Introduction
Seabirds occupy diverse habitats that include marine and terrestrial ecosystems where they find resources to feed, nest, breed, find shelter and moult (Schreiber and Burger, Reference Schreiber, Burger, Schreiber and Burger2001). In this dual environment, seabirds are exposed to parasites through their diet and eating habits (e.g. helminths present in fish species) (Randall and Bray, Reference Randall and Bray1983; Brandão et al., Reference Brandão, Moreira and Luque2014), nesting sites (e.g. ectoparasites in the nest material and soil) (Daturi, Reference Daturi1986) and through their interaction with other co-occurring congeneric and confamilial birds (e.g. bird-specific ectoparasites and pathogens such as viruses and bacteria) (McCoy et al., Reference McCoy, Boulinier, Schjørring and Michalakis2002).
There are several factors that can cause among colony variation in parasite diversity and level of infestations in seabirds (Jones and Shellam, Reference Jones and Shellam1999). Most seabird species form large colonies comprising up to thousands of breeding pairs (Schreiber and Burger, Reference Schreiber, Burger, Schreiber and Burger2001) and therefore colony size and especially the nest density can facilitate high infestations of both host (lice) and nest parasites (fleas and ticks) (Rivera-Parra et al., Reference Rivera-Parra, Levin and Parker2014; Ramos and Drummond, Reference Ramos and Drummond2017). For example, tick infestation has been found to be higher in Peruvian Boobies (Sula variegata), a seabird that nest in large groups than in blue-footed boobies (Sula nebouxi), which tend to cluster at relatively lower densities (Duffy and Campos de Duffy, Reference Duffy and Campos de Duffy1986). The presence of co-occurring congeneric and confamilial bird can further increase host and nest density, which can result in higher abundances and transmission of bird-specific parasites (Duffy, Reference Duffy1988). The level of parasite infestation in prey species can also vary spatially and may influence the risk of infection for seabirds that act as definitive hosts (Van der lingen et al., Reference Van Der Lingen, Weston, Ssempa and Reed2015; Levsen et al., Reference Levsen, Svanevik, Cipriani, Mattiucci, Gay, Hastie, Bušelić, Mladineo, Karl, Ostermeyer and Buchmann2018). A large scale study by Levsen et al. (Reference Levsen, Svanevik, Cipriani, Mattiucci, Gay, Hastie, Bušelić, Mladineo, Karl, Ostermeyer and Buchmann2018) recorded regional difference in parasite infestations of Anisakid nematodes in sardine (Sardina pilchardus) and other commercial fish species within the European fishing grounds. Several of these nematodes require seabirds as definitive hosts (Anderson, Reference Anderson2000). Climatic conditions on land and in the water can also affect parasite distribution. Nidicolous ectoparasite species (spend time in nests and shelters) are susceptible to mean temperature and precipitation (Marshall, Reference Marshall and Marshall1981a; Sonenshine, Reference Sonenshine and Sonenshine1993) while water temperature and salinity can effect most marine endoparasites in fish (Möller, Reference Möller1978). In addition, bird age and immune status are also important factors (De Lope et al., Reference De Lope, Møller and De la Cruz1998; Van Rensburg, Reference Van Rensburg2010). For example, a study on the seabird Kittiwake (Rissa tridactyla) recorded higher tick infestations on intermediate age chicks compared with younger and older chicks. The authors surmised that this may be due to the fact that intermediate aged chicks spend more time in the nests and are therefore more exposed to ticks (Boulinier and Danchin, Reference Boulinier and Danchin1996). Knowledge of the factors that drive parasite infestation is important as parasites can directly (cause stress, anaemia and reduced fitness) (Johnson and Clayton, Reference Johnson and Clayton2003; Bitam et al., Reference Bitam, Dittmar, Parola, Whiting and Raoult2010) and indirectly (transmit disease causing microbes such as protozoa, bacteria and viruses) affect the condition and survival of their host (Nuttall, Reference Nuttall1984). Although several studies have been conducted on parasites of seabird at the terrestrial-marine interface (e.g. Gauthier-Clerc et al., Reference Gauthier-Clerc, Jaulhac, Frenot, Bachelard, Monteil, Le Maho and Handrich1999; Frenot et al., Reference Frenot, De Oliveira, Gauthier-Clerc, Deunff, Bellido and Vernon2001; Carrera-Játiva et al., Reference Carrera-Játiva, Rodríguez-Hidalgo, Sevilla and Jiménez-Uzcátegui2014; Rivera-Parra et al., Reference Rivera-Parra, Levin and Parker2014), little is known about the factors that influence parasite loads on seabirds that naturally occur along the southern African coastline (Daturi, Reference Daturi1986; Duffy and Daturi, Reference Duffy and Daturi1987).
The African penguin (Spheniscus demersus) is endemic to the Benguela Upwelling Ecosystem (Crawford et al., Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Geldenhuys, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011) and the only penguin species that breeds in Africa (Shelton et al., Reference Shelton, Crawford, Cooper and Brooke1984). The species breeds in 28 colonies (24 islands and four mainland) (BirdLife International, 2016) distributed from central Namibia to the east coast of South Africa (Crawford et al., Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Geldenhuys, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). The suitability for breeding sites has been linked to the distribution and abundance of their primary prey: the Cape anchovy (Engraulis encrasicolus) and the South African sardine (Sardinops sagax) (Crawford et al., Reference Crawford, Hemming, Kemper, Klages, Randall, Underhill, Venter, Ward and Wolfaardt2006). During the breeding season (in South Africa normally extended from February to September/October; Crawford et al., Reference Crawford, Boonstra, Dyer, Upfold, Dann, Norman and Reilly1995; Crawford et al., Reference Crawford, Hemming, Kemper, Klages, Randall, Underhill, Venter, Ward and Wolfaardt2006), the adults spend most of the day catching fish at sea and return to the colonies in the evenings to feed the chicks and relieve their mate (Cooper, Reference Cooper1980). They lay two eggs that are incubated for about 40 days. After hatching, the chicks are under parental care and become independent after ca. 80 days (Williams and Cooper, Reference Williams and Cooper1984). Historically, African penguins were common on nearshore islands, but more recently the species also started to occupy mainsland areas. It is surmised that this movement may have been due to excessive harvesting of eggs (for human consumption) and guano (for fertilizer) on islands (Rand, Reference Rand1969; Whittington et al., Reference Whittington, Hofmeyr and Cooper1996), although a reduction in food resources along certain islands may have also contributed (Shelton et al., Reference Shelton, Crawford, Cooper and Brooke1984; Crawford et al., Reference Crawford, Altwegg, Barham, Barham, Durant, Dyer, Geldenhuys, Makhado, Pichegru, Ryan, Underhill, Upfold, Visagie, Waller and Whittington2011). Consequently, the species has suffered severe (>50% over three generations) and rapid population decline mainly on island colonies, and its conservation status is listed as endangered (BirdLife International, 2016). African penguins are parasitized by a diverse range of parasite taxa that include ectoparasites (soft ticks, lice and fleas), helminths (nematodes, cestodes and trematodes) and haemoparasites (Brandão et al., Reference Brandão, Moreira and Luque2014; Parsons and Vanstreels, Reference Parsons and Vanstreels2016). To date, most studies on the parasite of African penguins have been conducted on animals admitted to rehabilitation centres (e.g. Horne et al., Reference Horne, Bray and Bousfield2011; Yabsley et al., Reference Yabsley, Parsons, Horne, Shock and Purdee2012; Parsons and Vanstreels, Reference Parsons and Vanstreels2016) or have focused on parasites in nests at a single island colony (Daturi, Reference Daturi1986; Duffy and Daturi, Reference Duffy and Daturi1987). As yet, there is no empirical data on the parasites associated with African penguins and their nests across multiple colonies in South Africa. In addition, little is known with regards to the factors that drive among and within colony parasite infestations on African penguins and in their nests.
The aims of the study were: (1) to record the diversity and prevalence of parasites associated with African penguins and their nests at multiple colonies along the south-western coast of South Africa, and (2) to establish the effect of various host and environmental factors on parasite infestation patterns. We predict that penguin chicks will have higher parasite infestations compared with adults. This may be due to a combination of factors such as lower immunity and closer association with nests (and nest parasites) in chicks. We further predict that parasite abundance and prevalence will be positively related to nest density. Colonies with higher nest densities provide more resources (food and shelter) that can facilitate higher parasite infestations.
Materials and methods
Study site and design
The study was conducted at five African penguin colonies: three islands: Dassen-, Dyer- and Robben Island and two mainlands: Stony Point and Simon's Town (previously known as Boulders Beach) along the south-western coast of South Africa during 2016 and 2017 (Fig. 1; Table 1). Penguins (adults and chicks: 20 day-old and older) and their respective nests were randomly selected and sampled at the peak of the first breeding season between May and July (autumn/winter, i.e. cold and rainy season) each year. In addition, penguins and their nests were sampled at one colony (Stony Point) during a second breeding season in October–November (spring, i.e. warm and dry season) during 2016. Sixty penguins (20 adults and 40 chicks) and 40 nests were sampled at each colony in autumn/winter each year. At Stony Point, 105 penguins (22 adults and 83 chicks) and 109 nests were sampled in autumn/winter 2016, 103 penguins (eight adults and 95 chicks) and 81 nests were sampled in spring 2016 and 105 penguins (20 adults and 85 chicks) and 118 nests were sampled in autumn/winter 2017 (Table 1). Sampling was conducted during the day starting at 9:00 and ending at 16:00.

Fig. 1. Map of the selected African penguin colonies along the south-western coast of South Africa. Two mainland (Simon's Town and Stony Point) and three island colonies (Dassen-, Dyer- and Robben Island). Areas were plotted using GPS coordinates and QGIS open source Geographic Information System (http://qgis.osgeo.org).
Table 1. Locality, date of sampling, sample size, season and nest density at five African penguin colonies along the south-western coast of South Africa during 2016 and 2017

Parasite collection from penguins and nests
Each penguin (adult and chick) was examined for 8 min. Ectoparasites (fleas, lice and ticks) were collected by systematically brushing the plumage for 1 min around the pelvic area using a soft brush. Ectoparasites that occur on the face of the animals were also removed using forceps. Parasites were stored in 70% ethanol. A new brush and clean tweezers were used for each animal. A blood smear was made from a drop of blood collected from the dorsal aspect of the foot using a mechanical pipette attached to a 23-gauge needle. The blood smear was air-dried and fixed with methanol. Penguin chicks naturally defecate when handed. This allowed the collection of fresh faecal material, which were fixed in 10% formalin and kept cool until examination in the laboratory. Body mass (kg) was recorded for each penguin with a handheld electronic scale (25 kg/50 lb Sensation). Penguin nests were sampled for parasites by collecting 200 mL nest material (including soil) from the centre of the nest. Nest material was stored in plastic jars sealed with a lid and kept cool until further processing.
Parasite recovery and identification
Ectoparasites were extracted from the nest material using a modified Berlese funnel method (Southwood, Reference Southwood and Southwood1978). In a sealed unit naphthalene moth balls (100 g) were used as a repellent and hung above the nest material for 24 h (Daturi, Reference Daturi1986). Thereafter, each nest material sample was systematically examined using a dissecting microscope. The latter method was included due to the ineffectivity of the extraction method to remove all parasites. Parasites recorded by the two methods were combined. Ectoparasites were identified morphologically using taxonomic reference keys (Jordan, Reference Jordan1942; Von Keler, Reference Von Keler1952; Arthur, Reference Arthur1963; Kohls et al., Reference Kohls, Sonenshine and Clifford1965; Segerman, Reference Segerman1995; Banks and Palma, Reference Banks and Palma2003) and counted. Ectoparasite species were identified to species level and the life stage and sex was recorded. Thin blood smears were stained using an Eosin-Methylene Blue stain (RapiDiff kit) and examined by detecting presence of haemoparasites in 150 fields per slide under a light microscope (Leica Microsystems, Wetzlar, Germany) at 100× magnification (Palinauskas et al., Reference Palinauskas, Valkiūnas, Bolshakov and Bensch2008). Haemoparasites were identified to order level (Piroplasmorida/Haemospororida and Spirochaetales) based on morphological characters (Campbell and Ellis, Reference Campbell, Ellis, Campbell and Ellis2007; Peirce and Parsons, Reference Peirce and Parsons2012; Vanstreels et al., Reference Vanstreels, Braga and Catão-Dias2016). Faecal material (1 g) was examined for helminth eggs using qualitative techniques. Nematode and cestodes eggs were detected with the modified Wisconsin sugar flotation method (Nolan, Reference Nolan2006) (specific gravity of sugar solution >1.14). The sedimentation technique described by Hansen and Perry (Reference Hansen, Perry, Hansen and Perry1994) was used to detect trematodes, acanthocephalans and any eggs that did not float with the flotation technique. The helminth parasites were identified to genus level (Horne et al., Reference Horne, Bray and Bousfield2011; Carrera-Játiva et al., Reference Carrera-Játiva, Rodríguez-Hidalgo, Sevilla and Jiménez-Uzcátegui2014).
Nest density
Nest density was recorded by counting the total number of nests (non-active and active nests) and active nests only (nests containing eggs, chicks and/or adults) in a 15 × 15 m quadrant during the autumn/winter season each year. Five quadrants were randomly selected each year at all colonies apart for Stony Point. At the latter colony, 12 quadrants were selected each year.
Climate data
Data on the annual mean temperature (°C) and annual precipitation (mm) was obtained for each colony from WorldClim (Global Climate Data) using the function getData in the ‘raster’ package in R (Hijmans and van Etten, Reference Hijmans and van Etten2012). Remote censed data were selected due to the lack of local weather data at all the colonies.
Data analysis
To assess the effect of different parameters on parasite infestations, we considered the total number of parasites (i.e. parasites at all life stages) and combined the two flea species found in this study into one group. Since flea larvae only occur in the nest and adult fleas are found in the nest and on the host, we considered analysis at each life stage only for this ectoparasite in order to record differences. Morphological differentiation between flea species at the larval stage is notoriously difficult (Krasnov, Reference Krasnov and Krasnov2008) and as such the larvae of P. humboldti and E. gallinacea (recorded only at Dassen Island) could not be distinguished. Consequently, data on flea larvae from Dassen Island were not considered in the calculation of abundance and prevalence of total fleas (i.e. no available data).
The effect of penguin age (adult and chick), colony location (mainland and island) and colony (Dassen-, Dyer-, and Robben Island, Stony Point and Simon's Town) on parasite loads during the autumn/winter season were assessed primarily using generalized linear models (GLMs). Where needed the effect of penguin body mass (kg) and year (2016 and 2017) were corrected for in the models. Since parasite data were highly skewed with an excess of zeros, parasite data were first modified by adding the value of 1, then log transformed and rounded (Changyong et al., Reference Changyong, Hongyue, Naiji, Tian, Hua and Ying2014), followed by testing for overdispersion (GLM ‘quasipoisson’). To model data on parasite abundance, we used zero-inflated regression with a negative binomial residual distribution to correct for data overdispersion, using the zeroinfl function from the ‘pscl’ R package (Jackman, Reference Jackman2017). Whenever the model did not fit the data we transformed the abundance data into presence/absence format and used GLM with a binomial distribution [function glm()]. Parasite prevalence was assessed by GLM with a binomial distribution. Since we aimed at assessing the effect of different factors on parasite infections, we presented the full models with all independent variables in the main text. However, we also performed backward model selection based on Akaike information criterion (AIC), using the function step() in R, and compared the selected models with the corresponding full models using a χ 2 test. To compare cross-colony parasite mean abundance/prevalence in relation to nest density (total nest density and active nest density) and climatic factors (air temperature and precipitation) we used analysis of variance and Tukey HSD tests. The effect of nest density and climatic factors on parasite mean abundance (i.e. number of parasites of a particular species divided by the total number of hosts examined; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) and prevalence (i.e. number of infected hosts by a particular parasite species divided by the total number of hosts examined; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) was assessed using Pearson and Spearman correlation tests. Statistical analysis included Wilcoxon rank sum test and proportion test to compare parasite abundance and prevalence, respectively, between sampling seasons (autumn/winter and spring) at Stony Point. Seasonal differences in parasite prevalence were assessed using parasites from chicks because chicks were mainly sampled in the spring season at Stony Point and helminth parasites were only recorded for chicks. All statistical tests and plot design were conducted in R 3.4.3 (R Core Team, 2017).
Results
Three parasitic groups (ectoparasites, haemoparasites and helminth parasites) were recorded from 793 African penguins and 628 penguin nests at five colonies along the south-western coast of South Africa (Fig. 1). Ectoparasites comprised of two fleas (Parapsyllus humboldti and Echidnophaga gallinacea), a louse (Austrogoniodes demersus) and a soft tick (Ornithodoros capensis s. s.). Haemoparasites were morphologically consistent with the orders Piroplasmorida/Haemospororida and Spirochaetales. Four helminth genera were detected in chicks (Cardiocephaloides spp., Renicola spp., Contracaecum spp. and Cyathostoma spp.). Prevalence, mean abundance, mean intensity (i.e. average number of parasites of a particular species divided by the number of infected hosts; Bush et al., Reference Bush, Lafferty, Lotz and Shostak1997) and sex ratios of parasites associated with penguins, across the selected colonies, are provided in Table 2. Although mites (Acari) were recorded, they were mainly found in penguin nests and in high abundance. Mites are very specious and were not included in this study due to the taxonomic difficulty in distinguishing between parasitic and non-parasitic soil mites (Proctor and Owens, Reference Proctor and Owens2000). However, the importance of mite data is recognized and will be included in follow-up studies.
Table 2. Ectoparasites, haemoparasites and helminths recorded from African penguins at five colonies along the south-western coast of South Africa during 2016 and 2017

Sample sizes N = 793 (ectoparasites), 734 (haemoparasites) and 413 (helminths).
a Total prevalence, mean abundance and mean intensity of fleas excluded flea larvae from Dassen Island.
b Mean abundance and mean intensity of flea larvae excluded flea larvae from Dassen Island.
Ectoparasites on penguins
The most abundant and prevalent ectoparasite on penguins was P. humboldti (4.57 ± 0.2; 69.10%), while the second most abundant ectoparasite was O. capensis (s. s.) (0.51 ± 0.07; 16.65%) of which larvae were mainly collected (Table 2). This life stage was the most abundant on penguins at three of the five colonies (Supplementary Table S1). Parapsyllus humboldti also exhibited the highest mean intensity on penguins (6.61 ± 0.25), followed by E. gallinacea (4.95 ± 0.60). The infestation level of A. demersus was in general very low, and apart from its absence at Simon's Town, no pattern was evident. The sex ratios of the individual parasite taxa varied: P. humboldti recorded an equal sex ratio, E. gallinacea and A. demersus recorded a female-biased ratio, while only female O. capensis (s. s.) (three females infested three penguins) were recorded from penguins (Table 2).
Haemoparasites and helminth parasites of penguins
Piroplasmorida/Haemospororida was the most prevalent haemoparasite group (33.51%) compared with Spirochaetales (2.59%) in penguins. The helminth Cardiocephaloides spp. was the most prevalent genus (56.17%) in penguin chicks followed by Contracaecum spp. (12.83%) (Table 2).
Ectoparasites in nests
The prevalence, mean abundance, mean intensity and sex ratios of parasites recorded from penguin nests across the selected colonies are provided in Table 3. Only ectoparasites were recorded in nests, therefore the results are presented only for fleas and ticks. More than half of the nests were infested with P. humboldti (57.80%), of which the larval stage was the most abundant and prevalent (13.60 ± 1.49; 47.29%). The second most abundant and prevalent parasite was O. capensis (s. s.) (6.37 ± 1.90; 54.30%), of which nymphs were the most abundant and prevalent (2.5 ± 0.94; 39.81%) (Supplementary Table S2). Parapsyllus humboldti also recorded the highest mean intensity, in infected nests, (25.30 ± 2.52) of which larvae recorded the highest mean intensity (28.55 ± 2.90), followed by O. capensis (s. s.) (11.74 ± 3.48) of which tick larvae recorded the highest mean intensity (9.08 ± 3.02) (Table 3). Ectoparasite taxa in nests exhibited different sex ratios: P. humboldti recorded a female-biased ratio, E. gallinacea recorded an equal number of males and females, and O. capensis (s. s.) recorded a male-biased ratio in nests (Table 3).
Table 3. Ectoparasites recorded from nests of African penguins (N = 628) along the south-western coast of South Africa during 2016 and 2017

a Prevalence, mean abundance and mean intensity of flea larvae excluded flea larvae from Dassen Island.
b Total prevalence, mean abundance and mean intensity of fleas excluded flea larvae from Dassen Island.
Factors that influence parasite infestations
The outcome of regression models showed a strong effect of penguin age, colony location and colony (Table 4). The majority of the full models used in the analysis did not show significant differences from the best models selected according to the AIC (Supplementary Table S3). In particular, abundance of total ectoparasites, fleas (P. humboldti and E. gallinacean combined) and O. capensis (s. s.), and prevalence of Piroplasmorida/Haemospororida were significantly higher in chicks compared with adult penguins (Table 4). Interspecific variation in parasite infestations, on penguins, was recorded in mainland compared with island colonies. Penguins at mainland colonies recorded significantly higher abundances for total ectoparasites and fleas on penguins than island colonies. In addition, Piroplasmids/Haemospororida and Cardiocephaloides spp. were significantly more prevalent in penguins at mainland colonies compared with islands. A similar pattern was recorded in penguin nests with significantly higher abundance recorded for total ectoparasites, fleas (both life stages combined), adult fleas and flea larvae at mainland colonies compared with islands.
Table 4. Effect of colony location (mainland and island), colony (Stony Point, Simon's Town, Dassen-, Dyer- and Robben Island) and penguin age (adult and chick) on parasite infestation of African penguins and their nests during in the autumn/winter season (2016 and 2017)

Type of analysis: regression model ZINB (zero-inflated negative binomial), glm ‘binomial’ and proportion test.
Significant values: ***<0.001, **0.001–0.01, *0.01–0.05.
Parasite infestations also varied between colonies with Stony Point and Simon's Town generally harbouring significantly more parasites on penguins and in nests. In particular, total ectoparasite abundance was significantly higher on penguins at Stony Point and Simon's Town than most of the other colonies (Table 4). In addition, at Stony Point a significantly higher O. capensis (s. s.) abundance and higher Piroplasmorida/Haemospororida prevalence were recorded for penguins compared with most or all other colonies. Penguin chicks at Stony Point also recorded a significantly higher prevalence of Cardiocephaloides spp. compared with other colonies, though when compared with Simon's Town the difference in prevalence was not significant. Abundance of O. capensis (s. s.) in nests were also generally higher at Stony Point compared with the other colonies and significantly so for Simon's Town and Dassen Island. In contrast, penguins at Simon's Town recorded a significantly higher abundance of fleas on penguins compared with other colonies. In addition, this colony recorded significantly higher infestations of total ectoparasites, total fleas (both life stages combined), adult fleas and flea larvae in nests compared with the other colonies.
Cross-colony comparison of mean abundance and prevalence of parasites in autumn/winter in relation to nest density (total and active nest density) and climatic factors (air temperature and precipitation) revealed significant differences between colonies for some parasitic groups (Piroplasmids/Haemospororida F = 39.73, P < 0.001; Cardiocephaloides spp. F = 2.69, P < 0.05; total parasites in nest F = 4.12, P < 0.01; fleas in nest F = 9.605, P < 0.001; total parasites on penguins F = 14.27, P < 0.001; fleas on penguins F = 15.15, P < 0.001). The infestation levels of several parasite taxa correlated with nest density (total and active). In particular, the prevalence of Piroplasmids/Haemospororida in penguins was significantly positively correlated with total and active nest density (r = 0.97, P < 0.01 and r = 0.92, P < 0.05, respectively) (total nest density as example Fig. 2A). The prevalence of Cardiocephaloides spp. in penguin chicks was significantly positively correlated with total nest density (Fig. 2B) (r = 0.98, P < 0.01). Likewise, mean abundance of total ectoparasite and O. capensis (s. s.) in nests was significantly positively correlated with the density of active nests (Fig. 2C) (r Spearman = 0.9, P < 0.05) and total nests (Fig. 2D) (r Spearman = 0.9, P < 0.05), respectively. Flea abundance in nests follow a similar pattern as the total ectoparasites in nests, however the response was not significant. The infestation levels of two parasite taxa correlate with climate. In particular, mean abundance of A. demersus on penguins was significantly negatively correlated with annual mean ambient temperature and annual precipitation (r = −0.95, P < 0.05, and r = −0.92, P < 0.05, respectively), while prevalence of Contracaecum spp. in chicks was significantly positively correlated with annual mean ambient temperature and annual precipitation (r = 0.97, P < 0.01, and r = 0.94, P < 0.05, respectively).

Fig. 2. Pearson correlation between (A) Piroplasmids/Haemospororida prevalence and total nest density, and (B) Cardiocephaloides spp. prevalence and total nest density. Spearman correlation between (C) mean total nest ectoparasites and active nest density and (D) mean nest ticks (O. capensis s. s.) and total nest density of African penguins.
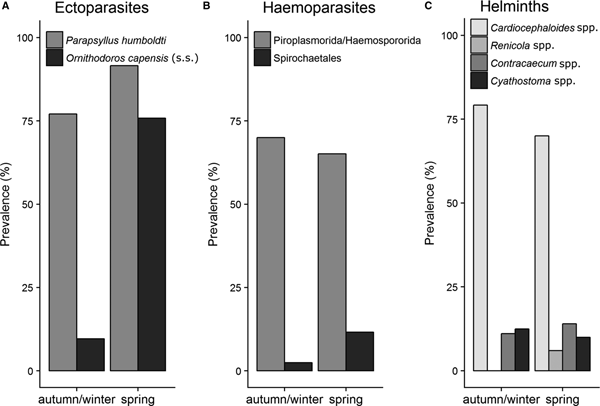
Several parasite taxa exhibited seasonal variation in infestations on penguin chicks at Stony Point during 2016. In particular, P. humboldti was significantly more prevalent (91.6 and 77.1% respectively, P < 0.05) and abundant (8.1 ± 0.7 and 4.6 ± 0.5 respectively, W = 2619.5, P < 0.001) on chicks in spring compared with autumn/winter. A similar, but stronger pattern was recorded for O. capensis (s. s.) prevalence (75.8 and 9.6% respectively, P < 0.001) and abundance (2.8 ± 0.4 and 0.1 ± 0.05 respectively, W = 1181.5, P < 0.001) on chicks [data for P. humboldti and O. capensis (s. s.) abundance not shown] (Fig. 3A). Spirochaetales were also more prevalent in penguins in spring compared with autumn/winter, but only marginally significant (11.6% spring and 2.5% autumn/winter, P = 0.05) (Fig. 3B). Helminth parasite infestations in chicks were not affected by season (Fig. 3C). Prevalence of ectoparasites in penguin nests also varied significantly across seasons. Parapsyllus humboldti was significantly more prevalent (68.0 and 42.2% respectively, P < 0.001) and abundant (8.14 ± 0.7 and 4.6 ± 0.5 respectively, W = 0.373, P value <0.001) in nests during spring compared with autumn/winter. Likewise, O. capensis (s. s.) was significantly more prevalent (95.1 and 56.0% respectively, P < 0.001) and abundant (13.9 ± 5 and 1.7 ± 0.3, respectively, P < 0.05) in nests in during spring compared with autumn/winter (Fig. 4).

Fig. 3. Prevalence of ectoparasites, haemoparasites and helminth parasites associated with African penguin chicks at Stony Point during two seasons (autumn/winter and spring) in 2016. Sample sizes N = 178 (ectoparasites), 166 (haemoparasites) and 122 (helminths).

Fig. 4. Prevalence of fleas and soft ticks in the nests of African penguins (N = 190) at the Stony Point colony during two seasons (autumn/winter and spring) in 2016.
Discussion
Parasite diversity and abundance associated with penguins and their nests
In this study, P. humboldti was the most prevalent and abundant parasite on penguins and in their nests. Most of the P. humboldti found on penguins were adults, while the larval stage dominated the nests. This is consistent with the life cycle of fleas, given that adult fleas mainly attach to the host for a blood meal while the larvae remain in the nest where they feed on organic matter (Bitam et al., Reference Bitam, Dittmar, Parola, Whiting and Raoult2010). The sex ratio of P. humboldti was equal on penguins and female-biased in nests (0.7:1). This pattern has previously been observed in nests of other avian species (e.g. passerines) and is consistent with the fact that female fleas live longer than males in natural populations and are thus more prevalent (Rothschild and Clay, Reference Rothschild and Clay1952; Shutler et al., Reference Shutler, Petersen, Dawson and Campbell2003). The genus Parapsyllus seems to be specifically targeting penguins (Clarke and Kerry, Reference Clarke and Kerry1993) with some species, such as P. longicornis, occurring on multiple penguin species (Murray et al., Reference Murray, Palma, Pilgrim and Sharp1990). In the case of P. humboldti the flea has been found on Humboldt penguins (Spheniscus humboldti) and in their nests in Chile and Peru (Segerman, Reference Segerman1995) and is frequently collected from African penguins in rehabilitation centres in South Africa (Parsons and Vanstreels, Reference Parsons and Vanstreels2016). It is currently the only species from the genus Parapsyllus in southern Africa (Segerman, Reference Segerman1995).
A species of flea not previously reported for African penguins, the sticktight flea (E. gallinacea) was attached to the eyelids and body of penguins and recorded in the nests at Dassen Island. Echidnophaga gallinacea has a worldwide-distribution (Boughton et al., Reference Boughton, Atwelland and Schoech2006) and infests an extensive variety of hosts, including poultry, domestic mammals and wildlife (Segerman, Reference Segerman1995; Bitam et al., Reference Bitam, Dittmar, Parola, Whiting and Raoult2010). The European rabbit (Oryctolagus cuniculus) is also a common host and the presence of the rabbit and sharing of burrows on the island would explain the flea's presence on African penguins (Dunnet and Nardon, Reference Dunnet and Nardon1974). Female fleas remain firmly attached on the host for long periods and have a high fecundity (Krasnov, Reference Krasnov and Krasnov2008). This would explain the female-biased ratio on the penguins (0.16:1).
As several other Austrogoniodes species are associated with penguin species (Pilgrim and Palma, Reference Pilgrim and Palma1982; Clarke and Kerry, Reference Clarke and Kerry1993), the presence of the chewing louse A. demersus on African penguins in the current study is not unexpected (Von Keler, Reference Von Keler1952; Banks and Palma, Reference Banks and Palma2003). The louse has also been recorded on Galápagos penguins (Spheniscus mendiculus) in the Galápagos Archipelago (Banks and Palma, Reference Banks and Palma2003). The on-host sex ratio for A. demersus was female-biased (0.38:1) and might be the result of the longevity of females and, the smaller size and active lifestyle of males (Marshall, Reference Marshall1981b). Lice transmission occurs by direct body contact between individuals such as between parents to offspring in the nest (Clayton and Tompkins, Reference Clayton and Tompkins1994, Reference Clayton and Tompkins1995) and between older chicks when they group together in the crèche stage (Banks et al., Reference Banks, Palma and Paterson2006).
The soft tick O. capensis sensu stricto (s. s.) infests several seabird species globally (e.g. Keirans et al., Reference Keirans, Hutcheson and Oliver1992; Dupraz et al., Reference Dupraz, Toty, Noël, Estrada-Peňa, González-Solís, Boulinier, Dujardin and McCoy2016). In South Africa, O. capensis (s. s.) has been collected from several seabird species such as Cape cormorant (Phalacrocorax capensis) (Peirce and Parsons, Reference Peirce and Parsons2012), great black-backed gull (Larus marinus) and African penguins (Theiler, Reference Theiler1959; Daturi, Reference Daturi1986). Ornithodoros capensis (s. s.) is nidicolous and is able to live in the host's shelter for long periods of time (with a maximum life span of 25 years) (Sonenshine, Reference Sonenshine and Sonenshine1991, Reference Sonenshine and Sonenshine1993; Vial, Reference Vial2009). All life stages (larvae, nymphs and adults) attach to the host for short periods (from a few minutes up to an hour) to feed (Oliver, Reference Oliver1989). The colonial lifestyle, repeated use of nests and high population densities that penguins reach, expose them to a greater abundance of ticks (Duffy, Reference Duffy1988; Mangin et al., Reference Mangin, Gauthier-Clerc, Frenot, Gendner and Le Maho2003). In our study, O. capensis (s. s.) was the second most abundant ectoparasites found on penguins. The presence of blood in larvae from penguins provides new evidence that larval stages from some Ornithodoros spp. do take blood meals. This life stage was also the most abundant on penguins in the majority of the selected colonies. In nests, O. capensis (s. s.) was also the second most prevalent and abundant parasite, and exhibited one of the highest mean intensity of infestation. Nymphs were the most prevalent and abundant life stage in all nests, while larvae recorded the highest mean intensity in nests. This agrees with a previous study on African penguin nests at Marcus Island (Daturi, Reference Daturi1986). In the current study, only female ticks were recorded on penguins, while in nests the tick showed a strong bias towards males. In many nidicolous tick species, males require fewer nymphal stages to emerge as adults (i.e. become adults sooner) compared with females (Sonenshine, Reference Sonenshine and Sonenshine1991). This could explain the presence of more male than female ticks in penguin nests. In fact, it is not unusual to find large numbers of male nidicolous ticks in the host nests (e.g. Argas arboreus in nests of cattle egrets (Bubulcus ibis); Guirgis, Reference Guirgis1971).
In this study, Piroplasmorida/Haemospororida (orders that include Babesia spp., Plasmodium spp. and Leucocytozoon spp.; Levine, Reference Levine1971; Atkinson, Reference Atkinson, Atkinson, Thomas and Hunter2008) were more commonly recorded in penguins (33.51%) compared with Spirochaetales (2.59%) (order that includes Borrelia spp.; Paster et al., Reference Paster, Dewhirst, Weisburg, Tordoff, Fraser, Hespell, Stanton, Zablen, Mandelco and Woese1991). This pattern supports previous studies and suggests that Piroplasmorida/Haemospororida are more prevalent in penguin species compared with Spirochaetales (Quillfeldt et al., Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011; Yabsley et al., Reference Yabsley, Parsons, Horne, Shock and Purdee2012). Ornithodoros spp. are known vectors of haemoparasites; it is possible that the high prevalence of Babesia-like inclusions in erythrocytes observed in this study is related to the presence of O. capensis on penguins and in their nests.
Helminth species from four genera (Cardiocephaloides, Renicola, Contracaecum and Cyathostoma) were recorded from penguin chicks. Previously, the trematodes Cardiocephaloides physalis and Renicola sloanei, and the nematodes Contracaecum sp., Contracaecum variegatum and Cyathostoma phenisci were recorded from African penguins (Horne et al., Reference Horne, Bray and Bousfield2011; Kanarek et al., Reference Kanarek, Horne and Zaleśny2013; Viljoen, Reference Viljoen2015). Most of these helminths have been associated with various penguin species, which may be related to their similarity in diet (Brandão et al., Reference Brandão, Moreira and Luque2014). Cardiocephaloides spp. was the most prevalent (56.17%) helminth genus recorded in the study followed by Contracaecum spp. (12.83%) and Cyathostoma spp. (3.87%). These results are supported by a previous study on African penguins along the south-western coast of South Africa (Viljoen, Reference Viljoen2015). Since the life cycle of the helminth parasites involves fish, squid and krill, it is likely that penguins acquired infection through their diet (Randall and Bray, Reference Randall and Bray1983; Horne et al., Reference Horne, Bray and Bousfield2011; Brandão et al., Reference Brandão, Moreira and Luque2014). The integrity of the immune system, type of diet and behaviour of penguins will however determine the degree of susceptibility to helminth infections (Diaz, Reference Diaz2006; Diaz et al., Reference Diaz, Cremonte and Navone2010; Carrera-Játiva et al., Reference Carrera-Játiva, Rodríguez-Hidalgo, Sevilla and Jiménez-Uzcátegui2014).
Factors that influence parasite infestations
Significantly more ectoparasites, and particularly fleas (P. humboldti and E. gallinacea) and ticks (O. capensis s. s.) were recorded on chicks compared with adult penguins. Chicks generally have a less developed immune system and are therefore more susceptible to parasitic infestations compared with adult penguins (van Rensburg, Reference Van Rensburg2010). In addition, chicks spend more time in or close to the nest (Sherley et al., Reference Sherley, Waller, Strauss, Geldenhuys, Underhill and Parsons2014) and are therefore more readily infested by nest-associated ectoparasites (fleas and soft ticks). Since ticks can act as vectors of haemoparasites, the significantly higher incidence of Piroplasmorida/Haemospororida in chicks compared with adult penguins, in the current study, could potentially be a reflection of the pattern observed for O. capensis (s. s.) (Peirce, Reference Peirce2000).
Parasite infestations were significantly higher in mainland compared with island colonies. Stony Point and Simon's Town, the two mainland colonies, exhibited the same parasite richness but higher abundance and prevalence of parasites on and in penguins and in their nests than on islands. The most likely explanation for this pattern for ectoparasites is the higher densities of both total and active nests on mainland compared with island colonies. Large colonies with nests at close proximity allow proliferation and transmission of ectoparasites (Brown and Brown, Reference Brown and Brown1986). Parasite species found in nests vary in host association, and in particular soft ticks are able to remain in nests regardless of the presence of a bird host (Duffy, Reference Duffy1988) while fleas are dependent on the presence of the host (Marshall, Reference Marshall and Marshall1981a). This was supported by our results, which showed a positive correlation between mean abundance of O. capensis (s. s.) in nests and total nest density (active and non-active together), while mean total ectoparasite abundance in nests (of which fleas represented 71.57%) correlated with the density of active nests. The positive correlation between total nest density and Piroplasmids/Haemospororida prevalence in penguins is most probably due to a higher abundance of O. capensis (s. s.) in colonies with higher nests densities. Coloniality in birds seems to facilitate elevated haemoparasite richness and prevalence (Tella, Reference Tella2002). From the current study it appears that higher total nest density can further aggravate the situation.
The trematode Cardiocephaloides spp. was significantly more prevalent in chicks at the two mainland colonies compared with islands. The complete life cycle of C. physalis, the most likely Cardiocephaloides species found in our study, has not been fully described but it is hypothesized that it uses the snail Burnupena spp. as its first intermediate host (Ukomadu, Reference Ukomadu2017). Rock lobsters such as the South Coast (Palinurus gilchristi) and West Coast rock lobster (Jasus lalandii) are the potential natural predator of Burnupena spp. Both lobster species are commercially fished between the west coast (Cape Point) and the southeast coast (East London) of South Africa (Fig. 1). This range includes the two mainland colonies (Stony Point and Simon's Town) and Dyer Island (Department of Environmental Affairs, 2013). This could affect the abundance of rock lobster in the area and facilitate a larger abundance of Burnupena spp. Further, the distribution of fish (second intermediate host) and specifically infected fish can also contribute to this pattern. Recently, studies on sardines (food source of penguins) did record higher densities of sardines and specifically higher densities of Cardiocephaloides spp. infected sardines along the south-western coast between Cape Point and Cape Agulhas (Mhlongo et al., Reference Mhlongo, Coetzee, Shabangu, Merkle, Hendricks and Geja2013; Van der lingen et al., Reference Van Der Lingen, Weston, Ssempa and Reed2015). From this evidence, it is possible that the presence of intermediate and definitive hosts facilitates a higher incidence of Cardiocephaloides spp. in penguins at the two mainland colonies. Although Dyer island falls within the abovementioned range, it is possible that the inaccessibility of the island to humans may facilitate a healthier lobster population in the immediate area around the island. This may explain why the incidence of Cardiocephaloides spp. in penguin chicks was lower compared with the two mainland colonies.
Infestation levels of O. capensis on penguins and in their nests were higher at Stony Point compared with other colonies. This pattern explains the higher incidence of Piroplasmids/Haemospororida in penguins from Stony Point. In contrast to other colonies the number of breeding pairs have consistently increased at Stony Point for the last 5 years to reach a size of 2388 in 2016 (2017 counts are yet unconfirmed) (CapeNature, DEA and SANParks, unpublished results). This influx of penguins may explain the higher total nest density (0.28 nests per m2) and O. capensis (s. s.) abundance on penguins and in nests at Stony Point compared with the other colonies. Interestingly, the Simon's Town colony had the second highest total nest density, but a higher active nest density (0.14 nests per m2), although only slightly, compared with Stony Point (0.13 nests per m2) (Table 1). The dependence of fleas on hosts (Marshall, Reference Marshall and Marshall1981a) would explain the higher abundance of total ectoparasites (of which fleas represented >70%) and fleas in active nests at Simon's Town compared with the other colonies.
Very limited deductions can be made from the relationship between the remote sensed climate data and parasite infestation. It appears that there was a significant negative relationship between A. demersus infestation on penguins and annual mean temperature and precipitation. Conversely, there was a positive relationship between prevalence of Contracaecum spp. in penguin chicks and annual mean temperature and precipitation. Although there are some evidence that chewing lice are severely affected by temperature and humidity near the host skin (Johnson and Clayton, Reference Johnson and Clayton2003) and that hatching time of Anisakidae larva (helminth family of Contracaecum spp.) is delayed in colder water temperature (Højgaard, Reference Højgaard1998) the sample sizes of the current study are too small to make firm deductions.
Seasonal variation in parasite infestation
Parapsyllus humboldti and O. capensis (s. s.) prevalence and abundance on penguin chicks and in nests were higher in spring compared with the colder and wet autumn/winter. Possible drivers of this pattern may be the absence of caring adults (e.g. providing allopreening and food) for the chicks and more favourable climatic conditions for parasites during spring. During the 21-day moult period (spring and summer) in South Africa (Crawford et al., Reference Crawford, Hemming, Kemper, Klages, Randall, Underhill, Venter, Ward and Wolfaardt2006) adults leave chicks unattended and unfed in the nests (Sherley et al., Reference Sherley, Waller, Strauss, Geldenhuys, Underhill and Parsons2014). A lack of food (Obendorf and McColl, Reference Obendorf and McColl1980) and allopreening (Brooke, Reference Brooke1985) will affect chick condition and parasite infestations. The warm and dry conditions associated with spring are also more favourable for tick development, which would facilitate higher infestation of O. capensis (s. s.) in nests in spring (Lees, Reference Lees1947). The higher incidence of O. capensis during spring could explain the higher incidence of Spirochaetales in penguins during spring compared with autumn/winter. Yabsley et al. (Reference Yabsley, Parsons, Horne, Shock and Purdee2012) also recorded a higher prevalence of Borrelia spp. in blood smears from African penguins during spring/summer months (October to February) compared with autumn/winter months (March to September) in South Africa.
The current study provides current information on the parasite diversity of natural occurring African penguins and their nests at colonies along the south-western coast of South Africa. In general, penguin chicks are more susceptible to parasite infestations during spring. Further, it is evident that the observed spatial variation in parasite infestations between colonies is driven by several factors. In particular, patterns recorded for ecto- and haemoparasites tend to be facilitated by nest density, while the availability of infected prey influences helminth infestations. Knowing and detecting changes in parasitic diversity and abundance can give insight into the possible intrinsic and extrinsic factors that may threaten the conservation of African penguins in the region.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018002159.
Author ORCIDs
Marcela P. A. Espinaze, 0000-0003-0809-4403; Sonja Matthee, 0000-0001-7289-6262; Cang Hui's, 0000-0002-3660-8160.
Acknowledgements
We are grateful to Drs Heloise Heyne, Nola Parsons, Ralph Vanstreels, Tertius Gous and Terry Galloway for taxonomic support during parasite identification. We are also grateful to Dr Guillaume Latombe for his help with climate data extraction. We would like to acknowledge the students and staff at Stellenbosch University, the personnel at SANCCOB, and the many fieldworkers and colony managers from CapeNature and SANParks that supported this project.
Financial support
This work was supported by the International Penguin and Marine Mammal Foundation, the National Research Foundation (NRF; GUN 85718 to S. Matthee; GUN 89967 to C. Hui) and Stellenbosch University. MPAE was awarded a scholarship from the Chilean National Scholarship Program for Graduate Studies (Becas Chile/2016 - 72170154) of the National Commission for Scientific and Technological Research (CONICYT). Any opinion, finding and conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
Conflict of interest
None.
Ethical standards
This research project was approved by the Animal Ethics Committee of the University of Stellenbosch (reference number SU-ACUD15-00114) and permit were obtained from the Division of Environmental Affairs (RES2016/95 and RES2017/02), the Threatened or Protected Species (TOPS) of the Biodiversity Act (07962), CapeNature (AAA007-00191-0056) and South African National Parks (CRC/2016-2017/038-2015/V1).