Article contents
Loomisite, Ba[Be2P2O8]⋅H2O, the first natural example with the zeolite ABW-type framework, from Keystone, Pennington County, South Dakota, USA
Published online by Cambridge University Press: 20 October 2022
Abstract
A new beryllophosphate mineral species, loomisite (IMA2022-003), ideally Ba[Be2P2O8]⋅H2O, was found from the Big Chief mine near Keystone, Pennington County, South Dakota, USA. It occurs as divergent sprays of very thin bladed crystals with a tapered termination. Individual crystals are found up to 0.80 × 0.06 × 0.03 mm. Associated minerals include dondoellite, earlshannonite, mitridatite, rockbridgeite, jahnsite-(CaMnFe) and quartz. No twinning or parting is observed macroscopically. Loomisite is murky white in transmitted light, transparent with white streak and silky to vitreous lustre. It is brittle and has a Mohs hardness of 3½–4, with perfect cleavage on {100} and {$\bar{1}$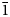 10}. The measured and calculated densities are 3.46(5) and 3.512 g/cm3, respectively. Optically, loomisite is biaxial (+), with α = 1.579(5), β = 1.591(5), γ = 1.606(5) (white light), 2V (meas.) = 82(2)° and 2V (calc.) = 85°. It is non-pleochroic under polarised light, with a very weak (r > v) dispersion. The mineral is insoluble in water or hydrochloric acid. An electron microprobe analysis, along with the BeO content measured with an ICP-MS, yields an empirical formula (based on 9 O apfu) (Ba0.96Ca0.06)Σ1.02[(Be1.96Fe0.06)Σ2.02P1.99O8]⋅H2O, which can be simplified to (Ba,Ca)[(Be,Fe)2P2O8]⋅H2O.
10}. The measured and calculated densities are 3.46(5) and 3.512 g/cm3, respectively. Optically, loomisite is biaxial (+), with α = 1.579(5), β = 1.591(5), γ = 1.606(5) (white light), 2V (meas.) = 82(2)° and 2V (calc.) = 85°. It is non-pleochroic under polarised light, with a very weak (r > v) dispersion. The mineral is insoluble in water or hydrochloric acid. An electron microprobe analysis, along with the BeO content measured with an ICP-MS, yields an empirical formula (based on 9 O apfu) (Ba0.96Ca0.06)Σ1.02[(Be1.96Fe0.06)Σ2.02P1.99O8]⋅H2O, which can be simplified to (Ba,Ca)[(Be,Fe)2P2O8]⋅H2O.
Loomisite is monoclinic, with space group Pn and unit-cell parameters a = 7.6292(18), b = 9.429(2), c = 4.7621(11) Å, β = 91.272(5)°, V= 342.47(14) Å3 and Z = 2. Its crystal structure is characterised by a framework of corner-sharing PO4 and BeO4 tetrahedra. The framework can be considered as built from the stacking of sheets consisting of 4- and 8-membered rings (4.82 nets) along [001] or hexagonal layers (63 nets) along [010]. The extra-framework Ba2+ and H2O are situated in the channels formed by the 8-membered rings. Topologically, loomisite represents the first natural example with the zeolite ABW-type framework, which is adopted by over 100 synthetic compounds with different chemical compositions.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © University of Arizona, 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: G. Diego Gatta
References
- 2
- Cited by


