Obesity is a global epidemic, and the World Health Organization recommends that all persons maintain a normal BMI( 1 ). However, there is ongoing discussion as to whether the WHO recommendations should apply to old (70–79 years) and very old (≥80 years) persons( Reference Dahl, Fauth and Ernsth-Bravell 2 – Reference Singh, Haddad and Tonstad 4 ). Among the older population, those who are underweight generally have a higher risk of death than those of normal weight( Reference Nagai, Kuriyama and Kakizaki 5 , Reference Winter, MacInnis and Wattanapenpaiboon 6 ), and some studies report a survival advantage for people in the overweight range( Reference Flegal, Kit and Orpana 7 – Reference Flicker, McCaul and Hankey 9 ).
These studies of the effect of BMI at older ages emphasise mortality, rather than quality of life or disability. However, obesity is also associated with an increased risk of CVD, cancer, hypertension, stroke, diabetes and other medical problems( Reference Nagai, Kuriyama and Kakizaki 5 , 10 , Reference Roberson, Aneni and Maziak 11 ), and weight loss in older persons may reduce morbidity from these diseases( Reference Elia 12 – Reference Felix and West 15 ). An alternative measure that incorporates both quality and quantity of remaining life is healthy life expectancy (HLE), which estimates the expected remaining number of years of life spent in a state of good health. Life expectancies continue to increase( Reference Collerton, Barrass and Bond 16 ), and research into HLE, as opposed to total life expectancy (TLE), helps to answer the crucial question of ‘whether the extra years gained year on year in life expectancy are healthy years’( Reference Robine and Jagger 17 ). Given the rapidly expanding ageing population( Reference Christensen, Doblhammer and Rau 18 ), there is growing interest in investigating the predictors of HLE in ageing populations, as well as TLE( Reference Robine and Jagger 17 , Reference Robine, Saito and Jagger 19 – Reference Crimmins, Hayward and Hagedorn 22 ). Steensma et al.( Reference Steensma, Loukine and Orpana 23 ) estimated TLE and HLE in adults aged 20–100 years. Although their results were in agreement with many studies in that overweight adults had a slightly longer TLE compared with normal-weight adults, they also found that these adults spent a greater proportion of these extra years in poorer health. Reynolds et al.( Reference Reynolds, Saito and Crimmins 24 ) found that obesity had little effect on TLE in adults aged over 70 years, but that the obese were more likely to become disabled, living a greater proportion of their remaining years with disability, and concluded that obesity-related death is therefore less of a concern than obesity-related disability in this age group. However, another study( Reference Diehr, O’Meara and Fitzpatrick 3 ) that looked at adults aged 65 years and over found that although underweight adults had worse outcomes than normal-weight adults, those who were overweight or obese rarely had worse, and sometimes had better, outcomes than normal-weight adults.
One possible explanation for the differing effects found in these studies may be the wide range of ages investigated. The effect of BMI may differ at extreme old age because of the changing relationship between BMI, disease processes and treatments( Reference Diehr, O’Meara and Fitzpatrick 3 ).
Effects may also differ by sex( Reference Flicker, McCaul and Hankey 9 ), and thus sex-specific estimates are also needed. Women live longer than men, and as the population ages older women will comprise an increasingly larger proportion of the Australian population( Reference Byles, Dobson and Pachana 25 ). Older women also make up a majority of the people with conditions such as dementia, sensory impairment and falls, and they are more likely to need aged care services( Reference Byles, Dobson and Pachana 25 ). Flicker et al.( Reference Flicker, McCaul and Hankey 9 ) found that women experienced lower mortality for all categories of BMI than men, and Nagai et al.( Reference Nagai, Kuriyama and Kakizaki 5 ) found that obese women had a higher risk of death than normal-weight women, but the effect was non-significant for men. Therefore, it is important to explore the effect of BMI on TLE and HLE exclusively in a cohort of very old women. The primary aim of this research is to investigate the relationship between BMI, TLE and HLE (as measured by subjective self-rated health), in a longitudinal study of women born in 1921–1926 with 18 years of follow-up. A secondary goal is to investigate the differences in TLE and HLE for obese women who lose weight.
Methods
Data
Data were from the Australian Longitudinal Study on Women’s Health – a nationally representative, prospective study of over 40 000 participants that commenced in 1996. Cohorts of women, born in 1973–1978, 1946–1951 and 1921–1926, were sampled from the Medicare Australia database and invited to complete the baseline postal survey. Further details on the establishment of the cohorts, and follow-up, have been published elsewhere( Reference Brown, Bryson and Byles 26 – Reference Dobson, Hockey and Brown 28 ). Following the baseline survey, women were resurveyed on a three-yearly basis, and after 2011 the 1921–1926 cohort was surveyed on a 6-monthly basis. This study presents data collected on the 1921–1926 cohort, who completed the baseline survey in 1996 (survey 1) when they were aged 70–75 years and subsequently followed up over 18 years.
At each of surveys 1 through 6, and the first four 6-monthly surveys (surveys returned before 8 November 2013), women were asked to rate their own health as excellent, very good, good, fair or poor. This rating was used to classify participants’ health at each time point as either ‘good’ (a response of excellent, very good, or good) or ‘poor’ (a response of fair or poor). BMI was calculated from self-reported weight and height, and classified according to the World Health Organization categories( 29 ) as underweight (BMI<18·5 kg/m2), normal weight (BMI≥18·5 and <25 kg/m2), overweight (BMI≥25 and <30 kg/m2) or obese (BMI≥30 kg/m2). Death dates were ascertained from the National Death Index (NDI)( Reference Powers, Ball and Adamson 30 ) and censored at the study cut-off date (31 March 2014), this being the last date of reliable death information.
Statistical analysis
TLE, HLE (time spent in a state of ‘good’ self-rated health) and unhealthy life expectancies (UHLE; time spent in a state of ‘poor’ self-rated health) were calculated from modelling the transitions between the three states (good health, poor health and death), over ten surveys (18 years follow-up), using discrete multi-state models implemented in the interpolated Markov chain (IMaCh) software, version 0.98q4( Reference Lièvre, Brouard and Heathcote 31 ). IMaCh was designed to estimate health expectancies( Reference Willekens and Putter 32 ) by estimating an illness–death model, with good health and poor health being ‘transient’ states (participants can move between these states between surveys) and death an ‘absorbing’ state( Reference Jackson 33 ). IMaCh partitions the time intervals between surveys into shorter steps( Reference Jagger, Matthews and Matthews 21 ) and then models the transition probabilities using multinomial logistic regression on age (and other covariates)( Reference Jagger, Matthews and Matthews 21 ). A step-length of 1 month was used to approximate the underlying continuous time process. Missing surveys are accommodated, as the intervals between each survey do not need to be equal( Reference Jagger, Matthews and Matthews 21 , Reference Crimmins, Hayward and Hagedorn 22 ). Baseline BMI was included in the models as a covariate; participants with missing baseline BMI were excluded from the analysis, but a sensitivity analysis was undertaken by backfilling missing baseline BMI with values from later surveys.
Given that weight loss in obese older persons may result in reduced morbidity, a secondary analysis was performed on the subset of obese women, in order to investigate whether weight loss in this group is associated with improved HLE. These women were classified into two groups – those whose BMI category did not change between surveys 1 and 2 and those whose BMI category decreased. TLE, HLE and UHLE were compared for the two groups.
Results
A total of 12 432 women completed the baseline survey. Participants with missing BMI (n 1313) values were excluded, leaving 11 119 women available for analysis, of whom 42 % were still alive at the study cut-off date (31 March 2014) and were censored. A sensitivity analysis was performed to determine the effect of missing BMI by backfilling missing baseline BMI with the nearest reported BMI value (up to survey 4), which recovered 467 women. The results of the sensitivity analysis (n 11 586, not reported) were not substantially different from the original analysis.
Lasagna plots( Reference Jones, Hockey and Mishra 34 ) were created (Fig. 1–4) to show the overall proportion of women in each state, and the changes between states, across BMI categories and across the first six surveys. These plots demonstrate that the normal-weight group has the highest proportion of women starting in good health (74·8 %), whereas the obese group has the smallest proportion (58·1 %). There are few backward transitions from poor to good health (recovery) across any BMI category. The underweight group appears to have the smallest number of transitions from good to poor health, with women being more likely to die than to transition to poor health. In contrast, the obese group appears to have the most transitions from good to poor health over time.

Fig. 1 Lasagna plot for women with normal BMI showing pattern of self-rated health status over surveys 1–6. ![]() , Poor health;
, Poor health; ![]() , good health;
, good health; ![]() , missing;
, missing; ![]() , deceased.
, deceased.

Fig. 2 Lasagna plot for women with underweight BMI showing pattern of self-rated health status over surveys 1–6. ![]() , Poor health;
, Poor health; ![]() , good health;
, good health; ![]() , missing;
, missing; ![]() , deceased.
, deceased.

Fig. 3 Lasagna plot for women with overweight BMI showing pattern of self-rated health status over surveys 1–6. ![]() , Poor health;
, Poor health; ![]() , good health;
, good health; ![]() , missing;
, missing; ![]() , deceased.
, deceased.

Fig. 4 Lasagna plot for women with obese BMI showing pattern of self-rated health status over surveys 1–6. ![]() , Poor health;
, Poor health; ![]() , good health;
, good health; ![]() , missing;
, missing; ![]() , deceased.
, deceased.
Fig. 5 displays the TLE, HLE and UHLE over time for each BMI category. Expected years of unhealthy life outnumber those of healthy life earliest for obese women (about the age of 82 years), and then about the age of 91 years for overweight women. In contrast, for normal-weight and underweight women, years of HLE exceed years of UHLE at all ages. The data for Fig. 5 are provided in the online Supplementary material.

Fig. 5 Total life expectancy (TLE), healthy life expectancy (HLE) and unhealthy life expectancy (UHLE) by BMI category. ![]() , TLE;
, TLE; ![]() , HLE;
, HLE; ![]() , UHLE.
, UHLE.
Overall (regardless of the initial state) TLE at the age of 75 years was not significantly different for normal-weight and overweight women (Table 1), although overweight women, compared with those of normal weight, did have significantly more unhealthy years (0·99; 95 % CI 0·56, 1·42 years) and fewer healthy ones (0·79; 95 % CI 0·37, 1·21 years). Obese women at the age of 75 years also experienced fewer healthy years (2·55; 95 % CI 2·07, 3·03 years) and more unhealthy years (1·46; 95 % CI 0·97, 1·95 years) than normal-weight women, but in addition they also lived fewer years in total (1·09; 95 % CI 0·41, 1·77 years). Compared with normal-weight women, underweight women had 3·38 (95 % CI 2·43, 4·33) fewer years in total, with 2·46 (95 % CI 1·59, 3·33) fewer years healthy, although UNHLE at the age of 75 years was not significantly greater.
Table 1 Total life expectancy (TLE), healthy life expectancy (HLE) and unhealthy life expectancy (UHLE) (years) at the age of 75 years by BMI, overall and by initial state (Years and 95 % confidence intervals)

Table 1 also displays the TLE, HLE and UHLE at the age of 75 years stratified by initial health state (good or poor health). TLE across all BMI categories was greater for women who began in good health than those who began in poor health, with roughly 2 years difference for normal weight, overweight and obese women, and 3 years difference for underweight women. HLE was much greater for those who began in good health compared with those who began in poor health across all BMI categories.
Table 2 displays the effect of BMI on transitioning between good health, poor health and death. Overweight and obese women were more likely to transition from good to poor health than normal-weight women (risk ratio (RR) 1·15; 95 % CI 1·08, 1·23 and RR 1·60; 95 % CI 1·46, 1·75, respectively), whereas the risk for underweight women was not significantly different. There was no difference in the risk of transitioning from good health to death for any BMI category, nor was there any difference in the risk of recovery. However, underweight women were almost twice as likely to die (from poor health) than normal-weight women (RR 1·90; 95 % CI 1·58, 2·28), whereas overweight women were slightly less likely to die (RR 0·84; 95 % CI 0·76, 0·91). There was no difference between normal-weight and obese women in the risk of transitioning from poor health to death.
Table 2 Risk ratios for the effect of BMI on transitioning between health states and death (Risk ratios (RR) and 95 % confidence intervals)
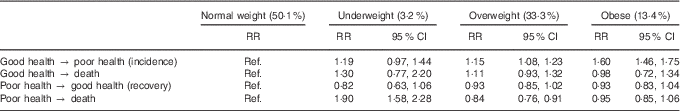
Ref., referent values.
In order to exclude the possibility that the increased risk of mortality for underweight women starting in poor health was because of pre-existing terminal disease, a second sensitivity analysis was performed, based on the sub-group of women who had survived to at least survey 2 (n 10 660). Any underweight women in this sub-sample had therefore been underweight for at least 3 years, which removes the possibility that their underweight status is a recent change resulting from terminal disease. The results of the sensitivity analysis (not reported) showed no substantive difference from the original analysis.
The results of the secondary analysis were not statistically significant, and are included in the online Supplementary material. A total of 1486 women were identified as obese at survey 1, and of these 229 decreased their BMI class at survey 2 (222 became overweight, seven became normal weight and no obese women became underweight). Overall, women whose BMI decreased tended to have slightly higher TLE and HLE. When stratified by initial health state, the biggest difference identified was for women who began the survey in poor health, with almost 1 year of additional HLE added for women who lost weight compared with women who did not. Although this result was not statistically significant because of the relatively small number of obese women who lost weight, the result is clinically significant.
Discussion
We found that, compared with normal-weight women, overweight women at the age of 75 years could expect to have almost one more unhealthy year (0·99; 95 % CI 0·56, 1·42 years) and fewer healthy ones (0·79; 95 % CI 0·37, 1·21 years). However, TLE at the age of 75 years for overweight women was not significantly longer than that for normal-weight women. There was a 16 % reduction in the risk of death for overweight women who started the study in poor health, but those who started the study in good health had no survival advantage. Indeed, these women were 15 % more likely to transition to poor health. Therefore, although being overweight may be slightly protective against death, especially for older women in poor health, it puts all women at a greater risk of poor health in later life, increasing unhealthy years and reducing healthy ones.
Obese women not only had significantly more unhealthy years (1·46; 95 % CI 0·97, 1·95 years) and fewer healthy years (2·55; 95 % CI 2·07, 3·03 years) but also lived 1 year less (1·09; 95 % CI 0·41, 1·77 years), although the increase in TLE appeared to be confined to those initially in good health.
It is clear from our results that there is generally no benefit from being obese, as there was no significant protective effect for death (Table 2), and obesity was associated with a 60 % increased risk of poor health (compared with normal-weight women). These findings support the work by DeCaria et al.( Reference DeCaria, Sharp and Petrella 35 ), who hypothesised that as obesity is associated with several diseases that increase functional disability, the increasing prevalence of obesity will lead to more unhealthy years of life, and greater health-care costs.
It has been suggested that extra weight over a certain age is protective against mortality because of its protective effects against osteoporotic fractures and cognitive decline, and the fact that it may act as an energy reserve, which protects protein-energy malnutrition( Reference Oreopoulos, Kalantar-Zadeh and Shrma 36 ). In our study, this benefit was not observed to extend to women who were obese, perhaps because obesity may have an impact on a person’s physical activity level, which is known to have a positive effect on mortality( Reference Gregg, Cauley and Stone 37 ). Overweight older people may still be able to move well and engage in physical activity, and therefore they are able to reap both the benefits of additional weight and physical activity. Thus, it may be that beyond a certain excess of weight the benefit may be negated by the concurrent reductions in physical activity. Obesity is also associated with high levels of metabolic dysfunction and chronic disease, which will also affect disability and self-rated health( Reference Burton, Foster and Hirsch 38 ).
Finally, our results support the literature that being underweight is linked to poorer health outcomes. Underweight women had the shortest TLE and HLE at 75 years, and were almost twice as likely to die from a state of poor health as normal-weight women. It is possible that this relationship could reflect underlying pre-existing terminal diseases for this BMI group, although results from the sensitivity analysis showed no substantive differences. Interestingly, underweight women had the shortest UHLE; however, this is probably a reflection of their overall shorter TLE, rather than being an advantage per se. For example, underweight women may die faster following disease onset, and thus spend fewer years in an unhealthy state.
This study demonstrates the importance of entering old age in a state of good self-rated health. Regardless of BMI status, TLE and HLE at 75 years were greater for those whose initial health status was good. In addition, no BMI group showed a significant benefit in terms of recovery (transitioning from poor to good health), and no greater risk of death if the initial health status was good.
One limitation of this study is the lack of adjustment for possible confounding variables, such as disease or physical activity. However, as yet this software cannot adjust for confounding factors but rather calculates HLE within the separate strata, which can result in small subgroup sizes. Future work will also investigate the effect of physical activity on TLE and HLE, and the interaction between physical activity and BMI. Another limitation of the study is that BMI was calculated from self-reported height and weight, with potential for under-estimation of weight and BMI category( Reference Stommel and Schoenbprn 39 ).
The secondary analysis examined weight reduction in the obese group. Although these results were not statistically significant, the increase in HLE of 1 year for obese women who started the study in poor health and who lost weight is clinically significant. Our results suggest that the greatest benefits of weight loss in the obese elderly may be achieved for those who are in poor health, whereas only modest gains may be achieved for those who are in good health. However, one complication of looking at BMI change as opposed to baseline BMI is that it can be difficult to differentiate between intentional weight loss (e.g., for obesity-related disorders) and unintentional weight loss (as a result of ill health)( Reference Elia 12 ). Dahl et al.( Reference Dahl, Fauth and Ernsth-Bravell 2 ) found that, compared with older persons whose BMI remained stable, the mortality hazard was 65 % higher for those whose BMI reduced over time, and 53 % higher for those whose BMI increased over time. In addition to the small sample size, the inability to identify intentional weight loss may have contributed to the non-significant findings of the secondary analysis. Weight loss in older adults (aged 70–79 years) can be associated with the loss of lean muscle mass( Reference Newman, Lee and Visser 40 ). However, Felix & West( Reference Felix and West 15 ) state that those obese older adults who are likely to be prescribed weight loss are also more likely to have increased lean mass and bone density, making any potential loss in these areas less of a concern. Further, weight loss in obese older adults has also been associated with improved physical function( Reference Sartorio, Lafortuna and Agosti 41 , Reference Villareal, Banks and Sinacore 42 ), reduced pain( Reference Messier, Loeser and Miller 43 ) and improved mental state and quality of life( Reference Napoli, Shah and Waters 44 ). In addition, many of these results have been shown to be enhanced when combined with exercise( Reference Villareal, Banks and Sinacore 42 – Reference Bouchonville, Armamento-Villareal and Shah 45 ).
The relationship between weight and HLE has important implications for nutrition for older people. Concerns are for obesity, which has serious implications for the older person’s health and quality of life, as well as increasing demands for health services, and for underweight which is very common in older age. Maintenance of lean body mass is important in both scenarios. Because of the possible complications of weight loss in obese older adults, it has been recommended that weight-loss intervention be individualised based on weight-loss history and medical conditions( Reference Bales and Buhr 13 ). We support the concept of individualised assessment, and further suggest that the benefits of a weight-loss intervention may not outweigh the risks for those obese older adults in good health, but that it may be beneficial for obese older adults in poor health. Many older people have insufficient dietary intakes of protein and other nutrients to maintain muscle mass, particularly those with chronic conditions. It is important that obese older persons who are prescribed weight loss, as well as underweight older adults, are provided with sufficient dietary advice with the aim of maintaining lean muscle mass. Older people also have reduced levels of physical activity, which further contributes to loss of muscle mass and strength( Reference Mithal, Bonjour and Boonen 46 , Reference Morris and Jacques 47 ). Under-nutrition is also determined by disease and psychosocial factors including living alone( Reference Schilp, Wijnhoven and Deeg 48 ).
The study used self-rated health, which has been found to be a significant predictor of survival in older Australians and other older populations, and may capture the complex and interacting nature of multiple diseases and varying disease severity among older persons, including frailty, and the subjective value the person places on their quality of life and well-being( Reference McCallum, Shadbolt and Wang 49 ).
Conclusion
This study is significant in determining the effect of BMI on HLE among old women. Although an overweight BMI was associated with slightly reduced mortality among those in poor health (compared with normal weight), among those in good health this excess weight increased the risk of poor health and resulted in fewer years spent healthy and more years unhealthy. Further, being obese had no beneficial effect on mortality, and it greatly increased the risk of poor health. However, weight loss in obese women may be beneficial in increasing HLE for those in poor health. Being underweight was associated with increased mortality. Finally, regardless of BMI, we show that it is important to enter old age in a state of good health. In a time when life expectancy continues to increase, it is important to discover whether these years are spent in good or poor health, as this has implications for both health-care utilisation and for the quality of life older persons can expect. It is therefore recommended that older women maintain a healthy weight, and that public health initiatives continue to promote health and prevent disease and disability in younger adults to ensure that as many as possible enter old age in good health.
Acknowledgements
The research on which this paper is based was conducted as part of the Australian Longitudinal Study on Women’s Health, the University of Newcastle and the University of Queensland. The authors would like to thank Nicolas Brouard, senior researcher at the Mortality, Health and Epidemiology Research Unit, Institut National d’Etudes Démographiques, for his technical advice on the software IMaCh.
The authors are grateful to the Australian Government Department of Health for funding and to the women who provided the survey data. This research was supported by infrastructure and staff of the Research Centre for Generational Health and Ageing, who are members of the Hunter Medical Research Institute. The authors acknowledge the assistance of the Data Linkage Unit at the Australian Institute of Health and Welfare (AIHW) for undertaking the data linkage to the NDI.
L. L. contributed to drafting all sections of the paper, prepped the paper for submission, performed data preparation and analysis and contributed to the literature review, interpretation of results and discussion; J. E. B. contributed to editing and critique of the entire paper, provided substantive expertise and contributed to the literature review, interpretation of results and discussion; C. J. contributed to editing and critique of the entire paper, provided substantive expertise, provided statistical expertise and contributed to the literature review, interpretation of results and discussion.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114516002403










