There is increasing evidence elucidating the role of diet during pregnancy on the growing fetus( Reference Blumfield, Hure and MacDonald-Wicks 1 , Reference Moore and Davies 2 ) and subsequently, in the offspring metabolic health in adulthood( Reference Maslova, Rytter and Bech 3 ). Maternal diet in pregnancy is suggested to contribute in the alteration of fetal outcomes( Reference Kjøllesdal and Holmboe-Ottesen 4 ), including birth weight( Reference Brantsæter, Haugen and Myhre 5 ), preterm delivery( Reference Englund-Ögge, Brantsæter and Sengpiel 6 ), low birth weight infants (<2500 g)( Reference Chen, Wu and Neelakantan 7 ) and small-for-gestational-age (SGA) births( Reference Thompson, Wall and Becroft 8 ). Meta-analyses( Reference Klemmensen, Tabor and Østerdal 9 – Reference Lu, Lu and Li 11 ) have examined the role of micronutrients in the maternal diet, including vitamin C( Reference Klemmensen, Tabor and Østerdal 9 ), iron( Reference Alwan, Greenwood and Simpson 12 ) and folate( Reference Kawai, Spiegelman and Shankar 13 , Reference Fekete, Berti and Trovato 14 ) in the development of adverse birth outcomes. Amongst dietary macronutrients, evidence has been restricted to exploring the use of protein-energy supplementation in pregnancy for improving offspring birth weight amongst low-income countries( Reference Imdad and Bhutta 15 – Reference Liberato, Singh and Mulholland 17 ). However, amongst high-income countries the prevalence of maternal and infant protein-energy under nutrition is low due to sufficient macronutrient consumption during pregnancy( Reference Blumfield, Hure and Macdonald-Wicks 18 ).
Although during pregnancy, in well-nourished women, the recommended dietary allowances of protein, carbohydrate (CHO) and fat are largely met( Reference Brantsæter, Haugen and Myhre 5 , Reference Blumfield, Hure and Macdonald-Wicks 18 , Reference von Ruesten, Brantsæter and Haugen 19 ), the influence of the source of energy intake: macronutrients during pregnancy on birth outcomes including birth weight remains unclear. The specific source of energy (dietary protein, fat and CHO) consumed may also have a differential impact on birth outcomes( Reference Moore and Davies 2 , Reference Moore, Davies and Willson 20 – Reference Lagiou, Tamimi and Mucci 23 ). Evidence remains inadequate and conflicting from previous observational studies( Reference Moore and Davies 2 , Reference Moore, Davies and Willson 20 – Reference Lagiou, Tamimi and Mucci 23 ) that investigated the potential association between energy composition of food consumed during pregnancy and birth weight. Studies have also explored the effect of macronutrient/energy-dense dietary patterns in pregnancy( Reference Englund-Ögge, Brantsæter and Sengpiel 6 , Reference Thompson, Wall and Becroft 8 , Reference Knudsen, Orozova-Bekkevold and Mikkelsen 24 ) on birth outcomes. These ‘western’ or ‘junk’ dietary patterns in the studies, included energy-dense food items, for instance, sweet snacks, desserts, bakery products and processed foods, were suggested to have negative implications on the quality of birth outcome. Amongst macronutrient sub-components, results remain conflicting in studies which explored the effect of fatty acids, including long chained PUFA (LC-PUFA) on birth outcomes( Reference De Giuseppe, Roggi and Cena 25 – Reference Mani, Dwarkanath and Thomas 29 ). In addition, no studies, to our knowledge, have explored the effect of dietary saccharides (monosaccharides, disaccharides, dietary fibres) during pregnancy on birth outcomes including birth weight or ‘customised’ birth weight centiles – computer generated antenatal growth charts for individual pregnancies that allow variation in the maternal characteristics, taking birth weights from previous pregnancies into consideration( Reference Gardosi, Chang and Kalyan 30 ). Customised birth weight centiles are used in this study as they set individual standards for fetal growth that allow better differentiation between optimal and abnormal growth in utero ( Reference Gardosi 31 ). This method adjusts for a number of variables including maternal height, weight, parity, sex of the baby, ethnicity, and across all gestational ages. Using this external adjustment is particularly useful for some categories, such as minor ethnic groups which require large numbers from which to derive precise model coefficients.
We aimed to investigate the association between intakes of specific dietary macronutrients (CHO, fat and protein, and their sub-components such as saccharides and fatty acids) during pregnancy in a well-nourished population and birth outcomes: birth weight, birth centile, SGA infants and large-for-gestational-age (LGA) infants.
Methods
Study design and population
The CAffeine and REproductive health (CARE) study prospectively recruited low risk pregnant women from two large teaching hospital maternity units in Leeds, UK from September 2003 to June 2006( Reference Boylan, Dolby and Greenwood 32 , Reference Greenwood, Alwan and Boylan 33 ). This study was designed to explore diet with a focus on maternal caffeine intake in relation to fetal growth. The inclusion criteria were pregnant women aged between 18 and 45 years and carrying singleton pregnancies accurately dated by ultrasound. Women with concurrent medical disorders, psychiatric illness, HIV infection, or hepatitis B infection were excluded. Participants completed a consent form indicating their willingness to participate in the study. They were interviewed by research midwives during their booking appointment in the antenatal clinic. Questionnaires for trimester 1 (8–12 weeks of gestation) and 3 (from 28 weeks of gestation) were interviewer-administered, and the questionnaire for trimester 2 (13–27 weeks of gestation) was self-administered( Reference Alwan, Greenwood and Simpson 34 ). Their demographic details (age, parity, maternal height, weight, socioeconomic status, and gestational age) were self-reported by means of an interviewer-administered questionnaire. Ethical approval was obtained from Leeds West Local Research Ethics Committee (LREC), Directorate of Research and Development, Leeds, LREC Ref 7260. Participants gave signed informed consent before enrolment into the study.
Dietary data
Out of 1289 participants in the original study, dietary information was available for 1196 women in trimester 1 and 575 women in trimester 2. The dietary intake was collected at home twice in a 24-h dietary recall( Reference Greenwood, Alwan and Boylan 33 – Reference Boylan, Cade and Kirk 35 ) administered by a trained research midwife; once during trimester 1 (8–12 weeks of gestation) and again during trimester 2 (13–27 weeks of gestation). Trained personnel entered the 24-h dietary recalls by using nutrient analysis package – ‘DANTE’ (Diet and Nutrition Tool for Evaluation). The nutrient analysis computed by this software package was based on the standard UK food composition tables by the Royal Society of Chemistry( Reference Holland, Unwin and Buss 36 ).
Primary exposures were macronutrients: protein, fat and CHO and their sub-components including fatty acids and saccharides. The CHO sub-components included mono-saccharides (glucose, fructose), disaccharides (sucrose, maltose and lactose), and complex sugars (starch, soluble fibre). The dietary fat sub-components included SFA, MUFA and PUFA. However, total protein was considered for sub-component analyses as the data for animal and vegetable protein, and amino acid contents were unavailable.
Other data
Questionnaires administered by trained midwives included information on confounders such as smoking habits, alcohol consumption and other information such as episodes of nausea. The multiple linear regression models were adjusted for smoking status( Reference Cogswell, Weisberg and Spong 37 ) and alcohol intake( Reference Nykjaer, Alwan and Greenwood 38 ) due to their adverse effects on infant and prenatal nutrition. Smoking status for trimesters 1 and 2 listed the frequency of smoking and was categorised into three: ‘non-smoker’, ‘current smoker’ and ‘occasional smoker – previously smoked everyday but do not smoke now’. The participant’s average alcohol consumption (units/d) (continuous variable) was measured during trimester 1 and 2. Physical activity was self-reported and was recorded into three categories: ‘no weekly physical activity’, ‘light/moderate physical activity’ and ‘vigorous physical activity (up to <20 min 1–2/week).’ Three questionnaires were administered to determine lifestyle behaviours with a focus on caffeine intake in pregnancy from 4 weeks before pregnancy until recruitment into the study at 8–12 weeks of pregnancy; the second covered the period 13–27 weeks; and the third included the period from 28–40 weeks of pregnancy( Reference Alwan, Greenwood and Simpson 34 ).
Outcome: birth weight, birth centile, small-for-gestational-age and large-for-gestational-age births
The information on antenatal pregnancy complications and delivery details (gestational age at delivery, birth weight, and sex of the baby) were obtained from the electronic maternity databases. The primary outcomes in our study were birth weight and birth centile. Birth weight was recorded in g in the electronic maternity database. The customised birth centiles were computed by using customised centile charts( Reference Gardosi 31 , 39 ) which accounted for the following factors: maternal weight, height, ethnicity, parity, gestational age at delivery and sex of the baby. Other outcomes additionally explored were SGA births and LGA births (refer supplementary material). These particular definitions were chosen as they are clinically relevant amongst at-risk infant groups. On the customised centile chart, SGA birth was defined as birth weight <10th centile( Reference Gardosi, Chang and Kalyan 30 , Reference Gardosi 31 , Reference Clausson, Gardosi and Francis 40 ), and LGA birth was defined as birth weight >90th centile( Reference Gardosi, Chang and Kalyan 30 , Reference Pasupathy, McCowan and Poston 41 ). Both of these outcomes accounted for the following variables: maternal height, weight, ethnicity, parity, gestational age at delivery and sex of the baby( Reference Gardosi 31 ).
Statistical analysis
We calculated the means and standard deviations, and absolute frequency distributions with percentages (n (%)) for demographic characteristics of interest (Table 1 in results). To examine associations between macronutrients or their sub-components, and birth weight/centile; multiple linear regression models (models 1 and 2) were designed for trimesters 1 and 2 separately. Each macronutrient and its sub-component model were adjusted for other energy contributing macronutrients and sub-components within the model. In order to help with the interpretation of birth centiles, we have additionally presented these results in actual birth weight in g. In the centile model (model 1) we made use of customised centile charts( Reference Gardosi 31 , 39 ) which automatically accounted for these variables: maternal height, weight, parity, ethnicity, gestational age at delivery and sex of the baby. The birth weight model (model 1) was adjusted for maternal height, weight, parity, ethnicity, gestational age at delivery and sex of the baby. All regression models (birth weight/centile models) under model 2 were additionally adjusted for participants’ alcohol consumption and smoking habits in pregnancy.
Table 1 Characteristics of the participants and their infants in the CAffeine and REproductive health study (Mean values and standard deviations; numbers and percentages)
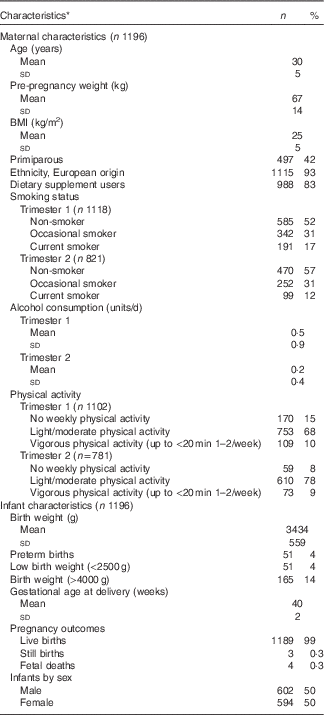
* Results of the descriptive statistics have been restricted to participants included in the later analyses.
We carried out logistic regression analyses to explore the OR for delivering an SGA/LGA infant. In the logistic regression models, SGA and LGA births were binary outcomes. Model 2 additionally adjusted for alcohol intake and smoking habits.
The results of the macronutrient consumption (CHO, fat and protein) were presented for 10 g/d increments, and sub-components of dietary fat and CHO were presented for 1 g/d increments. However, couple of sub-components consumed in higher amounts: starch and glucose intakes were presented for 10 g/d increments. The statistical significance level for the results was set at 5 %. All analyses were performed using Stata SE, version 13.1 (1985–2013; StataCorp).
Results
Baseline characteristics
The CARE study analyses included 1196 women in trimester 1, amongst which trimester 2 included 575 women (45 % lost to follow-up). The descriptive characteristics of 1196 participants in our analyses are similar to the remaining non-participants in the original cohort.
The mean age of the women in this cohort was 30 (sd 5) years, with (42 %, n 540) being primiparous (Table 1). A majority of the cohort were of European origin (93 %, n 1202). The mean BMI of the participants measured at baseline was 25 (sd 5) kg/m2. A majority of women (98 %, n 1171) were employed; one third (39 %, n 472) of the participants completed university degree as the highest level of education. Approximately half of the cohort (52 %, n 585) were non-smokers during pregnancy, and approximately 68 % (n 753) and 78 % (n 610) did light/moderate physical activity in trimesters 1 and 2 respectively. Amongst the neonates, the mean birth weight was 3434 (sd 559) g, and the mean gestational age at delivery was 40 weeks (sd 2). Around 4 %, (n 51) infants were termed low birth weight (<2500 g) and preterm births respectively, and approximately 14 % (n 165) were LGA (>90th centile) infants.
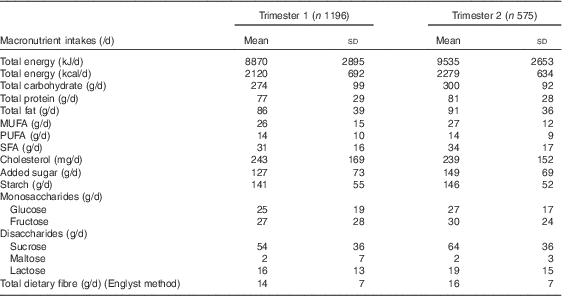
Mean total energy intake per day of the participants in trimesters 1 and 2 were 8870 (sd 2895)kJ (2120 (sd 692) kcal) and 9535 (sd 2653)kJ (2279 (sd 634) kcal), respectively (Table 2). During trimester 1, the mean total CHO, protein and fat intakes per day were 274 (sd 99), 77 (sd 29) and 86 (sd 39) g, respectively. However, during trimester 2 the mean total CHO, protein and fat intakes per day slightly increased to 300 (sd 92), 81 (sd 28) and 91 (sd 36) g, respectively. There was a slight increase in mean added sugar intake per day from 127 (sd 73) g in trimester 1 to 149 (sd 69) g in trimester 2.
Table 2 Dietary macronutrient intakes of the CAffeine and REproductive health study participants in trimesters 1 and 2 (Mean values and standard deviations)
Relationship between macronutrients, and birth centile/birth weight
We observed associations between trimester 1 macronutrient intake and both birth centile and birth weight (Table 3). In trimester 1, there was a positive association between CHO consumption and birth centile/birth weight. The fully adjusted models (model 2) indicated that a higher intake of CHO (10 g/d increment) was associated with a higher birth centile (0·2; 95 % CI 0·1, 0·4; P=0·002) and a higher birth weight (4 g; 95 % CI 1, 7; P=0·003). Conversely, a higher total fat intake (10 g/d increment) at this stage of pregnancy was negatively associated with birth centile (−0·7; 95 % CI −1·2, −0·1; P=0·008) on the customised centile chart. However, on further adjusting the model for alcohol intake and smoking habits (model 2), higher fat intake (10 g/d increment) was not associated with birth centile (−0·5; 95 % CI −1·0, 0·0; P=0·06) in spite of narrow CI. When we explored its relation with birth weight, fat consumption (10 g/d increment) was negatively associated with birth weight (−8g; 95 % CI −16, −0·3; P=0·04) in the fully adjusted model (model 2). Amongst other macronutrients, protein intake was not associated with birth centile or birth weight after adjusting for smoking status and alcohol intake, but it had wide CI.
Table 3 Association between macronutrients (g) in trimester 1 and 2, and birth centile/birth weight (Birth centiles/birth weights and 95 % confidence intervals)
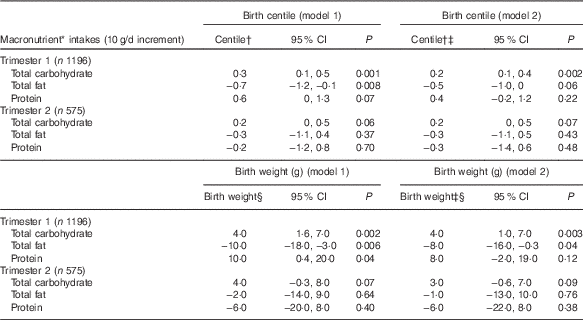
* Mutually adjusted for other energy contributing macronutrients.
† Adjusted using customised growth charts for maternal weight, height, ethnicity, parity, gestational age at delivery, sex of baby.
‡ Additional adjustment for average alcohol intake and smoking status.
§ Adjusted for maternal weight, height, ethnicity, parity, gestational age at delivery, sex of baby.
The odds of delivering a SGA infant were positively associated with a high-fat consumption (10 g/d increment), unadjusted OR 1·05 (95 % CI 1·00, 1·10; P=0·03). However, after adjusting the model (model 2) the odds of delivering a SGA infant (adjusted OR 1·03, 95 % CI 0·98, 1·09; P=0·14) were not associated with a high fat intake (10 g/d increment). Our analyses showed no evidence of an association between macronutrient intake, and the risk of giving birth to LGA infants.
Relationship between macronutrient sub-components, and birth centile/birth weight
In trimester 1 (model 2) (Table 4 and 5), among the complex CHO sub-components, higher starch intake (10 g/d increment) was positively associated with birth centile (0·3; 95 % CI 0·0, 0·7; P=0·05) but not with birth weight (5 g; 95 % CI −0·6, 10; P=0·08). Amongst saccharides, higher lactose intake (1 g/d increment) was associated with a higher birth centile (0·1; 95 % CI 0·0, 0·2; P=0·03) and not with higher birth weight (2 g; 95 % CI −0·1, 4 g; P=0·06). In trimester 2 (model 2), higher glucose (10 g/d increment) consumption was positively associated with a higher birth weight (52 g; 95 % CI 4, 100; P=0·03). Lactose intake (1 g/d increment) was positively associated with a higher birth centile (0·2; 95 % CI 0·0, 0·4; P=0·01) and birth weight (5 g; 95 % CI 2, 7; P<0·001). Amongst fat sub-components in trimester 1 (model 2), a higher PUFA intake (1 g/d increment) was negatively associated with birth weight (−4g; 95 % CI −8, 0·1; P=0·05) but not with birth centile.
Table 4 Association between macronutrient sub-components during trimester 1 and 2, and birth weight (Birth weights and 95% confidence intervals)
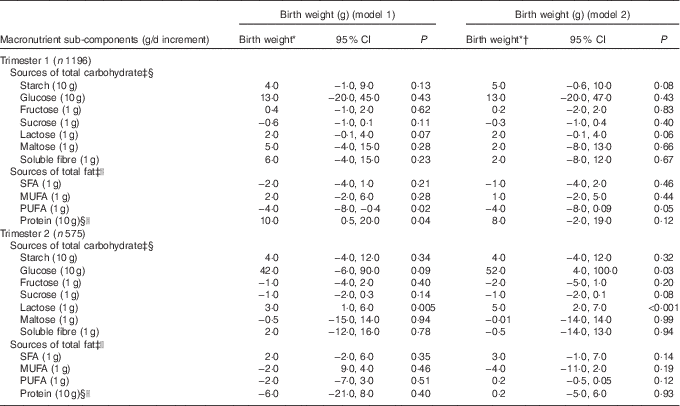
* Adjusted for maternal weight, height, ethnicity, parity, gestational age at delivery, sex of baby.
† Additional adjustment for average alcohol intake and smoking status.
‡ Adjusted for dietary protein intakes.
§ Adjusted for dietary fats intakes.
|| Adjusted for carbohydrate intakes.
Table 5 Associations between macronutrient sub-components in trimesters 1 and 2, and birth centile (Birth centiles and 95 % confidence intervals)
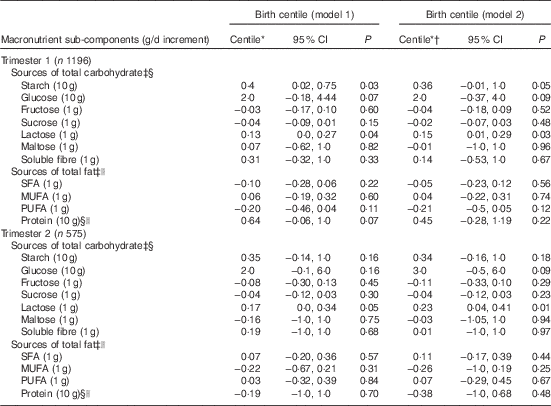
* Adjusted using customised growth charts for maternal weight, height, ethnicity, parity, gestational age at delivery, sex of baby.
† Additional adjustment for average alcohol intake and smoking status.
‡ Adjusted for dietary protein intakes.
§ Adjusted for dietary fats intakes.
|| Adjusted for carbohydrate intakes.
Discussion
This analysis has shown that dietary macronutrient composition and its sub-components could be associated with birth outcomes. To our knowledge, this is the first observational study to explore relationships between dietary macronutrient sub-components in pregnancy and birth outcomes, including birth weight and birth centile. These associations were mostly observed in trimester 1. A possible explanation for this might be that placentation is established and the fetal growth programmed in trimester 1( Reference Kroener, Wang and Pisarska 42 – Reference Smith 44 ). Up to 11 weeks of gestation, the embryo develops in a stable nutritional environment. This may explain why the associations seem to weaken or disappear in trimester 2. Early pregnancy reflects infant organ developmental stages, where the overall energy intake may be less important than the quality of diet. So it might be that the diets high in CHO and fat might just reflect poorer quality diets. In addition, 45 % women in trimester 2 (n 575) were lost to follow-up as fewer women responded to the request for a second 24-h dietary recall, as communication at this point with the women was by post rather than a study visit. Despite this, the size of the estimates and CI were similar between trimesters 1 and 2. In trimester 2, glucose and lactose were associated with increasing birth weight, this might be attributed to the increased availability of free maternal glucose ready to be utilised as a primary source of energy to meet fetal demands required for organ growth during this period( Reference Brantsæter, Olafsdottir and Forsum 45 – Reference Hay 49 ).
Higher intakes of total CHO during trimester 1 was associated with higher birth weight and an increase in birth centile. This finding in our study is in agreement with literature. A study reported similar associations between low contribution of CHO to total energy during pregnancy and thinness at birth( Reference Langley-Evans and Langley-Evans 50 ). Another study reported that high percentage (%) of energy from CHO in the diet could be associated with high offspring birth weight( Reference Moore, Davies and Willson 20 ).
Interestingly, amongst monosaccharides, we observed that in trimester 2 additional consumption of dietary glucose was associated with heavier birth weight. A similar association was observed in a study( Reference Kerssen, de Valk and Visser 51 ) amongst pregnant women with type 1 diabetes mellitus. They reported an association between increased maternal glucose levels amongst diabetic pregnant women and LGA offspring. In our study, we observed that high intake of starch was associated with increased odds of delivering LGA infants. According to a study( Reference Butte 52 ) which compared normal v. pregnant women with gestational diabetes mellitus (GDM), participants who consumed a CHO-rich diet were likely to have high blood glucose levels, and an increased risk of delivering LGA offspring. Randomised controlled trials (RCT) have reported possible effects of a high CHO intake v. a low CHO intake amongst women with GDM and increased risk of macrosomia( Reference Combs, Gunderson and Kitzmiller 53 , Reference Walsh, McGowan and Mahony 54 ). A possible explanation for these results could be that high CHO intakes could lower maternal insulin sensitivity, making higher levels of free glucose available for placental circulation, subsequently activating fetal glycogenesis( Reference Clapp 55 ). Pedersen( Reference Pedersen 56 ) attributed the role of maternal hyperglycaemia to this birth outcome which reportedly caused increase in fetal insulin levels and led to fetal hyperglycaemia.
A high lactose intake might be attributed to high milk and dairy product intake by the women. The Danish National Birth Cohort study( Reference Olsen, Halldorsson and Willett 57 ) explored the association between maternal milk and dairy products consumption with birth weight among 50 117 mother–infant pairs and found that higher dairy consumption promoted higher birth weight. Another study came to a similar conclusion suggesting a decreased risk of SGA( Reference Olmedo-Requena, Amezcua-Prieto and Luna-Del-Castillo 58 ). Additional lactose consumption (in the form of dairy products) leading to a higher birth weight could also be related to higher iodine levels found in milk and dairy sources in the UK( Reference Bath, Hill and Goenaga Infante 59 , Reference Yang and Huffman 60 ). Iodine levels could influence birth weight( Reference Rydbeck, Rahman and Grandér 61 , Reference Andersen, Olsen and Wu 62 ) through a role in controlling metabolic rate and development of body structures( Reference Xiao, Sun and Li 63 ). The lactose association observed may also be indirectly attributed to the level of placental calcium transferred to the fetus( Reference Kovacs 64 ), increasing bone calcification during skeletal development, and overall birth weight( Reference Sabour, Hossein-Nezhad and Maghbooli 65 ).
Unlike previous studies( Reference Blumfield, Hure and MacDonald-Wicks 1 , Reference Moore, Davies and Willson 20 , Reference CucÓ, Arija and Iranzo 22 ) which reported an association with protein, our study did not find any evidence of an association between protein and birth weight/centile, and LGA/SGA. Although we found a positive association between protein intake and birth weight under model 1 during trimester 1, no association was observed after adjusting for alcohol and smoking habits, but the CI were wide. Our study participants were adequately nourished, hence this might be the reason we did not notice any effects. A study( Reference Moore, Davies and Willson 20 ) suggested that the energy contribution from protein in the diet is associated with increased birth weight and placental weight. They considered the type of protein such as animal/vegetable protein but their results were of low statistical power, and did not adjust for mother’s alcohol consumption. However, in support of our finding, a study( Reference Chong, Chia and Colega 21 ) in Asia found no evidence of an association between protein intake in pregnancy and offspring weight.
Our analyses suggest that total fat intake and its sub-components such as PUFA were associated with lower birth weight and birth centile. However, our result conflicts with a South-Asian study( Reference Mani, Dwarkanath and Thomas 29 ) which reported a positive association between dietary fat intake in pregnancy and increased birth weight. Contradicting results from other studies( Reference Moore, Davies and Willson 20 , Reference Chong, Chia and Colega 21 , Reference Lagiou, Tamimi and Mucci 23 ) reported no association between them; an observational study( Reference Moore, Davies and Willson 20 ) explored the relation between energy percentage (%) from total dietary fat and birth weight, and suggested no evidence of an association after adjusting for other energy contributing nutrients. Our analysis adjusted for alcohol, as it is associated with increased risk of lower birth weight( Reference Chiaffarino, Parazzini and Chatenoud 66 – Reference Nykjaer, Alwan and Greenwood 68 ) and fat-rich foods are often consumed with alcohol. Conversely, the study by Moore et al.( Reference Moore, Davies and Willson 20 ) did not adjust for alcohol consumption during pregnancy. However, amongst RCT on animal models, there is no evidence suggesting an association between a high-fat diet in pregnancy and changes in birth weight. Previous studies( Reference Khan, Dekou and Douglas 69 , Reference White, Purpera and Morrison 70 ) based on animal models explored the effect of a high-fat diet in pregnancy on the development of offspring metabolic disorders including hyperinsulinaemia, blood pressure, and changes in serum leptin levels. An RCT( Reference White, Purpera and Morrison 70 ) amongst pups, explored the effects of high-fat diet on offspring and suggested that maternal adiposity and not dietary fat per se, was associated with increased offspring weight, and metabolic disorders such as hyperinsulinaemia which could further persist through adulthood. During trimester 1, higher PUFA intake was associated with lower birth weight of infants. Three studies( Reference Blumfield, Hure and MacDonald-Wicks 1 , Reference Newman, Bryden and Fleck 71 , Reference Nuernberg, Breier and Jayasinghe 72 ) discussed the ‘anti-obesogenic’ property of PUFA during pregnancy which reportedly prevented extra fat mass deposits in the fetus. Ethical issues make studies of this nature challenging in humans such as acidosis and ketosis in response to low-CHO-high-fat diets, alterations in cholesterol and free fatty acid metabolism in pregnancy. Further studies are needed to validate our result.
The CARE cohort was a well-nourished group; the participants’ average dietary macronutrient intake/d during trimesters 1 and 2 largely met the estimated average requirements of energy recommended during pregnancy in the Committee on Medical Aspects of Food Policy report by the Department of Health, UK( 73 ), and the intakes were similar to those found in other studies involving pregnant women( Reference Moore, Davies and Willson 20 , Reference Langley-Evans and Langley-Evans 50 , Reference Chen, Tint and Fortier 74 ). Our previous publication of results made use of the specially designed questionnaire to capture caffeine intake, which demonstrated that maternal caffeine intake was inversely associated with birth weight( Reference Boylan, Dolby and Greenwood 32 ). We chose to use the 24-h dietary recall which was also collected, to measure the whole dietary intake of our participants in detail on a specific day. Alternative approaches such as a FFQ were not available for the whole diet in this sample and require participants to subjectively average out a potentially varied diet over a longer period of time. A number of validation studies( Reference Hartman, Brown and Palmgren 75 – Reference Karvetti and Knuts 78 ) have shown that 24-h dietary recall is a well-established method which correlates well with true usual intake, and are adequate and suitable to large populations rather than individuals. Though this method is less suited to episodically consumed foods, it has been shown to work well for commonly consumed foods and nutrients, particularly macronutrients, present in most food items that are the subject of this current research( Reference Hartman, Brown and Palmgren 75 , Reference Beer-Borst and Amadò 76 ).
The estimates of change in birth weight by macronutrient intake are small because we have chosen a small macronutrient increment/d (10 g is 1/10th of a standard deviation). Using a larger increment for all macronutrients, such as 100 g/d, equivalent to 1 sd, would be associated with an increase in birth weight of about 40 g. Such a change in birth weight might have a modest impact on preterm infants or those already having low birth weight, but need not be of great concern to infants with a better starting point. Furthermore, it is essential to consider that small effects on a population level could be important( Reference Rose 79 ), through shifting the whole distribution of birth weights, higher or lower depending on the type of macronutrient consumed.
Strengths and weaknesses
Our study had some strengths to be considered. This is a large cohort comprising of 1196 pregnant women, from whom dietary data were collected on two occasions during their pregnancy, that is in trimesters 1 and 2. Diet was assessed using an interviewer led 24-h recall; allowing detail of food types and amounts to be recorded. The regression models were carefully adjusted for potential confounders: alcohol intake, smoking habits, maternal height, weight, parity, ethnicity and sex of the baby. We had detailed dietary information, including values of macronutrient sub-components including saccharides and fatty acids.
There are few limitations to any study which explores nutritional intake. For sub-components, the nutrient values computed in the software using the food composition database( Reference Holland, Unwin and Buss 36 ) may not be accurate or complete. A couple of studies( Reference Deharveng URC, Slimani and Southgate 80 , Reference Cowin and Emmett 81 ) reported issues of missing values for nutrients in databases, including McCance and Widdowson’s food composition database( Reference Cowin and Emmett 81 ). Energy intake estimations from food items and beverages of the participants were based on memory recall and are subjected to mis/under-reporting and bias( Reference Meltzer, Brantsaeter and Ydersbond 82 – Reference Brantsaeter, Haugen and Alexander 84 ). Some studies suggest use of a combination of dietary assessments to cross check the dietary information for correct quantity estimation, measurement uniformity and frequency of consumption( Reference Byers 85 , Reference Hebert, Clemow and Pbert 86 ), however, this is more common where food frequency questionnaires are the main dietary measure. Dietary data in our study was recorded only for trimesters 1 and 2. Data were unavailable for type of protein (animal/vegetable) and amino acid content, which led us to include total protein in the regression models.
Conclusion
These results show that dietary macronutrient composition during pregnancy is associated with birth weight outcomes. CHO and its sub-components such as lactose, glucose and starch were associated with increasing offspring birth weight. Conversely dietary fat and its sub-component – PUFA were associated with decreasing birth weight. Offspring birth weight could be supported through carefully balanced fat and CHO intakes during pregnancy.
Acknowledgements
The authors would like to thank the CARE study participants, members of the CARE Study Group: Sinead Boylan, Vivien A. Dolby, Alastair W. M. Hay, Sara F. L. Kirk, Susan Shires and James D. Thomas, James Walker, Kay L. M. White and Christopher P. Wild from the Centre for Epidemiology and Biostatistics, University of Leeds, research midwives: Viv Dolby and Heather Ong, members responsible for designing the nutritional methods: Sinead Boylan, Sara Kirk, and for database management: Neil Hancock, James Thomas, Ellen Hill and nutritionist students.
This work was supported by the Food Standards Agency, UK (contract no. T01032/33). The Food Standards Agency had no role in the design, analysis or writing of this article.
S. S. S. undertook the project, formulated the research question, performed the statistical analyses of the data and wrote all the drafts of the manuscript. D. C. G. helped formulate the research question and designing the study, supervised the analyses and commented on all the drafts. N. A. B. S. helped formulate the research question and study design, and commented on all the drafts. J. E. C. was the principal investigator of the original CARE Study, formulated the study design and the research question, supervised the analyses and commented on all the drafts.
None of the authors has any conflicts of interest to declare.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003609








