studies suggest that infant gut microbiota composition may influence cognitive milestones and behavioral phenotypes as early as the first years of life. This is important in the context of playing an important role in laying the foundation for subsequent social-emotional well-being and psychiatric disorders (Brown et al., Reference Brown, Copeland, Sucharew and Kahn2012).
Temperament, which reflects, in part, emotional development, can be observed across the lifespan, including in the first year of life (Rothbart, Reference Rothbart2007). Temperament refers to biologically based individual differences in responsivity to stimuli, reactivity, self-regulation, and activity (Rothbart, Reference Rothbart2007) and it is a relatively stable way of acting and reacting over time and across situations (De Pauw & Mervielde, Reference De Pauw and Mervielde2010). However, as the central nervous system continues to develop, particularly during the early years, environmental factors influence immunological, metabolic, and stress regulation processes as well as brain and behavioral development. These influences are likely mediated, at least in part, through epigenetic mechanisms, highlighting the potential significance of environmental factors in understanding the developmental processes of temperament (Gartstein & Skinner, Reference Gartstein and Skinner2018). It is also important to note that temperament characteristics are to some extent going through some alterations in early childhood (Pesonen et al., Reference Pesonen, Räikkönen, Heinonen, Komsi, Järvenpää and Strandberg2008) and these alterations are likely to be relevant for later psychosocial development. Among the potential environmental factors that can affect temperament development, the gut microbiome emerges as a relevant research subject, which could in turn further our understanding of the complex relationship between environment and temperament.
Higher negative reactivity, which means the tendency to experience and display negative emotions such as anger, sadness, and fear, is an especially relevant temperament trait, and a possible transdiagnostic risk factor that has consistently been associated with various types of psychopathology such as internalizing and externalizing symptoms, autism spectrum disorder and attention deficit hyperactivity disorders later in life (De Pauw & Mervielde, Reference De Pauw and Mervielde2010; Hankin et al., Reference Hankin, Davis, Snyder, Young, Glynn and Sandman2017; Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020; Nigg, Reference Nigg2006). The trajectory of fear reactivity, which refers to inhibited behavior, particularly in novel situations, can be differentiated from other negative reactivity traits for its later emergence and peak at around one year of age (Gartstein et al., Reference Gartstein, Bridgett, Rothbart, Robertson, Iddins, Ramsay and Schlect2010; Putnam & Stifter, Reference Putnam and Stifter2002). Fear reactivity and especially shyness is suggested to predict later anxiety and risk for internalizing problems (Buss & McDoniel, Reference Buss and McDoniel2016; Clauss et al., Reference Clauss, Avery and Blackford2015; Feng et al., Reference Feng, Shaw and Silk2008). Thus, higher negative reactivity and fear reactivity can be considered intermediate phenotypes crucial for later mental health. Additionally, due to its early emergence, it could be a potential target phenotype for early intervention with focus on gut microbiota, which could be seen as a part of the biological and environmental factors influencing temperament expression in infancy.
Previous studies on the gut microbiota and temperament have been conducted in toddlers (Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Xie et al., Reference Xie, Wang, Zou, Wu, Fan, Dai, Liu and Bai2022) and in infants (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Carlson et al., Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021; Fox et al., Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022; Loughman et al., Reference Loughman, Ponsonby, O’Hely, Symeonides, Collier, Tang, Carlin, Ranganathan, Allen, Pezic, Saffery, Jacka, Harrison, Sly and Vuillermin2020; Wang et al., Reference Wang, Chen, Yu, Liu, Zhang and Bai2020; Zhang et al., Reference Zhang, Huang, Lu, Mao and Cao2023). Christian et al. (Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015) found that differences in the overall composition of microbiota between subjects, at child age of 2 years, was associated with parent-reported extraversion/surgency, a trait related to positive reactivity, at the age of 2 years. However, they found no associations between alpha diversity, i.e. the species diversity within a subject, and negative reactivity. Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) reported that greater alpha diversity at the age of 2.5 months was associated with lower mother-reported negative reactivity at 6 months. Similarly, recent studies have repeated earlier findings at a different age points including association between community composition at child age of 1-3 weeks and parent-reported extraversion/surgency at child age 12 months (Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Fox et al., Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022). In addition, a negative trend between alpha diversity at child age 2 months and parent-reported negative reactivity at child age 12 months was reported (Fox et al., Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022). Finally, Carlson et al. (Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021) showed that lower alpha diversity at 1 month of age was associated with higher observed fear reactivity at 1 year of age. They also found association between community composition and fear reactivity at 1 year of age. Most recent studies did not find any associations between gut microbiota composition and negative or fear reactivity (Xie et al., Reference Xie, Wang, Zou, Wu, Fan, Dai, Liu and Bai2022; Zhang et al., Reference Zhang, Huang, Lu, Mao and Cao2023).
Regarding temperament, the focus has predominantly been on parent-reported measurements without incorporating laboratory-observed measurements. However, combining reported and observed measurements would be beneficial since previous studies have provided mixed findings regarding for example anger proneness which is considered a component of negative reactivity (Ollas-Skogster et al., Reference Ollas-Skogster, Rautakoski, Bridgett, Kataja, Karlsson, Karlsson and Nolvi2023). Both parent-reported and laboratory-observed measures of child temperament have their own advantages, which is why using them in combination is recommended.
Temperament and gut microbiota associations have also differed by sex. Early rodent studies have indicated that microbiome modulation has differential effects on brain functioning and behavior depending on sex (Clarke et al., Reference Clarke, Grenham, Scully, Fitzgerald, Moloney, Shanahan, Dinan and Cryan2013; Cryan et al., Reference Cryan, O’Riordan, Cowan, Sandhu, Bastiaanssen, Boehme, Codagnone, Cussotto, Fulling, Golubeva, Guzzetta, Jaggar, Long-Smith, Lyte, Martin, Molinero-Perez, Moloney, Morelli, Morillas and Dinan2019; Org et al., Reference Org, Mehrabian, Parks, Shipkova, Liu, Drake and Lusis2016). However, human studies investigating sex-specific brain and behavior associations are limited (Carlson et al., Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021). Emerging data suggests differing association profiles between gut microbiome composition and temperament between girls and boys in humans (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015), making it important to continue expanding on previous studies by examining the potential role that sex has on these associations. Since only a few studies have considered possible sex differences in these associations in humans (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Wang et al., Reference Wang, Chen, Yu, Liu, Zhang and Bai2020) we believe that this calls for more investigation.
With few notable exceptions (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Carlson et al., Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Fox et al., Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022; Loughman et al., Reference Loughman, Ponsonby, O’Hely, Symeonides, Collier, Tang, Carlin, Ranganathan, Allen, Pezic, Saffery, Jacka, Harrison, Sly and Vuillermin2020; Wang et al., Reference Wang, Chen, Yu, Liu, Zhang and Bai2020; Xie et al., Reference Xie, Wang, Zou, Wu, Fan, Dai, Liu and Bai2022; Zhang et al., Reference Zhang, Huang, Lu, Mao and Cao2023), studies linking gut microbiota composition and function (metabolites) and temperament development through the early development in humans are still few in number. We aim to add to this field by utilizing a larger sample size and using multiple age points of temperament assessment, which is important from child developmental perspective. Notably, we applied both observed and reported measures of temperament, which is unique and considerably enhances the temperament phenotype assessment (Ollas-Skogster et al., Reference Ollas-Skogster, Rautakoski, Bridgett, Kataja, Karlsson, Karlsson and Nolvi2023). Finally, we added measures of microbial metabolites, which has not been reported in infant populations in the context of temperament earlier. We specifically selected SCFAs as there is emerging evidence linking them with various physiological processes of the host including immune regulation and maturation of the brain (O’Riordan et al., Reference O’Riordan, Collins, Moloney, Knox, Aburto, Fülling, Morley, Clarke, Schellekens and Cryan2022). SCFAs are a known type of metabolite that offer a unique opportunity to explore its potential associations with early developmental phenotypes, including temperament traits, which have not been extensively studied before. By focusing on SCFAs among other gut microbiota measures we aim to investigate the potential complex relationship between these microbial metabolites and the developing brain, which underlie behavioral temperament. In addition to that, we believe that possible sex-specific associations should be included as a distinct question since emerging data suggests differing association profiles between gut microbiome composition and temperament between girls and boys in humans (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015).
Thus, we aim to study how gut microbiota diversity and composition as well as concentrations of microbially metabolized SCFAs at child age 2.5 months associate with laboratory-observed at child age 8 months and parent-reported at child age 12 months negative reactivity and fear in a relatively large samples of infants. Here, we focus on the aspects of negative reactivity as it has been shown to be a transdiagnostic risk factor relating to various later mental health problem (De Pauw & Mervielde, Reference De Pauw and Mervielde2010; Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020; Nigg, Reference Nigg2006). Likewise, we investigate the possibility of sex-specific associations between gut microbiota and negative reactivity and fear, and we hypothesize that girls and boys will have different associations profiles. Sex differences in the associations between gut microbiota composition and temperament have not been extensively explored in humans, with a few notable exceptions (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Wang et al., Reference Wang, Chen, Yu, Liu, Zhang and Bai2020). It is worth noting that Aatsinki et al., and Christian et al., explored these associations at different age points compared to our current study. However, Wang et al., who examined the same age group as out study, did not find any significant differences between boys and girls. Given the scarcity of research in this area and the disparity in age groups, there is insufficient evidence to support a definitive hypothesis regarding the potential differential associations between girls and boys.
Methods
Procedures
The subjects of this study were part of the larger FinnBrain Birth Cohort Study (Karlsson et al., Reference Karlsson, Tolvanen, Scheinin, Uusitupa, Korja, Ekholm, Tuulari, Pajulo, Huotilainen, Paunio and Karlsson2018). The data was collected in the areas of Turku and Åland Islands in Finland. The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland (ETMK 59/1801/2013, ETMK57/180/2011, ETMK 121/2801/2013). Subcohort partially overlaps with one utilized in previous Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) study. However, it is important to note that the reported negative and fear reactivity measurements in this study are collected at a later time point (12 months), which differs from the time point used in the study by Aatsinki et al., which was 6 months. Additionally, this study incorporated observational measurements at 8 months, which were not included in the previous study. Children with at least one form of temperament assessment and successfully collected and analyzed stool samples were included in the analysis (observed sample n = 150 and reported sample n = 276). Mothers provided a written consent of their own and their child’s behalf prior any measurements and samplings Mothers were asked about their age at birth and education at baseline (gestational week 14), number of siblings (at 12 months postpartum) and breastfeeding at child age 2.5 months. Breastfeeding was based on maternal reports of breastfeeding over the first year of infant’s life. Information regarding birth such as duration of pregnancy (weeks), maternal pre-pregnancy body mass index (BMI), mode of delivery (vaginal or C-section) and antibiotic intake during the first months was drawn from the National Birth Registry provided by the National Institute for Health and Welfare (www.thl.fi). Participant characteristics shown in Tables 1 and 2.
Table 1. Reported and observed samples participant characteristics and temperament traits by infant sex
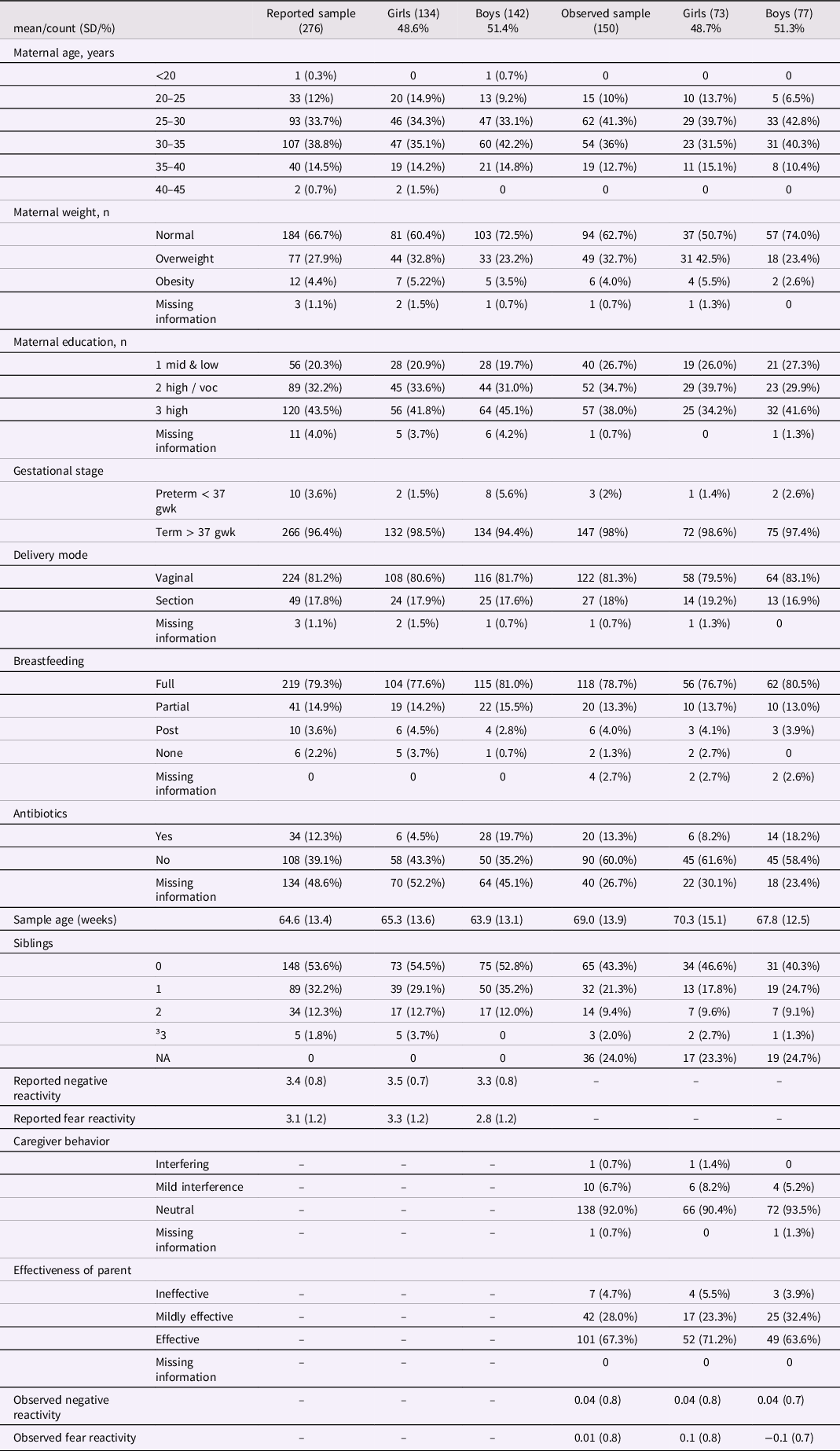
Table 2. Linear regression models for alpha diversity and negative/fear reactivity variables for the whole study population and for girls and boys separately
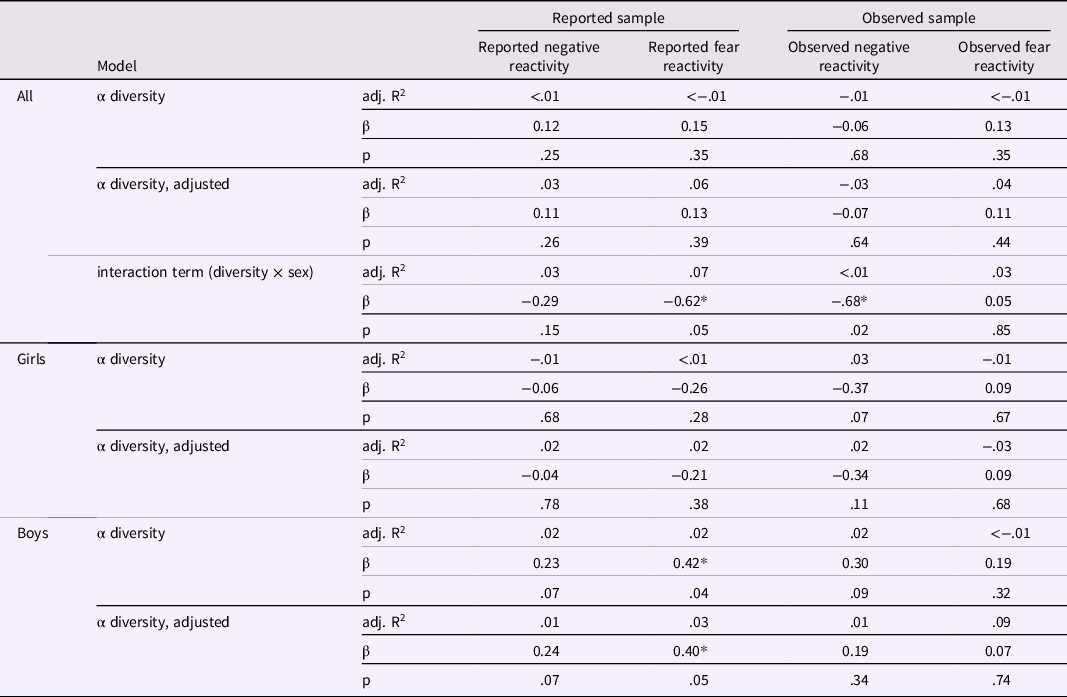
Results adjusted for delivery mode and sex for reported negative and fear reactivity and for delivery mode, maternal age and sex for observed negative reactivity and for delivery mode, maternal age, sex and caregiver behavior for observed fear reactivity. * p < .05.
Infant negative reactivity and fear reactivity
Infant negative reactivity and fear were assessed using two methods. First at 8 months the observations were made using tasks from the Infant Laboratory Temperament Assessment Battery (Lab-TAB) prelocomotor (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1999). At 12 months, negative reactivity and fear were assessed using maternal reports of Infant Behavior Questionnaire-Revised Short Form (IBQ-R SF) (Putnam et al., Reference Putnam, Helbig, Gartstein, Rothbart and Leerkes2014).
Observed negative reactivity and fear
Observed negative reactivity. Negative reactivity was observed during the Lab-TAB episode “Gentle arm restraint by parent”, which tends to elicit frustration/anger, but also sadness (Goldsmith & Rothbart, Reference Goldsmith and Rothbart1999). During this episode, the child is first allowed to play with a toy with their mother for 15–30s after which the mother gently restrains the infant’s arms for 30 s, preventing their child from interacting with the toy. The trial is then repeated, and both restraint trials are divided into six 5-second epochs and scored using different indicators of negative reactivity. The indicators coded in this study were facial anger (on a scale from 0 to 3), facial sadness (0–3), distress vocalizations (0–5) and bodily struggle (0–4). Effectiveness of the parent during the observations was coded on a 0–2 scale for the whole episode. The temperament indicators of infant negative reactivity were standardized and collapsed into an averaged sum variable called “observed negative reactivity”. Cronbach’s alpha for overall negative reactivity factor in this study was 0.74. Altogether, the inter-rater reliability for the sum variable was acceptable (Mean of Cohen’s Kappa for sum of all episodes = 0.67 and for indicators; facial anger = 0.72, for facial sadness = 0.63, for distress vocalizations = 0.78 and for bodily struggle = 0.57, and inter-rater r for facial anger = 0.80–0.96, for distress vocalizations = 0.90–0.99, for facial sadness = 0.64–0.90 and for bodily struggle = 0.69–0.94).
Observed fear reactivity. Fear reactivity was observed during the episode called “Masks”. The stimuli in this episode contains four masks (evil queen, old man, glow-in-the-dark vampire, and gas mask) that are each shown to the child for 10s with 5s interval in between the masks. The sequence of the masks was fixed. Each of the four 10s mask trials are divided into two 5s scoring epochs. The indicators for each epoch used in this study were facial fear (on a scale from 0 to 3), bodily fear (0–3), escape behavior (0–3) and distress vocalizations (0–5). Caregiver behavior was coded for interferences on a 0-2 scale for the whole episode. The indicators of infant fear reactivity were coded into a sum variable called “observed fear reactivity”. Internal consistency for overall fear reactivity in this study was good (α = 0.88). The inter-rater reliability for the episode was also good (Mean of Cohen’s Kappa for sum of all episodes = 0.79 and for indicators; facial fear = 0.73, for bodily fear = 0.8, for escape behavior = 0.74 and for distress vocalizations = 0.83, and inter-rater r for facial fear = 0.98, for bodily fear = 0.97, for escape behavior = 0.95, and for distress vocalizations = 0.98).
Reported negative reactivity and fear
Mother-reported negative reactivity and fear reactivity. Negative reactivity was assessed using the IBQ-R SF that contains 91 items to which the caregiver responds based on how often their child has expressed a specific behavior in everyday situations during the past week or two weeks (Putnam et al., Reference Putnam, Helbig, Gartstein, Rothbart and Leerkes2014). The items in the questionnaire form 14 scales which in turn form three temperament factors called Positive Affectivity/Surgency, Negative Affectivity and Orienting/Regulatory Capacity. The main dimension Negative Affectivity and its subscale Fear were used in this study, and they were referred to as “reported negative reactivity” and “reported fear reactivity”. The responses to each item ranged on a scale from 1 to 7. Cronbach’s alpha for negative reactivity was 0.85 and for fear reactivity α = 0.76.
Infant gut microbiota analysis
Infant fecal samples were collected by the parents at home at approximately 2.5 months postpartum (mean sample age: 65.4 days, sd: 13.4). Parents received a written as well as oral instructions to collect the samples into sterile collection tubes, mark the date and time of the sample taking, store them immediately at below + 4°C, and bring them to the laboratory as soon as possible within 24 hours (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019). Only the samples that were delivered back within 48 hours were included in the analyses. In the research facilities (Microbiome Biobank, Turku University Hospital, Clinical Microbiology), samples were thoroughly homogenized, divided into the aliquots and frozen at −75°C until DNA extraction. We adapted and modified the targeted SCFA analysis from previously work (Trimigno et al., Reference Trimigno, Khakimov, Mejia, Mikkelsen, Kristensen, Jespersen and Engelsen2017). Whilst we used the same column as Trimigno et al., we optimized other parameters such as septum purge flow and split flow, carrier gas, and GC oven program to fit our instrument. More detailed information described in Supplemental Materials.
Covariates
Maternal age was divided into categories with five-year interval (less than 20, 20–25, 25–30, 30–35, 35–40 and 40–45) and weight into three categories: normal (BMI less than 25), overweight (BMI between 25 and 35) and obesity (BMI over 35). Further, maternal education was divided into three categories (1 = basic education to upper secondary level, 2 = vocational tertiary, referring to applied university of polytechnics, 3 = university degree or higher). Duration of the pregnancy was divided into two categories: preterm (less than 37 gestational weeks) and term (over 37 gestational weeks) and the mode of delivery was categorized either vaginal birth or cesarean section. Mothers reported breastfeeding status at child age of 2.5 months postpartum, and reports were divided into four categories: no breastfeeding, cessation before timepoint, partial breastfeeding or full breastfeeding. Number of siblings was divided into four categories: no siblings, one sibling, two siblings or three or more siblings. Caregiver behavior during Masks task was coded for interference and coded into three categories for the whole episode (0 = interfering, 1 = mild interference, 2 = not interfering). We encoded all covariates as categorical variables, including the continuous variables maternal age and BMI. The categorization facilitates the discovery of potential non-linear or non-monotonous effects that could be missed by a simple parametrized models for continuous covariate. A secondary benefit is that the utilized differential abundance testing method supports categorical covariates, and the presentation and interpretation of results can be simplified when all covariates are treated categorically. We do not expect major benefits from the continuous modeling of the covariate in this case.
Statistical analyses
Statistical analyses were conducted using R 3.6.3 software (R Core Team, 2020). Alpha diversity index (Shannon Index) was calculated with phyloseq R package from the ASV abundance matrix (McMurdie & Holmes, Reference McMurdie and Holmes2013). Beta diversity index (Bray-Curtis dissimilarity) was calculated using the ASV abundance matrix and community composition analyses were conducted with vegan R package (Oksanen et al., Reference Oksanen, Kindt, Legendre, O’Hara, Stevens, Oksanen and Suggests2007) and microbiome R package (Shetty & Lahti, Reference Shetty and Lahti2019). Differential abundance analyses were conducted using DESeq2 R package (Love et al., Reference Love, Huber and Anders2014). The two samples with available mother-reports of negative reactivity (n = 276) and observations of negative reactivity (n = 150) were analyzed separately. This was done to retain maximal statistical power in the analyses. Temperament measurements were normally distributed (Shapiro-Wilk p > .05) except for reported fear (Shapiro-Wilk p < .001). However, the distribution of reported fear reactivity was very close to normal based on the observation of skewness estimate (0.43) and visual estimation, and thus was used in the analyses without transformations.
Gut microbiota alpha diversity, community composition (i.e., beta diversity), genus abundance and SCFA concentrations were used as independent variables in the analyses. For the sample with reported outcomes, overall reported negative reactivity and its subscale fear were used as dependent variables, whereas for the sample with observed outcomes, overall observed negative reactivity and overall observed fear reactivity were the dependent variables in the analyses. Based on association testing and theoretical framework, covariates in the reported sample were sex and mode of delivery and in observed sample sex, mode of delivery and maternal age for negative reactivity and sex, mode of delivery, maternal age, and caregiver behavior for fear reactivity (detailed information about covariate selection in Supplemental Materials). For observed sample, breastfeeding status showed significant association with community composition. However, breastfeeding status was excluded from the primary analyses for its potential mediating effect between negative reactivity and diversity for being affected by child temperament (more detailed explanation in Supplemental Materials). The robustness of the association for covariate selection was tested by including breastfeeding as a covariate for observed sample and the results of the sensitivity analyses with breastfeeding as a covariate can be found in Supplemental Tables 1 & 2. Alpha diversity analyses were repeated with rarefied data set (see Supplementary Table 3.) and the data set was rarefied to minimum depth with rarefy_even_depth command from phyloseq-package with default options.
The analyses were conducted separately for alpha diversity, community composition and differential abundances of each genus as well as SCFAs. First, associations between alpha diversity and negative reactivity features were investigated using Spearman’s correlations. Then, for each dependent variable, two linear regression models (unadjusted and adjusted for the covariates) were built. As a last step, an interaction term (alpha diversity by sex) was included in the model to investigate sex by microbiome interactions in predicting infant negative reactivity. In addition, linear regression models were built separately for boys and girls (e.g., stratified analysis) to further examine the sex differences in the association between community composition and temperament.
Associations between community composition and each negative reactivity outcome were assessed using the Permutational Multivariate Analysis of Variance (PERMANOVA) based on Bray-Curtis dissimilarities. Function adonis from vegan R package (Oksanen et al., Reference Oksanen, Kindt, Legendre, O’Hara, Stevens, Oksanen and Suggests2007) and 999 permutations were used. To investigate sex differences, associations between community composition and negative reactivity outcomes were also conducted for boys and girls separately to examine the sex differences. Correlations between the first three PCoA loadings and negative reactivity measurements for boys were conducted. DESeq2 R package was used to test which genera related to axes that were correlated with negative reactivity (Love et al., Reference Love, Huber and Anders2014).
DESeq2 R package was used for differential abundance analysis of genera. Differential abundance analyses were conducted only adjusted for covariates to reduce the number of analyses. Covariates were the same as in other analyses. In line with the other analyses of this study, differential abundance analysis of taxa was performed for boys and girls separately to examine the sex differences in the associations. Deseq2 adjusts p-values for multiple testing with Benjamini-Hochberg procedure. Overall, p ≤ .05 after adjusting for multiple analyses was considered significant.
Associations between SCFA concentrations and negative reactivity and fear reactivity were investigated first using Spearman’s correlation. A sum of the major SCFA (butyric, propionic and acetic acid) concentrations was investigated in addition to raw concentration values. Adjusted linear regression models were built for those SCFA variables and temperament measurements that correlated statistically significantly.
Results
Participant characteristics
There was no correlation between parent-reported and observed negative reactivity (r = −.03, p = .71) or parent-reported and observed fear reactivity (r = .001, p = .99). The analyses were done on two partially distinct samples because the overlap between these groups was relatively small (n = 120), and we wanted to maximize the statistical power in the analysis. In both reported and observed samples, 49 % of study subjects were girls and 81 % were born vaginally, and majority were born full-term (Table 1.). Obese mothers had a higher proportion of girls than boys in the observed sample (p = 0.02) but not in the reported sample (p = .12).
Associations between covariates, gut microbiota parameters and negative reactivity and fear measurements
We studied the associations between gut microbiota parameters and temperament traits and covariates. Within the reported subcohort, alpha diversity (Shannon index) was not associated with any of the covariates (p > 0.05 for all). Gut microbiota profile assessed with Bray-Curtis dissimilarity was associated with delivery mode (PERMANOVA F = 3.19, R2 = 0.03, p = .01). Girls had higher scores for both the reported negative reactivity (Kruskal-Wallis χ2 = 4.86, df = 1, p = .03) and fear reactivity (Kruskal-Wallis χ2 = 13.82, df = 1, p < .001). Sex and delivery mode were covariates in analysis.
Within the observed subcohort, lower Shannon index was associated with higher maternal age (Kruskal-Wallis χ2 = 8.48, df = 3, p = .04), and community composition was associated with delivery mode (F = 3.19, R2 = 0.03, p = .01). Caregiver behavior that is also assessed during the fear observation was associated with higher observed fear reactivity (Kruskal-Wallis χ2 = 8.54, df = 2, p = .01). No evidence on gender difference in observed negative reactivity or fear was noted. Covariates for the observed subcohort were sex, delivery mode and maternal age for negative reactivity and sex, delivery mode, maternal age and caregiver behavior for fear.
Delivery mode can be seen as a confounder for the reported subsample since it was associated with both gut microbiota and temperament. For the reported subsample, sex was associated with reactivity measures but not gut microbiota measures. For observed sample, delivery mode, maternal age and breastfeeding status associated with gut microbiota. Caregiver behavior associated with observed fear reactivity.
Shannon index and negative reactivity and fear
Shannon index was not associated with maternal reports of negative reactivity or fear (Table 2). Further, there was no evidence on Shannon index being associated with the maternal reported temperament traits in either unadjusted or adjusted (for sex and mode of the delivery) linear regression models (Table 2), Shannon index correlated significantly with sex (p = .05) suggesting sex-specific associations between Shannon index and reported fear reactivity (Table 2). To further examine sex-specific interactions, linear regression models were performed for boys and girls separately (Figure 1; Table 2). For boys, higher Shannon index was associated with increased reported fear reactivity both in the unadjusted analysis (b = .42, p = .04) and when adjusted for delivery mode (b = .40, p = .05). We tested the robustness of the association for the choice of diversity index as well as rarefication. Associations remained qualitatively similar regarding significance level effect sizes and direction when different diversity index or rarefication were used and similar regarding direction when community richness indices were used (Supplementary Table 3).

Figure 1. Alpha diversity (Shannon index) and reported negative reactivity. ( a ) Reported fear reactivity. ( b ) Observed negative reactivity. ( c ) And observed fear reactivity. ( d ) Regression lines displayed by sex.
Similarly, there were no associations between Shannon index and laboratory-observed negative reactivity or fear (Table 2). Shannon index was not associated with observed negative reactivity or fear in either the unadjusted or the adjusted (for sex, mode of delivery and maternal age for negative reactivity; and additionally, for caregiver behavior when conducting models for fear reactivity) linear regression models (Table 2). For observed negative reactivity, a diversity by sex interaction was observed (Figure 1.). However, when built separately for boys and girls, linear regression models showed no significant associations between Shannon index and observed negative reactivity or fear (Table 2). The interaction remained significant when using Gini-Simpson but not inverse Simpson nor richness as alpha diversity index (Supplementary Table 3). Rarefication did not change the conclusions (Supplementary Table 3).
Community composition and negative reactivity and fear
Community composition (i.e., beta diversity) based on Bray-Curtis dissimilarities was not associated with reported or observed temperament traits with or without covariates (Table 3; Figure 2). Community composition and temperament measurements were further investigated separately for boys and girls to investigate potential differential associations. For boys, the reported negative reactivity was associated with community composition (F = 2.07, R2 = 0.01, p = .02) and the association remained when adjusted for delivery mode (F = 2.02, R2 = 0.01, p = .02). Similarly, for boys in the observed sample, the observed negative reactivity was associated with community composition (F = 2.0, R2 = 0.03, p = .02) and the association remained when adjusted for delivery mode and maternal age (F = 2.26, R2 = 0.03, p = .01) (Table 3; Figure 3). However, this association did not remain when using Unifrac or weighted Unifrac distances (p > .38). This suggests that boys may have different microbial community composition based on the differences in reported and observed negative reactivity. For girls, community composition was not associated with either reported or observed temperament traits (Table 4). When including breastfeeding in a statistical model as a covariate, the results did not change substantially regarding significance level or effect size (F = 2.19, R2 = 0.03, p = .02) (Supplemental Table 2).
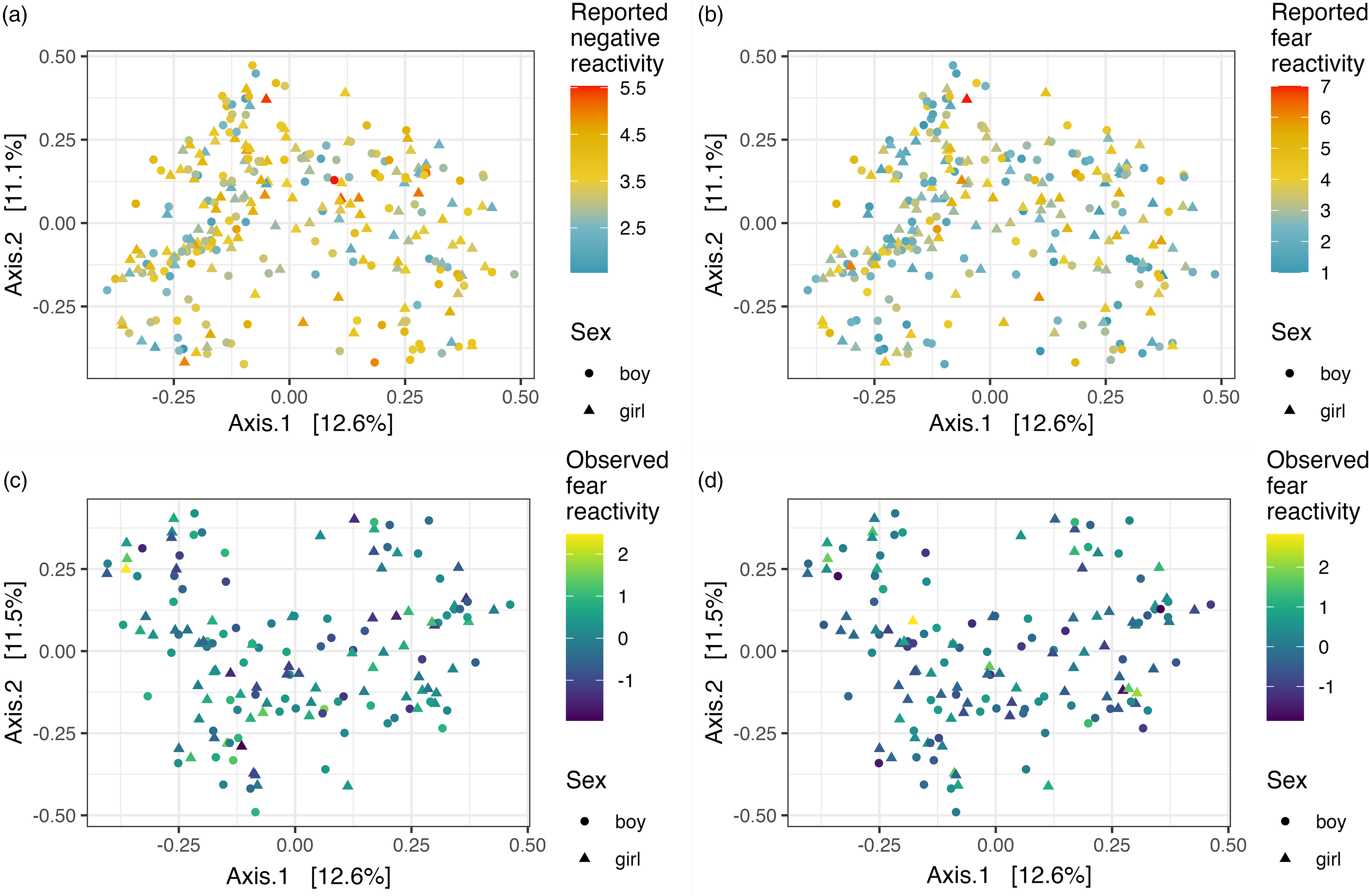
Figure 2. PCoA plots illustrating Bray-Curtis dissimilarity between samples and reported negative reactivity. ( a ), Reported fear reactivity. ( b .), Observed negative reactivity. ( c ) And observed fear reactivity ( d ).

Figure 3. PCoA plots for boys illustrating community composition in three axis (with two out of three total axes illustrated in each plot) that explained the largest variation on Bray-Curtis dissimilarity and reported negative reactivity. ( a ) And observed negative reactivity ( b ).
Table 3. PERMANOVA results between community composition and negative/fear reactivity for the whole study samples and for girls and boys separately
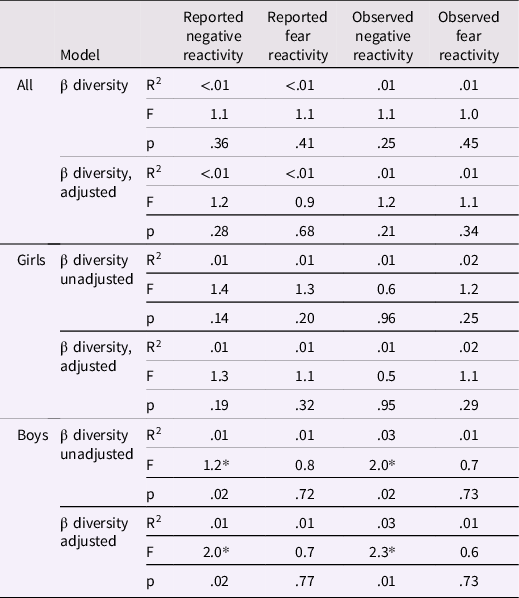
Adjusted for delivery mode and sex for reported negative and fear reactivity and for delivery mode, maternal age and sex for observed negative reactivity and for delivery mode, maternal age, sex and caregiver behavior for observed fear reactivity. * p < .05.
Table 4. Genera associated with negative and fear reactivity
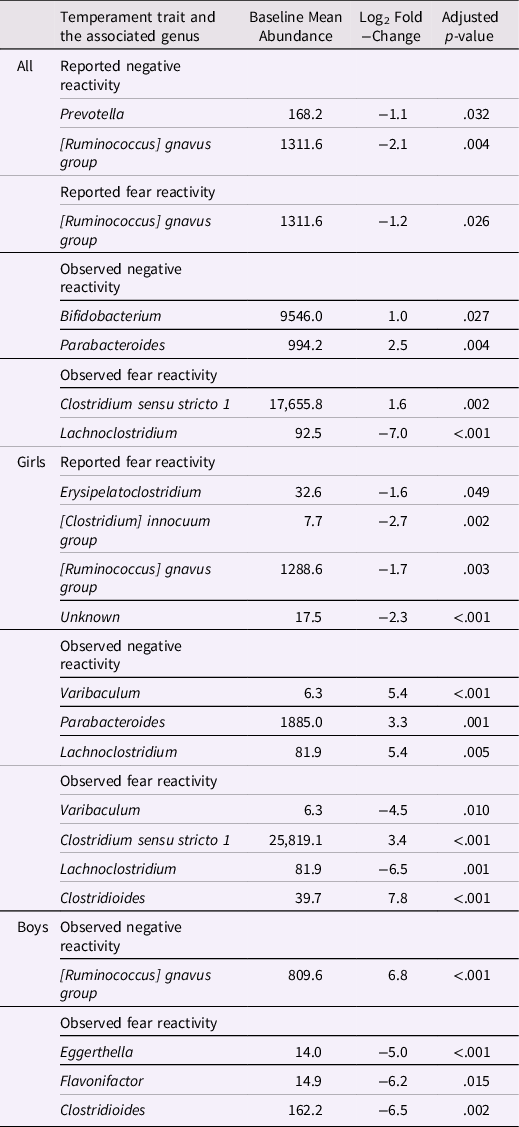
Differential abundance analyses were conducted only adjusted for covariates to reduce the number of analyses. Adjusted for delivery mode and sex for reported negative reactivity and fear and for delivery mode, maternal age and sex for observed negative reactivity and for delivery mode, maternal age, sex and caregiver behavior for observed fear reactivity.
To further elucidate the factors related to community composition findings we tested whether the first three PCoA axis loadings associate with negative reactivity in boys. PCoA axis 1 and 3 associated with reported negative reactivity (ρ = .22, p = .02; ρ = −.18, p = .04, respectively) and axis 3 associated with observed negative reactivity (ρ = −.32, p = .02). Further, axis 1 had positive associations with genera Bilophila and Escherichia-Shigella and negative associations with Flavonifactor, Veillonella, Citrobacter and Klebsiella (p < .05). Axis 3 was positively associated with genera Collinsella, Bacteroides, Parabacteroides, [Ruminococcus] gnavus group and negatively associated with genera Actinomyces, Bifidobacterium, Prevotella, Enterococcus, Streptococcus, Gemella, Staphylococcus, Clostridium sensu stricto 1, Veillonella, Escherichia-Shigella and Klebsiella (p < .05).
Differential abundances and negative reactivity and fear
The associations between bacterial abundances at genus level and both reported and observed negative reactivity and fear are shown in Table 4. For example, reported negative reactivity showed a negative association with genera Prevotella and [Ruminococcus] gnavus group (Table 4). Similarly, reported fear reactivity showed negative association with [Ruminococcus] gnavus group (Table 4). Furthermore, observed negative reactivity was positively associated with genera Bifidobacterium and Parabacteroides and observed fear was positively associated with genus Clostridium sensu stricto 1 and negatively with genus Lachnoclostridium (Table 4). Statistically significant associations are shown in Figure 4.
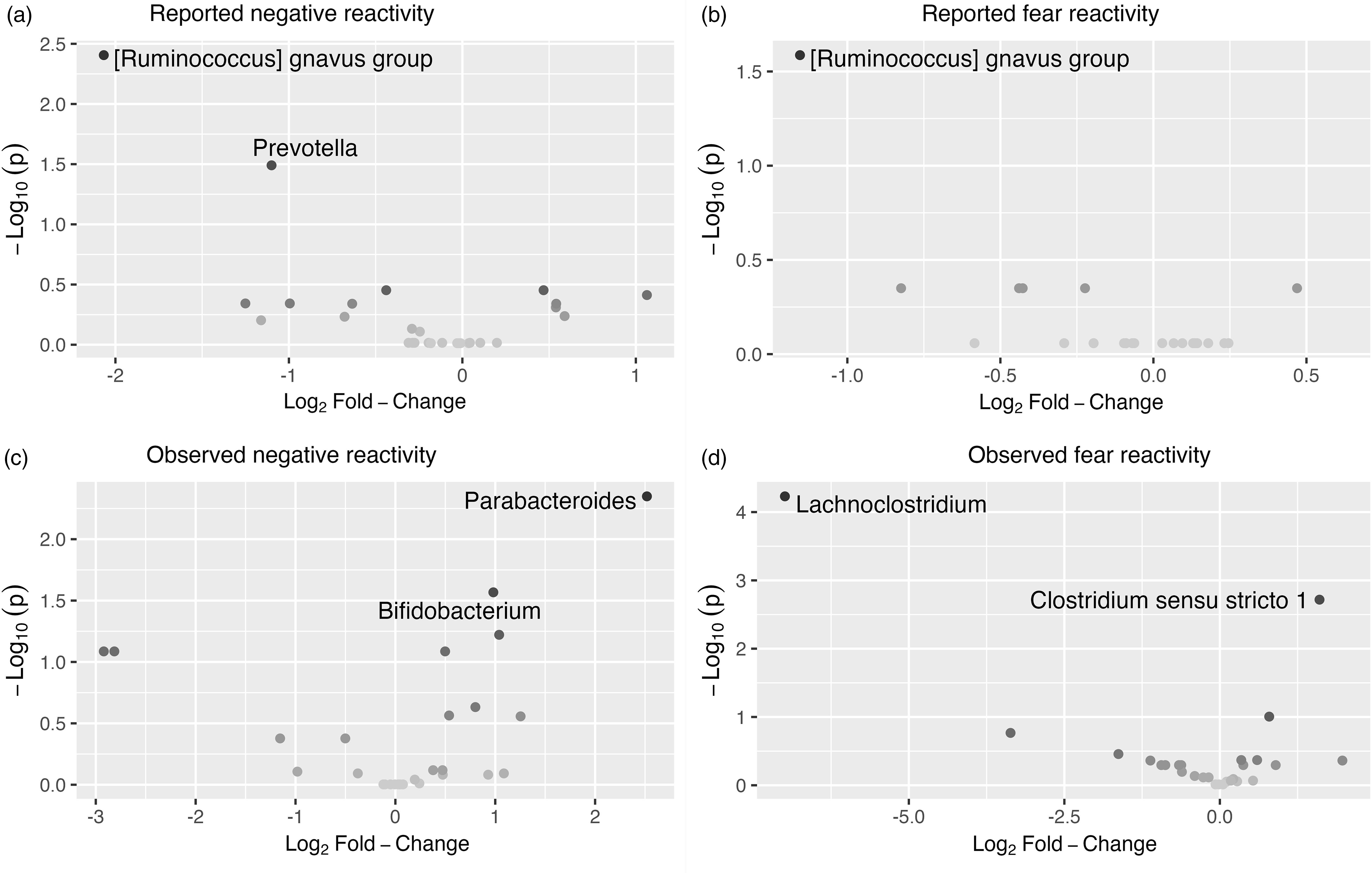
Figure 4. Volcano plots showing associations between genera and reported. ( a ) And observed. ( c ) Negative reactivity as well as reported. ( b ) And observed. ( d ) Fear reactivity. The statistically significant genera are labeled.
When stratified for boys and girls, a more nuanced pattern emerged (Table 4). For girls, reported negative reactivity was not linked with genera but reported fearfulness had negative associations with genera Erysipelatoclostridium, [Clostridium] innocum group and [Ruminococcus] gnavus group as well as some unknown genus (p < 0.05 for all). In addition, for girls, observed negative reactivity positively associated with Parabacteroides, Varibaculum and Lachnoclostridium. Observed fear reactivity in turn was positively associated with genera Clostridium sensu stricto 1 and Clostridioides and negatively associated with genera Varibaculum and Lachnoclostridium (Table 4). For boys, neither of the reported temperament measurements were associated with genera. On the contrary, for boys, the observed negative reactivity was positively associated with genus [Ruminococcus] gnavus group and observed fear reactivity was negatively associated with Eggerthella, Flavonifactor and Clostridioides. These statistically significant associations for girls are shown in Supplementary Figure 5 and for boys in Supplementary Figure 6.
Short-chain fatty acids and negative reactivity and fear
The sum variable of the major SCFAs did not correlate with either reported of observed temperament measurements. Out of all SCFA, only Isobutyric acid showed a negative correlation with observed negative reactivity (ρ = −.28, p = .02). This association remained when one outlier was removed (ρ = −.25, p = .04). However, the strength of the findings attenuated in a linear model with log-transformed isobutyric acid concentration (b = −0.80, p = .08, unadjusted; b = −0.81, p = .10, adjusted for sex, mode of delivery and maternal age). We found no evidence for isobutyric acid by sex interaction (b = −0.85, p = .40). There was a weak signal for isobutyric acid correlating with negative reactivity among girls (ρ = −.38, p = .06) and after removing one outlier, the significance of this correlation decreased further (ρ = −.32, p = .12). However, in an adjusted linear model, log-transformed isobutyric acid concentration was associated with observed negative reactivity (b = −2.07, p = .008) and the association remained when one outlier was removed (b = −2.40, p = .03). SCFAs and reported negative reactivity and fear as well as observed fear reactivity showed no correlations. Figure for Isobutyric acid and observed negative reactivity are included in the supplemental materials (Figure 7).
Discussion
A few previous studies have reported associations between gut microbiota composition and child temperament traits (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019; Carlson et al., Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021; Christian et al., Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015; Fox et al., Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022; Loughman et al., Reference Loughman, Ponsonby, O’Hely, Symeonides, Collier, Tang, Carlin, Ranganathan, Allen, Pezic, Saffery, Jacka, Harrison, Sly and Vuillermin2020; Wang et al., Reference Wang, Chen, Yu, Liu, Zhang and Bai2020), which are constitutional individual differences in reactivity, activity and self-regulation (Rothbart, Reference Rothbart2007). In the current study, we leveraged a larger sample of infants to investigate the potential associations between gut microbiota composition and both observed and parent-reported negative reactivity. We found that gut microbiota diversity and composition is associated with negative reactivity especially in boys. Moreover, the isobutyric acid may associate with negative reactivity and could be a potential mechanistic route, however, replications are warranted. Negative reactivity is related to childhood emotional and behavioral problems, and fear reactivity, an aspect of negative reactivity that has specific relevance for anxiety-related tendencies later in life, and these traits are potential transdiagnostic factors relating to various later mental health problems (De Pauw & Mervielde, Reference De Pauw and Mervielde2010; Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020; Nigg, Reference Nigg2006).
Unlike what we expected based on our previous study (Aatsinki et al., Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019), we found no major associations between either alpha diversity (Shannon index) or community composition and negative reactivity and fear in the whole study population. However, in line with our hypotheses, we found sex-specific associations with alpha diversity. Higher alpha diversity was associated with higher reported fear reactivity for boys but not for girls. Interestingly, since Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) studied mother-reported fear reactivity at the child age of 6 months, and our focus was on mother-reported fear at 12 months, in the current study the reported fear reactivity increased instead of decreasing with higher alpha diversity for boys. Six months of age is approximately the time in development where the trait of fear first emerges, increasing during the second half of first year and peaking at around 10-12 months of age (Gartstein et al., Reference Gartstein, Bridgett, Rothbart, Robertson, Iddins, Ramsay and Schlect2010; Putnam & Stifter, Reference Putnam and Stifter2002). The associations may diverge between these age points, since some individuals may remain at the same (initially high) levels of fear, whereas others may increase in fear until one year of age, leading to a different inter-individual rank order at these different age points. Thus, differing results could be explained by general (and dynamic) developmental trajectories of temperament trait fear in early life. In addition, the development of fear reactivity is shown to have differing trajectories for girls and boys, with girls displaying steeper increases in fear (Gartstein et al., Reference Gartstein, Bridgett, Rothbart, Robertson, Iddins, Ramsay and Schlect2010). This could also explain the differing results in comparison to Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) study, assuming that boys were expressing lower levels of fear reactivity at 6 months of age than girls.
Similarly, we found sex-specific associations between community composition and negative reactivity. For boys, community composition was associated with reported and observed negative reactivity; thus, boys expressing more negative reactivity seem to differ from boys expressing less negative reactivity based on their microbial community compositions. In line with Christian et al. (Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015), our findings were more consistent and stronger among boys than girls. However, Christian et al. (Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015) found differences in community composition in relation to positive reactivity but not with negative reactivity, whereas in this study, the associations were found in relation to negative reactivity. This difference may be explained by the differential continuity of temperament across infancy and childhood. Differential continuity refers to the different phenotypic presentation of the same underlying trait at different points of development (Caspi & Roberts, Reference Caspi and Roberts2001). Interestingly, prior studies have found associations between infant negative reactivity and surgency/extraversion in toddlerhood (Casalin et al., Reference Casalin, Luyten, Vliegen and Meurs2012; Pesonen et al., Reference Pesonen, Räikkönen, Heinonen, Komsi, Järvenpää and Strandberg2008); some traits related with negative reactivity in infancy, such as anger and frustration, may in toddlerhood develop on to more active behavior with approach tendencies that start to reflect the surgency/extraversion dimension (Rothbart et al., Reference Rothbart, Derryberry and Hershey2000). This interpretation would be also supported by a recent study by Fox et al. (Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022) which found association between community composition at child age 1-3 weeks and surgency/extraversion at 12 months of age.
We found associations solely using Bray-Curtis dissimilarity and not with Unifrac or Weighted Unifrac. Since Bray-Curtis dissimilarity measures unshared microbial abundances and Unifrac in turn unshared microbial taxas between two samples and Weighted Unifrac takes into account both abundances and taxas (Bastiaanssen et al., Reference Bastiaanssen, Quinn and Loughman2022) differing results here could be explained by the difference of these indices. Our results refer to differences in microbial abundances instead of specific taxas in relation to negative reactivity for boys. Furthermore, it is important to acknowledge that the effect sizes are small, which is to be expected given the multifactorial nature of temperament. Consequently, the impact of gut microbiota on temperament is limited at best, resulting in small-scale effects. This is in line with other studies, which have shown that the observed effect sizes in beta diversity are relatively small in population based studies (Falony et al., Reference Falony, Joossens, Vieira-Silva, Wang, Darzi, Faust, Kurilshikov, Bonder, Valles-Colomer, Vandeputte, Tito, Chaffron, Rymenans, Verspecht, De Sutter, Lima-Mendez, D’hoe, Jonckheere, Homola and Raes2016).
Overall, previous studies conducted in humans and other animals have suggested sex-specific effects of gut microbiota on brain and behavior (Jaggar et al., Reference Jaggar, Rea, Spichak, Dinan and Cryan2020). For instance, Clarke et al. (Reference Clarke, Grenham, Scully, Fitzgerald, Moloney, Shanahan, Dinan and Cryan2013) found that male mice were more prone to gut microbiota induced alterations in the hippocampal serotonergic system. Similarly, Leclercq et al. (Reference Leclercq, Mian, Stanisz, Bindels, Cambier, Ben-Amram, Koren, Forsythe and Bienenstock2017) found that prenatal antibiotic treatment reduced anxiety-like behavior in male but not female mice, while on the other hand (Champagne-Jorgensen et al., Reference Champagne-Jorgensen, Mian, Kay, Hanani, Ziv, McVey Neufeld, Koren and Bienenstock2020) found that prenatal penicillin treatment decreased anxiety-like behavior in female but not in male mice. Our findings together with this body of research suggest that gut microbiota diversity and composition may have sex-specific associations with the brain structure and functioning, but more research on human subjects is needed to confirm the findings.
Additionally, differential abundance analysis showed a set of associations between different gut bacterial genus and temperament. Higher reported negative reactivity was associated with a decrease of abundances in genera Prevotella and [Ruminococcus] gnavus group. Similarly, higher reported fear in the whole sample and for girls when analyzed separately for boys and girls was linked with a decrease in genera [Ruminococcus] gnavus group. This suggests that the effect found in the whole sample is driven by the association in girls (but not for boys) since the effect size and statistical significance are notably stronger among girls compared to the whole sample. These results highlight again the importance of considering gender as a potential influential factor in interpreting these results.
In one prior study, Loughman et al. (Reference Loughman, Ponsonby, O’Hely, Symeonides, Collier, Tang, Carlin, Ranganathan, Allen, Pezic, Saffery, Jacka, Harrison, Sly and Vuillermin2020) found that decrease in the abundance of the genus Prevotella in infancy was associated with the risk at having more internalizing and externalizing problems at 2 years of age, although Prevotella abundance was not related to preceding temperament assessment, which contradicts findings of the present study. In turn, R. gnavus is a prevalent genus in the infant gut (Nilsen et al., Reference Nilsen, Madelen Saunders, Leena Angell, Arntzen, Lødrup Carlsen, Carlsen, Haugen, Heldal Hagen, Carlsen, Hedlin, Monceyron Jonassen, Nordlund, Maria Rehbinder, Skjerven, Snipen, Cathrine Staff, Vettukattil and Rudi2020; Sagheddu et al., Reference Sagheddu, Patrone, Miragoli, Puglisi and Morelli2016) and in adults, it is found to be less abundant in patients with major depressive disorder (Jiang et al., Reference Jiang, Ling, Zhang, Mao, Ma, Yin, Wang, Tang, Tan, Shi, Li and Ruan2015). Interestingly, for boys, higher observed negative reactivity was positively associated with R. gnavus. Given that higher negative reactivity is a risk factor for later depressive symptoms (Bould et al., Reference Bould, Araya, Pearson, Stapinski, Carnegie and Joinson2014; Compas et al., Reference Compas, Connor-Smith and Jaser2004) our finding corresponds to the study of Jiang et al. (Reference Jiang, Ling, Zhang, Mao, Ma, Yin, Wang, Tang, Tan, Shi, Li and Ruan2015). In the absence of the prior findings in children and the known differences between child and adult gut microbiome, the interpretation of these findings requires caution. A more recent rodent study has also found initial results that R. gnavus could potentially influence brain function through the production of metabolites (Coletto et al., Reference Coletto, Latousakis, Pontifex, Crost, Vaux, Perez Santamarina, Goldson, Brion, Hajihosseini, Vauzour, Savva and Juge2022). This could be one mechanism trough which different genera could affect central nervous system.
An increase in observed negative reactivity was associated with increase in abundance of genus Bifidobacterium and Parabacteroides. Similar findings were found in study by Carlson et al. (Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021), where greater abundance of genus Bifidobacterium at 1 year of age was associated with higher observed fear reactivity using the same assessment as in the present study at 1 year of age. Bifidobacterium and Parabacteroides are prevalent genera in the new-born gut (Bäckhed et al., Reference Bäckhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary, Li, Xia, Xie, Zhong, Khan, Zhang, Li, Xiao, Al-Aama, Zhang, Lee, Kotowska, Colding and Wang2015). In particular, Bifidobacterium is considered an important part of infant gut microbiota and healthy development (Lawson et al., Reference Lawson, O’Neill, Kujawska, Gowrinadh Javvadi, Wijeyesekera, Flegg, Chalklen and Hall2020) and essential for hypothalamic-pituitary-adrenal system (Sudo et al., Reference Sudo, Chida, Aiba, Sonoda, Oyama, Yu, Kubo and Koga2004). Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) found positive association between Bifidobacterium at 2.5 months and reported surgency (positive reactivity) and similarly, Fox et al. (Reference Fox, Lee, Wiley, Lagishetty, Sandman, Jacobs and Glynn2022) reported an association between Bifidobacterium at age 1–3 weeks and reported surgency/extraversion at age 12 months. Christian et al. (Reference Christian, Galley, Hade, Schoppe-Sullivan, Kamp Dush and Bailey2015) in turn found a positive correlation between sociability, a trait belonging to extraversion/surgency continuum, and Parabacteroides for boys. Associations between the principal (PCoA) axes and taxa abundances were similar with decrease in abundance of Bifidobacterium associating with PC3, which in turn correlated negatively with both reported and observed negative reactivity. I.e., higher negative reactivity correlates negatively with PC3 that is associated with less Bifidobacterium suggesting its beneficial role in the gut. Although we recognize that this is descriptive as unsupervised ordination in a single data set has limited generalizability to other data sets, it illustrates the features that show the strongest association with the overall community composition. Taken together, Parabacteroides and Bifidobacterium have been repeatedly related to emotional reactivity in childhood, although both positive and negative reactivity have been implicated, calling for caution in the interpretation.
First, our differential abundance findings could again be related to the differential continuity from negative reactivity in infancy to surgency/extraversion in toddlerhood explained above. Second, the findings could be explained by our as well as Carlson et al.'s (Reference Carlson, Xia, Azcarate-Peril, Rosin, Fine, Mu, Zopp, Kimmel, Styner, Thompson, Propper and Knickmeyer2021) study employing only one observed situation in the assessment of trait-like negative reactivity. Thus, it is possible that child frustration in a restricting situation could be age-appropriate and normative when observed in simply one situation since the same association is not seen with reported traits. More variation in the long-term negative reactivity could be detected when assessing the child across multiple experimental situations. Thus, combining several observations measuring negative reactivity could give a better depiction of observed negative reactivity.
Additionally, an increase in observed fear reactivity was linked with an increase in genus Clostridium sensu stricto 1 and decrease in genus Lachnoclostridium abundance. Similarly, for girls only, an increase in observed fear reactivity was associated with increase in Clostridium sensu stricto 1 and a decrease in Lachnoclostridium as well, but these associations were not observed among boys; thus, the associations among girls might be driving the associations observed among the whole sample. Lachnoclostridium has been connected to the incidence of adenomas (Liang et al., Reference Liang, Li, Nakatsu, Chen, Yau, Chu, Wong, Szeto, Ng, Chan, Fang, Sung and Yu2020). In addition, Aatsinki et al. (Reference Aatsinki, Kataja, Munukka, Lahti, Keskitalo, Korja, Nolvi, Häikiö, Tarro, Karlsson and Karlsson2022) found that higher abundance of Clostridium, genus belonging to the same family Clostridiaceae as Lachnoclostridium, was associated with attention bias towards fearful faces. Further studies are needed to replicate and extend these findings, as well as elucidate the role of different genera belonging to the family Clostridiaceae in these associations.
Several of the associated genera, such as Prevotella and Lachnoclostridium and Clostridium, are able to produce SCFA from dietary fibers (Dalile et al., Reference Dalile, Van Oudenhove, Vervliet and Verbeke2019). Specifically, Prevotella, Bifidobacterium, and Clostridium species are known to produce acetate (Koh et al., Reference Koh, De Vadder, Kovatcheva-Datchary and Bäckhed2016). Nevertheless, we did not observe any associations between temperament traits and the major SCFA. However, we did observe a negative correlation between isobutyric acid and negative reactivity, although the associations attenuated in the adjusted model. Moreover, the results suggest a possible sex-specific association in girls. Isobutyric acid is a branched chain fatty acid that is usually a product of protein fermentation in the gut (Koh et al., Reference Koh, De Vadder, Kovatcheva-Datchary and Bäckhed2016). More specifically, the depletion of readily available carbohydrates for fermentation may induce protein fermentation in the gut. As a result, systemic SCFAs levels may be affected, including the BCFA isobutyric acid. Relatively little is known of the health implications of branched short-chain fatty acids in the infant gut, although a link with immune system functioning and breast milk composition has been suggested (Pekmez et al., Reference Pekmez, Larsson, Lind, Vazquez Manjarrez, Yonemitsu, Larnkjær, Bode, Mølgaard, Michaelsen and Dragsted2020; Tanabe et al., Reference Tanabe, Sakurai, Nakanishi, Kato, Kawasaki, Nakano, Yamaide, Taguchi-Atarashi, Shiko, Takashima, Watanabe, Ochiai, Ohno, Fukuoka, Shimojo and Mori2021). Animal studies have shown that reduction induced by nutritional modifications in caucal isobutyric levels decreased depression- and anxiety-like behaviors, which suggests direct effects that isobutyric acid might have on behavior (Burokas et al., Reference Burokas, Arboleya, Moloney, Peterson, Murphy, Clarke, Stanton, Dinan and Cryan2017). Overall, there is evidence from animal studies suggesting SCFAs role as a mediator between gut microbiota and brain functioning via gut hormones or vagal pathways (Dalile et al., Reference Dalile, Van Oudenhove, Vervliet and Verbeke2019). However, the influence of isobutyric acid on human health and/or immune functioning is insufficiently known. Our results may indicate that isobutyric acid is a target metabolite linking gut microbiota composition and negative emotionality in humans but warrant for replication.
Certain strengths and limitations of this study should be noted. The strengths of this study include a relatively large sample size as well as two different standardized measurements of infant negative reactivity and fear together with inclusion of SCFA measurement. Another strength is a fecal sample from an early developmental timepoint, when the majority of infants were breastfed. This reduced the amount of variation caused by solid nutrition. However, since the breastfeeding status was based on maternal reports, we cannot rule out the possibility of some formula usage which may introduce latent or unmeasured variation in the breastfeeding variable. We also gave careful consideration to important covariates. Nevertheless, this study also has certain limitations. Despite the use of both reported and observed negative reactivity measurements, which reduces the impact of parent bias in temperament ratings, observed negative reactivity measurements were conducted using only one episode in controlled setting, which may not offer very versatile description of negative reactivity. Additionally, observed and reported negative and fear reactivity were assessed at different time points, although the comparison with the findings of Aatsinki et al. (Reference Aatsinki, Lahti, Uusitupa, Munukka, Keskitalo, Nolvi, O’Mahony, Pietilä, Elo, Eerola, Karlsson and Karlsson2019) with partially overlapping dataset was possible and diminished this limitation. Another limitation is the use of 16S rRNA sequencing that does not provide as high of a resolution on the functional capacity of microbiota. Use of shot-gun sequencing or metabolomics would potentially provide more detailed information. Additionally, we used a single fecal sample. Longer follow-ups with several measurement points would offer more detailed information about development of this association.
Taken together, the results of this study add to growing literature of the associations between gut microbiota composition and temperament and early childhood socio-emotional development. We found evidence for sex-specificity in the associations between gut microbiota diversity and composition and negative reactivity, which highlights the importance of including sex as a moderator in the future research. Differences in gut microbiota composition could result in differing effects on brain and behavior depending on child sex, and a better understanding of these relationships could provide new intervention targets to promote beneficial brain development and mental health to be tested in experimental and intervention studies. Another consideration is the effect of temperament development trajectories and its effect on these associations. The emergence and development of fear reactivity is not linear and shows sex-specific trajectories (Gartstein et al., Reference Gartstein, Bridgett, Rothbart, Robertson, Iddins, Ramsay and Schlect2010), and measurements from different age points complicate the interpretation of the findings. Temperament measurements were also associated with relative abundances of certain genera, such as [Ruminococcus] gnavus group, Clostridium sensu stricto 1, Clostridium and Bifidobacterium that have been previously associated with different neurodevelopmental outcomes. Moreover, our results suggest a link between isobutyric acid concentration and negative emotionality, especially in girls. Future research should employ longitudinal follow-ups, including several measurement points of temperament and gut microbiota to further assess the complexity and trajectories of the phenomena.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423001396.











