Introduction
Levodopa-carbidopa intestinal gel infusion (LCIG) is an established therapy for Parkinson disease (PD) complicated by motor fluctuations due to the erratic absorption of levodopa tablet. Reference Nyholm1 Multiple studies demonstrated the clinical efficacy of this advanced therapeutic option, with a significant impact on health-related quality of life. Reference Nyholm, Klangemo and Johansson2,Reference Olanow, Kieburtz and Odin3 With increased LCIG adoption worldwide, potential complications due to abnormal vitamin absorption or metabolism have been reported in patients treated with LCIG and associated with peripheral nerve toxicity, as confirmed by a higher than expected prevalence of polyneuropathy. Although underpowered, reported prevalence ranges from 20% to 70% in some studies. Reference Santos-García, de la Fuente-Fernández and Valldeoriola4–Reference Uncini, Eleopra and Onofrj7
Literature data currently available suggest careful monitoring of PD patients in treatment with LCIG, prophylactic administration of vitamins as a strategy to minimize the risk of levodopa-mediated neuronal toxicity, and additional and prompt supplementation with B-complex vitamins at the first clinical sign of polyneuropathy. Reference Santos-García, de la Fuente-Fernández and Valldeoriola4–Reference Merola, Romagnolo, Zibetti, Bernardini, Cocito and Lopiano8
Neurologists are, however, not familiar with vitamins physiology, pathophysiology in deficiency states and corresponding management. In addition, there are no established guidelines with respect to vitamin monitoring and supplementation.
Survey among Healthcare Professionals Treating LCIG Patients
On February 1, 2020, 56 nurses, neurologists, gastroenterologists, and neurology fellows from across Canada with an interest in PD and LCIG gathered in Toronto to exchange best practices. Before (by email) and during (by electronic poll) the meeting, an anonymous survey was conducted in order to capture the attitude of the attendees with respect to vitamins monitoring and supplementation. The survey was anonymous but 38 respondents specified their role (gastroenterologists: 13.2%, neurologists: 42.1%, nurses: 39.5%, and other: 5.3%) as well as the Canadian province of provenience (AB: 5.3%, BC: 7.9%, MA: 5.3%, NB: 5.3%, NL: 10.5%, ON: 28.9%, and QC: 31.6%). The survey identified a large inconsistency among health professionals mirroring the lack of established recommendations at the national and international level (Figure 1). In particular, 15.6% of responders indicated that they don’t order any test before starting LCIG while the majority (53.1%) only rely on blood tests (mainly vitamin B12, B6, and complete blood count in 96.1%, 52.9%, and 47.1%, respectively). In addition, most responders stated that they did not supplement patients with vitamins on a regular basis. Variable opinions were collected in terms of testing frequency and prescription of nerve conduction studies (Figure 1). Finally, the survey identified knowledge gaps such as not recognizing that supplementing vitamins may impact the interpretation of laboratory test (15.6% of survey respondents) and that folate deficiency can only be sufficiently identified when vitamin B12 is normal (31.3%).

Figure 1: Survey among healthcare professionals treating LCIG patients. On February 1, 2020, 56 nurses, neurologists, gastroenterologists and neurology fellows from across Canada with an interest in PD and LCIG gathered in Toronto to exchange best practices. Before (by email) and during (by electronic poll) the meeting, an anonymous survey was conducted in order to capture the attitude of the attendees with respect to vitamins monitoring and supplementation. Abbreviations: *: e.g. patients at risk, patients with pre-existing neuropathy, etc., **: multivitamins provided, #: multiple choices allowed, ##: 97% of the interviewees do not order NCS in non-symptomatic patients (e.g., without neuropathy), CBC: complete blood count, INR: international normalized ratio, LCIG: levodopa-carbidopa intestinal gel, MMA: methylmalonyl CoA, NCS: nerve conduction studies, Pts: patients.
The importance of the topic as well as the results of the survey motivated us to write this manuscript having two aims: (1) to summarize the current knowledge on vitamins physiology, testing and clinical impact of their deficiency/excess; and (2) to propose an opinion-based recommendations for clinicians treating PD patients treated with LCIG.
Vitamins
Vitamins are organic micronutrients that must be supplied in the diet to maintain adequate health. They most often are named by an alphabetical letter and number which identifies structural or functional similarity and/or order of identification. Vitamins can also be classified as fat soluble (A, D, E, K) or water soluble (B, C). Fat soluble vitamins are absorbed, transported, and stored for longer periods of time. In contrast, water soluble vitamins often function as coenzymes, are retained for shorter periods of time and have higher level of urinary excretion. Reference Shenkin, Roberts, Burtis and Bruns9 Suboptimal vitamin levels lead to significant and life-threatening health consequences. Thus, quantitative estimates of daily vitamin intake have been established by the Institute of Medicine. 10 These daily requirements may change in disease states, including states of hypermetabolism or malabsorption which can lead to increased demand for micronutrients. Laboratory quantification of vitamins or alternative functional markers can aid in identifying nutritional status. However, to interpret the laboratory results appropriately, it is important to understand the limitations of these tests and the associated result. While the test can provide a quantitative number, the extent of vitamin depletion before significant physiological changes occurs is poorly characterized. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11 The section below will outline the function, absorption, metabolism, physiological consequences of deficiency, and laboratory testing/analysis for 3 vitamins that are reduced with LCIG therapy.
Vitamin B6
Chemistry and Function
Vitamin B6 exists in three natural forms: pyridoxine (PN), pridoxamine (PM), and pridoxal (PL). Each form becomes phosphorylated to a pridoxal-5’-phosphate (PNP, PMP, and PLP), with PLP being the major form that acts as a coenzymes for synthesis, catabolism, and interconversion of amino acids. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11 Vitamin B6 can function in more than 100 different metabolic reactions of macronutrients including proteins, carbohydrates, and lipids. Some of these reactions include the formation of amines (epinephrine, norepinephrine, serotonin, y-aminbutyrate), sphingomyelin formation, DNA synthesis via folate cycle, trans-sulfuration pathway of homocysteine (Figure 2), biosynthesis of heme and co-enzyme for AST and ALT. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11

Figure 2: Homocysteine metabolism. 5-MTHF, 5-methyltetrahydrofolate; 5,10-MTHF, 5,10-methylene tetrahydrofolate; BHMT; betaine homocysteine methyltransferase; CBS, cystathionine beta-synthase; CT, cystathionine; MS, methionine synthase, MTHFR, methyl tetrahydrofolate reductase; SHMT, serine hydroxymethyltransferase.
Dietary Source and Absorption
Vitamin B6 is found in fortified cereal or oatmeal, potatoes, bananas, watermelon, chicken, turkey, liver, tuna, and trout with a bioavailability of 75%. 10 Sources with lower amounts of vitamin B6 include milk, eggs, and green leafy vegetables. Reference Kant and Block12 Once ingested, the phosphorylated forms of vitamin B6 are hydrolyzed in the intestinal lumen by alkaline phosphatase (ALP) whereas the non-phosphorylated forms are readily absorbed and then transported to the liver. Pyridoxal kinase then phosphorylates the vitamers (chemically similar to the vitamin) in the liver with PLP being the principle tissue form (and the primary lab measurement) and PL being the predominant circulating form. The primary catabolite is 4-pyridoxic acid (4-PA) that is then excreted in the urine, although other forms are also found in urine. Limited amounts of B6 are also excreted in feces. 13
Consequences of Deficiency or Excess Intake
Currently, both deficiency and excess intake are very rare in non-PD subjects. Classical symptoms of vitamin B6 deficiency include seborrheic dermatitis, microcytic anemia, seizures, peripheral neuropathy, depression, and confusion. 13 Excessive intake of vitamin B6 can be neurotoxic and induce photosensitivity. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11
Laboratory Testing and Interpretation
Vitamin B6 can be measured directly or indirectly. Direct measurements of PLP in red blood cells (RBC) or plasma can be assessed using high performance liquid chromatography (HPLC). Given that PLP is the principle tissue form, plasma PLP is correlated with tissue storage levels and thus a marker of long-term vitamin B6 levels. However, it also reflects B6 intake as it slowly increases with supplementation taking approximately 10 d to reach a steady state. Reference Lui, Lumeng, Aronoff and Li14 Contrary to plasma PLP which remain acutely unaffected by dietary intake, RBC-PLP shows rapid increases within 1 h of ingestion and decreases 4 h post ingestion. Reference Reynolds and Raiten15 Plasma PLP is the most common marker used to assess vitamin B6 status, however levels can be modified by inflammation, albumin concentration, ALP, and alcohol consumption. Reference Brussaard, Löwik, Van Den Berg, Brants and Bemelmans16 Given that plasma PLP binds to albumin in the circulation, conditions that reduced albumin (i.e., pregnancy or inflammation) lead to reductions in plasma PLP. Reference Ueland, Ulvik, Rios-Avila, Midttun and Gregory17,Reference Vasilaki, McMillan, Kinsella, Duncan, O’Reilly and Talwar18 Interestingly, in inflammation and hypoalbuminemia, RBC-PLP has an opposite correlation and is increased. Reference Talwar, Quasim, McMillan, Kinsella, Williamson and O’Reilly19 Plasma PLP, but not RBC-PLP, is inversely associated with total ALP, where patients with hypophosphatasia have extremely elevated levels of PLP, and children with elevated ALP in rickets have very low plasma PLP. Reference Iqbal, Brain, Reynolds, Penny and Holland20,Reference Reynolds, Reynolds and Lorenc21 From a pre-analytical perspective, samples should be collected protected from light to prevent reduced stability of the analyte and falsely low results.
Another direct assessment of vitamin B6 is 4-PA in plasma or urine that can be assessed by HPLC or liquid chromatography tandem mass spec (LC-MSMS) as a measure of short-term status. Plasma 4-PA increases up to 50-fold within 3 d after supplementation. Reference Bor, Refsum and Bisp22 Unlike plasma PLP, plasma 4-PA is not influenced by inflammation but is elevated in critically ill patients. Reference Bates, Schneede, Mishra, Prentice and Mansoor23,Reference Huang, Chang and Huang24 Additional limitations to interpretation of plasma 4-PA include the large intra-individual variability and increased levels in renal dysfunction. Reference Ueland, Ulvik, Rios-Avila, Midttun and Gregory17 Similarly, urine 4-PA increases rapidly with dietary intake and declines rapidly with lack of intake, whereas PLP levels will remain elevated for months following reduced intake. A 24 h urine sample is not required given that a random urine 4-PA samples can be assessed as a PA:creatinine ratio although there is evidence of circadian variation. Reference Ueland, Ulvik, Rios-Avila, Midttun and Gregory17,Reference Zempleni25
Indirect assessment of vitamin B6 include RBC AST and ALT activity. Vitamin B6 is a required coenzyme AST and ALT and thus levels would be reduced in vitamin B6 deficiency. However, studies have showed variable results Reference Bates26 and therefore this type testing is not commonly performed. Furthermore, AST and ALT assays available for routine testing commonly incorporate vitamin B6 into the assay hindering the ability to use in assessment of B6 deficiency. An alternative indirect marker of vitamin B6 is homocysteine levels which is discussed in further detail below.
Vitamin B9 (Folate)
Chemistry and Function
Folate is a generic name for a family of water-soluble compounds that are derived from pteroic acid and exists in various forms with multiple glutamate residues attached and different functional groups. The predominant circulating form is 5-methyltetrahydrofolate (5-methyTHF) and upon uptake into peripheral cells, is demethylated to THF which helps to retain folate within the cell. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11 The THF is converted to 5,10-methyleneTHF which is important for DNA synthesis (Figure 2). When 5,10-methyleneTHF is depleted or not available, this results in megaloblastic anemia and neuropathy, which can occur with vitamin B12 deficiency and is known as the methyl trap (discussed below in vitamin B12 deficiency).
Folate coenzymes also play a role in amino acid metabolism due to the transfer of a methyl group to homocysteine, thereby forming methionine. Reference Simpson, Bailey, Pietrzik, Shane and Holzgreve27 This reaction is carried out by methionine synthase (Figure 3A) which also requires vitamin B12, thus linking the metabolic pathways of both vitamins. The resulting methionine is converted to S-adenosylmethionine (Figure 2) which methylates over 100 compounds including RNA, DNA, protein, and phospholipids, and in doing so can regulate gene expression. Reference Simpson, Bailey, Pietrzik, Shane and Holzgreve27,Reference Razin28

Figure 3. Metabolic reactions involving cobalamin coenzymes. A . Conversion of homocysteine to methionine linking vitamin B12 (methylcobalamin) and folate metabolic pathways. B . Conversion of MMA to succinylCoa. THF; tetrahydrofolate.
Dietary Source and Absorption
Folate is abundant in green leafy vegetables, orange juice, legumes, fortified cereal, liver, green beans, and green peas. 10 Food-derived folate has a bioavailability of 50%, whereas folic acid, the term used for synthetic folate found in supplements, has a bioavailability of 85%. Reference Sauberlich, Kretsch, Skala, Johnson and Taylor29,Reference Pfeiffer, Caudill, Gunter, Osterloh and Sampson30 Folate found in food is converted to a monoglutamate form in the intestine and, once absorbed, is reduced and methylated to form 5-methylTHF which circulates bound to either albumin or folate binding protein. Folate is stored in the liver as well as other tissues, and excreted from the body either via urine or feces. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11
Consequences of Deficiency or Excess Intake
Folate deficiency causes symptoms indistinguishable to B12 symptoms including hematological and neurological symptoms (discussed below), and further leads to open neural tube defects during embryonic development. Reference Kilker, Mersereau and Mulinare31 Excess folate supplementation (>10 mg/day) has been shown to increase or induce neuropathy in vitamin B12 deficient patients. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11
Laboratory Testing and Interpretation
Folate can be measured directly in either serum or RBC. Serum folate is an indicator of short-term folate status and does not reflect folate storage. Serum levels decline within days of reduced dietary intake although tissue storage may be normal. Given that folate levels are concentrated inside the RBC, a small amount of hemolysis can cause falsely elevated serum folate levels. Reference Snow32 RBC folate is a measure of long-term or tissue storage status, and it is less sensitive to changes in dietary intake. Given that folate is taken up into the developing RBC in the bone marrow and not by mature RBCs, the RBC folate reflects ∼120-day status. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11 RBC folate however has significantly higher analytical variation partially due to pre-treatment steps which include RBC lysing agent, lysis time, deconjugation steps, and non-linear dilution. Reference Wright, Finglas and Southon33–Reference Nelson, Klausner and Branda35 RBC folate is also normalized to hematocrit and has a negative correlation with Hb oxygenation, which provide additional variables to consider with interpretation of data. Reference Farrell, Kirsch and Herrmann36,Reference Gunter, Bowman, Caudill, Twite, Adams and Sampson37 Further limitations of the test include false negatives occurring in cobalamin deficiency, Reference Snow32 time consuming laboratory process and test cost. Thus, debate remains regarding whether to use serum or RBC folate in assessing deficiency. Neither test is standardized and both show high inter-laboratory variability with one study showing 35% for RBC-folate and 28% for serum folate. Reference Refsum, Smith and Ueland38
As an indirect measurement, plasma homocysteine levels can be measured by HPLC, enzymatic assay, immunoassay or gas chromatography mass spectrometry (GC-MS) methods. Reference Shenkin, Roberts, Burtis and Bruns9 Homocysteine is not a specific marker given that levels are increased in vitamin B6, B12, and folate deficiency. Levels are also elevated in patients with renal insufficiency, hypovolemia, hypothyroidism, psoriasis, and inherited metabolic disorders. Reference Snow32 False elevations in homocysteine levels can also occur with improper sample handling or collection and thus preanalytical considerations are important in obtaining an accurate value. Patient preparations may include fasting. Large protein dietary intake may increase levels up to 15%. Once samples are collected, they should be kept on ice and centrifuged within 1 h to avoid false elevations. Reference Hortin39 Reference intervals will vary between labs due to lack of standardization and may also require age-specific reference intervals given that homocysteine progressively increases with age. Reference Hunt40
Vitamin B12
Chemistry and Function
Vitamin B12 is a group of essential water-soluble vitamins also known as cobalamins. These vitamins contain a cobalt atom at the center of tetrapyrrole rings with nucleoside chains attached. Modification to the side group results in 5 types: methylcobalamin (predominant physiological form), adenosylcobalamin (cytosolic form), hydroxocobalamin (treatment for cyanide poisoning), cyanocobalamin (most stable form), and aquocobalamin. Reference Shenkin, Roberts, Burtis and Bruns9 Vitamin B12 is a cofactor for two important enzymes: (1) methionine synthase and (2) L-methylmalonyl-CoA mutase (Figure 3). The first reaction is required for the conversion of methylmalonic acid (MMA) to succinyl CoA in the mitochondria, important for branched-chain amino acid catabolism. The second reaction is required for the conversion of homocysteine to methionine in the cytosol, important for protein synthesis. Reference Shenkin, Roberts, Burtis and Bruns9 A deficiency in vitamin B12 would result in an elevation of homocysteine and MMA.
Dietary Source and Absorption
Main dietary sources of vitamin B12 include red meat, chicken, fish/shellfish, milk, yogurt, eggs, cheese, liver, and fortified cereals, with a bioavailability of approximately 50%. 13,Reference Simpson, Bailey, Pietrzik, Shane and Holzgreve27 Dietary B12 is released from food in the stomach and binds to the salivary protein haptocorrin. This complex travels to the duodenum where haptocorrin is digested by pancreatic enzymes and B12 binds to intrinsic factor (IF) secreted by gastric parietal cells. The B12-IF complex is carried into the ileum where it partakes in receptor mediated absorption and enters into the bloodstream bound to either holotranscobalamin (6%–20%) or holohaptocorin (80%–94%), with the former form being the bioactive form. Reference Sukumar and Saravanan41 Vitamin B12 bound to holotranscobalamin is taken up and stored in the liver, and continuously excreted into the bile and reabsorbed. The liver has a capacity to store between 1 and 5 mg, which is a sufficient amount for 3–6 years based on an excretion rate of 0.1% into the feces or urine. 10 Thus, depletion of stores would require reduced intake (i.e., strict vegan diet) for several years. Reference Quinlivan42
Consequences of Deficiency or Toxicity
Vitamin B12 deficiency causes a functional folate deficiency given that the metabolic pathways converge with methionine synthase also plays a role in folate metabolism by converting methylTHF to THF (Figure 2). This can be referred to as the methyltrap, since the reaction involves the transfer of a methyl group that can no longer occur. Folate thus remains in the 5-methylTHF form which cannot be retained in the cell. This results in a pattern of elevated serum folate (essentially inactive) and low cellular and tissue folate Reference Snow32,Reference Tisman and Herbert43,Reference Stabler44 which can be restored with B12 supplementation (no known adverse effects) if there isn’t an additive non-functional folate deficiency. For this reason, measuring folate in vitamin B12 deficiency is not useful and should only be assessed after vitamin B12 levels are determined to be normal. A consequence of identifying folate deficiency without checking B12 status includes subsequent folate supplementation that can mask vitamin B12 deficiency since the anemia will resolve however the neurological abnormalities may still progress. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11
Vitamin B12 deficiency results in symptoms that are indistinguishable from folate deficiency. General symptoms include lethargy, hair loss, weight loss, glossitis, and diarrhea. Reference Quinlivan42,Reference Healton, Healton and Savage45 Hematological symptoms relating to anemia include skin pallor, knuckle hyperpigmentation, and dyspnea. The resultant macrocytic, megaloblastic anemia is believed to be due to the decrease in DNA synthesis caused by impaired folate metabolism (via the methyltrap). B12 deficiency also leads to demyelination of the CNS, leading to neurological symptoms that include paresthesia, peripheral neuropathy, gait abnormalities, and cognitive decline or depression. Reference Quinlivan42 For unknown reasons, presence of hematological and neurologic dysfunction have an inverse relationship with neurological symptoms sometimes presenting in the absence of hematological changes. Reference Lindenbaum, Healton and Savage46,47
Laboratory Testing and Interpretation
Vitamin B12 can be measured directly or indirectly. Direct measurement of total B12 levels employs competitive protein binding assays. Most commercial assays are immunometric and employ an intrinsic factor binder to assess total B12 levels. Reference Shenkin, Roberts, Burtis and Bruns9 Assays that measure bioactive B12 (bound to holotranscobalamin) are not widely available for clinical use in most countries, although a recent report from the National Institute for Health and Care Excellence suggests this type of test may improve diagnostic accuracy. Reference Brokner, Hager and Lindberg48 Recall that only 6%–20% of total B12 is bioactive, Reference Sukumar and Saravanan41 and thus a normal total serum B12 within the reference interval may not reflect adequate B12 status. Similarly, low serum B12 may not indicate deficiency but instead be due to low levels of holohaptocorin (inactive form) which can occur in benign neutropenia, multiple myeloma and leukemic reticuloendotheliosis, or other conditions with low total granulocyte mass. Reference Roberts, Taylor, Sodi, Rifai, Horvath and Wittwer11 Furthermore, B12 has been shown to have large inter-individual variability indicating that the ‘normal’ range between individuals can vary significantly. Reference Devalia, Hamilton, Molloy and British Comm49 Although it has been proposed that B12 levels <148 pmol/L (200 ng/l) have high sensitivity for diagnosing B12 deficiency, lack of standardization complicates the use of a universal cut-point and thus each lab will have an independent reference interval. Reference Snow32,Reference Bain, Wickramasinghe, Broom, Litwinczuk and Sims50 Due to these limitations, following up with indirect testing of B12 can be performed when B12 falls within the reference range but there is strong clinical suspicion of B12 deficiency.
Indirect measurements of B12 deficiency include elevations of MMA and homocysteine. Reference Snow32 While homocysteine is elevated in other vitamin deficiencies, MMA is more specific given that it is unaffected by folate and B6 deficiency. MMA, however, may be increased in renal insufficiency, hypovolemia, patients over 65 yrs and inherited metabolic defects. Reference Bates, Schneede, Mishra, Prentice and Mansoor23,Reference Snow32 In the laboratory, MMA can be measured by GC or LC-MS in urine or serum, Reference Shenkin, Roberts, Burtis and Bruns9 and may be available as an individual test or part of an organic acid panel. Limitations of MMA testing include lack of test availability and cost.
Table 1 provides a synopsis of the physiology and pathophysiology of vitamins B6, B9, and B12.
Table 1. Function, dietary source, symptoms of deficiency and lab testing for vitamin B6, B7 and B12

Abbreviations: *: it might vary depending on the lab; **: note that costs can vary significantly between labs and may change on an annual basis. Factors that contribute to cost differences for the same test between labs include the instrument, sample type, stability of reagents/calibrations, labor, and sample testing volume; ALT, alanine transaminase; AST, aspartate transaminase; CAD, Canadian dollars; MCV, mean corpuscular volume; MMA, methylmalonic acid; PLP, pyridoxal 5’-phosphate; PMP, pyridoxamine 5’-phosphate; PNP, pyridoxine 5’-phosphate; RBC, red blood cells.
LCIG and Vitamin Depletion
Oral Levodopa therapy has been identified to be a risk factor for polyneuropathy in PD, and is associated with decreased levels of vitamin B12, vitamin B6 and folate, and increased levels of MMA and homocysteine. Reference Müller, Laar and Cornblath5,Reference Rispoli, Simioni and Capone51,Reference Toth, Brown, Furtado, Suchowersky and Zochodne52 In non-PD patients, vitamin B6 deficiency-related neuropathy is often predominantly sensory, painful, and suggestive of small-fiber involvement. On the other hand, vitamin B12-related neuropathy often has a substantial motor involvement, which can also involve de dorsal columns of the spinal cord, suggesting the involvement of large, myelinated fibers. These clinical differences haven’t been studied and reported in PD patients, in whom an axonal sensorimotor polyneuropathy is usually seen. Reference Uncini, Eleopra and Onofrj7,Reference Duan, Ladenheim, Cutler, Kruman, Cadet and Mattson53–Reference Cossu and Melis55 The reason for these differences when comparing LCIG patients to the general non-PD population, as well as the rare occurrence of B6-related epilepsy (personal observation) and encephalopathy Reference Manca, Cossu and Murgia56,Reference Oppo, Melis, Melis and Cossu57 is presently unknown. A possible reason relies on the presence of subclinical axonal neuropathy associated with longstanding PD and individual brain susceptibility caused by the underlying neurodegeneration. Reference Merola, Rosso and Romagnolo58 Another probably overlooked scenario is the worsening of dysautonomia due to the involvement of visceral nerves in LCIG patients (personal observation), once again possibly related to underlying PD-related neuropathy.
There are two main hypotheses on how levodopa induces polyneuropathy. The first hypothesis entails the idea that levodopa increases demand on vitamin B stores leading to vitamin depletion. This is based on the theory that levodopa depletes methyl group reserves in the homocysteine methionine cycle (Figure 4), which continuously drives the cycle forward and leads to a reduction in the essential vitamins that are required within the cycle. Reference Müller, Laar and Cornblath5,Reference Loens, Chorbadzhieva, Kleimann, Dressler and Schrader59 The second hypothesis is that carbidopa irreversibly binds to and permanently deactivates Vitamin B6 and B6-dependent enzymes. Vitamin B6 is a known co-factor for the decarboxylation of levodopa to dopamine, thereby depleting vitamin B6 reserve. Reference Loens, Chorbadzhieva, Kleimann, Dressler and Schrader59–Reference Daidone, Montioli and Paiardini61
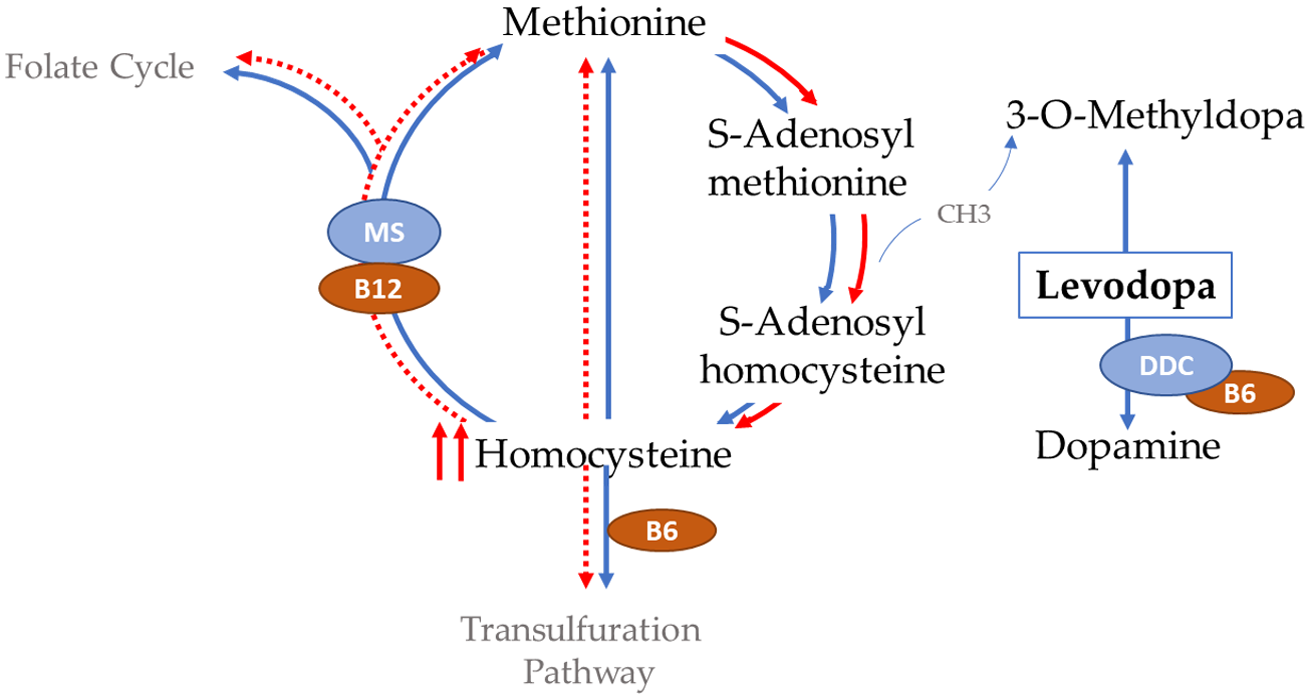
Figure 4. Levodopa depletion of methyl reserves from the homocysteine metabolic pathway. DDC, dopamine decarboxylase; MS, methionine synthase. Adapted from Refs.5,59
Why vitamin deficiency might be particularly severe with LCIG as compared to oral levodopa is unknown. At the moment, two – not mutually exclusive – reasons have been hypothesized:
-
1) the methylcellulose gel in LCIG may hamper jejunal membrane functions, promoting vitamin malabsorption, Reference Santos-García, de la Fuente-Fernández and Valldeoriola4,Reference Rajabally and Martey62,Reference Jugel, Ehlen, Taskin, Marzinzik, Müller and Klostermann63 and thus limiting the absorption of dietary vitamins Reference Loens, Chorbadzhieva, Kleimann, Dressler and Schrader59,Reference Jugel, Ehlen, Taskin, Marzinzik, Müller and Klostermann63 ;
-
2) the continuous intestinal delivery of LCIG may increase bioavailability of levodopa thereby leading to increased vitamin depletion in absence of ‘metabolic rest’ during which these vitamins can be used for other physiological functions. Reference Rispoli, Simioni and Capone51 For example, LCIG continuous delivery could saturate the folate mediated one-carbon pathway involved in the levodopa metabolism, leading to excessively high plasma levels of homocysteine. Reference Müller, Laar and Cornblath5–Reference Uncini, Eleopra and Onofrj7 The association between homocysteine and the risk of peripheral neuropathy has been confirmed by a recent literature review. Reference Romagnolo, Merola, Artusi, Rizzone, Zibetti and Lopiano64 Homocysteine exerts a direct neurotoxic effect mediated by mitochondrial dysfunction, glutamatergic excitotoxicity, inflammatory reactions, and DNA repair mechanisms impairment. Reference Uncini, Eleopra and Onofrj7,Reference Duan, Ladenheim, Cutler, Kruman, Cadet and Mattson53–Reference Cossu and Melis55 Accordingly, the use of catechol-o-methyltransferase inhibitors has been associated with a lower incidence of levodopa-associated peripheral neuropathy, likely due to the positive effect of these medications on homocysteine production. Reference Cossu, Ceravolo and Zibetti54 Vitamin B6 and B12 are also involved in the pathogenic mechanisms associated with the potential toxicity associated with LCIG. Reduced levels of these essential vitamins can result in neuronal damage through elevation of homocysteine plasma levels, reduction of succinyl-CoA availability, and alteration of vitamin-dependent RNA methylation, which may lead to an impairment in the production of axonal proteins. Reference Roos65,Reference Walerych, Venkataraman and Johnson66 Of relevance, almost all patients with PD-associated polyneuropathy have been found to have increased levels of MMA. Reference Toth, Breithaupt and Ge67
More insights in this respect will occur by exploring the occurrence of vitamin deficiency in patients receiving subcutaneous levodopa infusion (currently in phase III trials).
Recommendations and Conclusions
In absence of guidelines and gold standards, Tables 2 and 3 summarize our recommendations on the basis of published data, costs, and our clinical experience. First of all, patients and caregivers should be informed about the risk of vitamin deficiency before starting LCIG. They should also be educated on the signs and symptoms raising the suspicion of a vitamin deficiency. In addition, possible contributors to vitamin depletion should be taken into account, such as treatment with isoniazid or methotrexate (causing vitamin B6 and folate deficiency, respectively), history of bariatric surgery, atrophic gastritis or chronic use of proton pump inhibitors.
Table 2: Recommendations for monitoring, prophylaxis, and treatment of vitamin-related LCIG complications
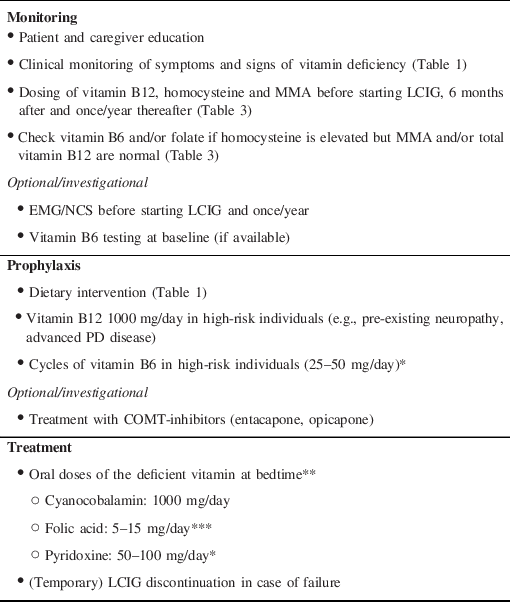
Abbreviations: *: excessive doses of vitamin B6 can be neurotoxic and induce photosensitivity; **: after disconnecting LCIG to minimize the interactions, in resistant cases (e.g., lack of improvement after 1 month) alternative routes need to be used (IM, IV, SC); ***: excessive folate supplementation can increase or induce neuropathy in vitamin B12 deficient patients; COMT: catechol-o-methyltransferase; LCIG: levodopa-carbidopa intestinal gel; MMA: methylmalonic acid.
Table 3: Synopsis of laboratory results in vitamin B6, B9 (folate), and B12 deficiency*
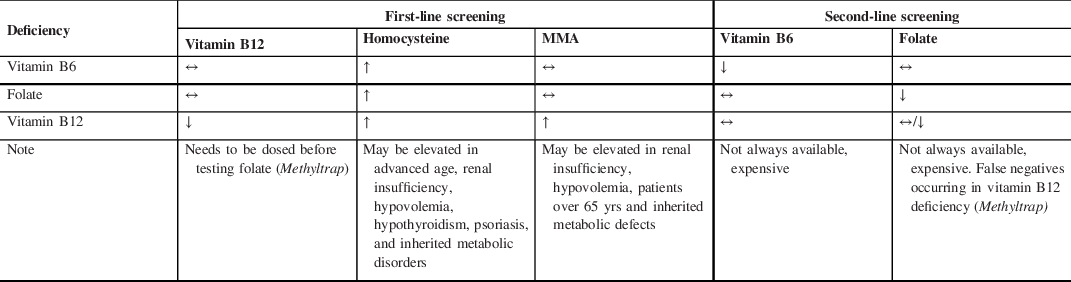
Abbreviations: *Cell blood count is not useful as neurological symptoms sometimes presenting in the absence of hematological changes; MMA, methylmalonic acid.
Second, vitamin B12, homocysteine, and MMA should be tested before starting LCIG, six months after and once/year thereafter (Table 3). Vitamin B6 and folate testing is not universally available and/or costly but it should be considered if homocysteine is elevated but MMA and/or total vitamin B12 are normal. Alternatively, at least one (either Vitamin B6 or folate) should be tested and if normal, one can infer that non-tested one is deficient. It is indeed important to diagnose a deficiency before supplementing particularly for Vitamin B6 as its excessive supplementation is neurotoxic too. Finally, in certain circumstances (e.g., patients at risk of or already presenting with progressive signs of neuropathy), additional testing upfront may be performed in order to have a deeper assessment of patient’s condition in a timely fashion.
Prophylaxis of vitamin deficiency is very important and should be started as soon as LCIG is implemented, possibly even before. Dietary recommendations are important (Table 1) and possibly enough in most patients. Nevertheless, a subgroup of patients is at higher risk and should receive Vitamin B12 regularly and cycles of B6 (e.g., 1-month supplementation every 1–2 months). High-risk categories are patients with pre-existing neuropathy for other causes, such as diabetes or advanced PD itself, or dietary restriction, for example, in case of swallowing issues. Folate supplementation is not needed as it is rarely deficient.
Finally, once diagnosed a vitamin deficiency should be readily treated (Table 3) and accompanied by clinical and laboratory monitoring. Resistant cases should receive non-oral routes of administration and possibly discontinue LCIG, even temporarily.
In conclusion, more work is certainly needed to establish evidence-based guidelines but we hope that this contribution would function as a guide for clinicians and will inspire a scientific discussion among experts. A number of unknowns need to be addressed in particular the protective effect of catechol-o-methyltransferase inhibitors, the risks in patients with a pre-existing neuropathy and the complications – if any – of subcutaneous levodopa infusion currently being evaluated in clinical trials.
Acknowledgments
Authors are grateful to Kataka Medical Communication (Montréal, QC, Canada) for having provided the survey data. This review was partly funded by the University of Toronto/University Health Network Chair in Neuromodulation and Multidisciplinary Care to AF.
Statement of Authorship
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
JT: 1B, 1C, 3A
TN: 1C, 3B
YYP: 1C, 3B
AM: 1C, 3B
TM: 1C, 3B
OS: 1C, 3B
VK: 1B, 1C, 3B
AF: 1A, 1B, 1C, 3A, 3B
Financial Disclosures/Conflict of Interest/Funding
Dr. Taher reports honoraria from Abbvie, outside the submitted work. Dr. Naranian has nothing to disclose. Dr. Poon has nothing to disclose. Dr. Merola reports grants from Lundbeck, grants and personal fees from Abbvie, personal fees from Abbott, personal fees from Theravance, outside the submitted work. Dr. Mestre reports personal fees as consultant and speaker from Abbvie, outside the submitted work. Dr. Suchowersky has nothing to disclose. Dr. Kulasingam has nothing to disclose. Dr. Fasano reports grants, personal fees and non-financial support from Abbvie, during the conduct of the study; personal fees from Abbott, grants and personal fees from Boston Scientific, personal fees from Ceregate, personal fees from Inbrain, grants and personal fees from Ipsen, grants and personal fees from Merz, personal fees from Sunovion, grants, personal fees and non-financial support from Medtronic, outside the submitted work.









