Chronic inflammation in the airways is a central feature in asthma and other respiratory diseases and is mostly driven by oxidative stress(Reference Hosseini, Berthon and Wark1,Reference MacNee2) . The imbalance between endogenous oxidant and antioxidant substances can be influenced by diet, in particular fruits and vegetables, which are rich in antioxidants. Epidemiological evidence suggests that a diet rich in these foods might have a beneficial effect on asthma and lung function in adults and children(Reference Hosseini, Berthon and Wark1,Reference Garcia-Larsen, Del Giacco and Moreira3–Reference Uddenfeldt, Janson and Lampa6) .
Extensive experimental evidence shows that flavonoids, which are polyphenols synthesized by plants, have antioxidant, anti-allergic and anti-inflammatory effects(Reference Ferk, Misik and Nersesyan7–Reference Rice-Evans, Miller and Paganga13). It is known that flavonoids can reduce the activation of the cyclooxygenase gene, which is involved in the inflammatory response(Reference Lago, Toledo-Arruda and Mernak14,Reference Smith, DeWitt and Garavito15) , and they might act as an inhibitor of lipoxygenase, whose products are involved in the pathogenesis of a series of inflammatory diseases, including rhinitis(Reference Ribeiro, Freitas and Tome16). Lipoxygenase uses peroxyl radical complexes for the metabolism of arachidonic acid and flavonoids might act as antioxidants by neutralizing the radicals(Reference Prochazkova, Bousova and Wilhelmova17).
In spite of the extensive and promising experimental evidence suggesting a potential benefit of flavonoids on airways disease, epidemiological studies examining this association are still scant and limited to few flavonoid subclasses(Reference Santana, Pinheiro and Mernak18). In Finnish adults, higher intakes of quercetin, naringenin and hesperetin were associated with a reduced risk of asthma(Reference Knekt, Kumpulainen and Jarvinen19). In a case–control study conducted in London, dietary intake of three major flavonoids (catechins, flavonols and flavones) was not associated with asthma or chronic bronchitis(Reference Garcia, Arts and Sterne20).
GEIRD (Genes Environment Interaction in Respiratory Diseases), a population-based multi-case–control study, aimed to provide evidence on environmental exposures, history of disease, treatment, genetic information and measurements of markers of inflammation in individuals affected by asthma, allergic rhinitis and chronic obstructive pulmonary disease (COPD)(Reference de Marco, Accordini and Antonicelli21). In this population, we investigated the association of dietary intake of six major classes of flavonoids and risk of asthma, chronic bronchitis and rhinitis.
Materials and methods
Study design
GEIRD, a population-based multi-case–control study, is a two-stage project which involves seven Italian centres(Reference de Marco, Accordini and Antonicelli21). The first stage aimed to find probable cases and controls through a screening questionnaire on respiratory symptoms. The questionnaire was mailed to pre-existing random cohorts (the Italian Study on Asthma in Young Adults (ISAYA)(Reference de Marco, Poli and Ferrari22), the Italian arm of the European Community Respiratory Health Survey (ECRHS-Italy)(Reference de Marco, Verlato and Zanolin23)) and a new random sample from the general population (20–84 years of age, male/female = 1/1). The second stage aimed to ascertain the respiratory condition of the individuals through a clinical visit. Individuals invited to clinics were a random sample of those who reported signs suggestive of rhinitis and those free from any respiratory symptoms, and all individuals who reported signs suggestive of chronic bronchitis (CB), COPD or asthma. In this stage, each individual underwent a computer-assisted clinical interview, lung function tests, reversibility test, methacholine test and skin prick test (SPT), and dietary information was collected through a self-administered FFQ. All measurement protocols agreed with international guidelines (www.geird.org)(Reference de Marco, Accordini and Antonicelli21). In the present analysis, only the individuals with information on the FFQ recruited in the years from 2007 to 2010 in the centres of Pavia, Torino, Sassari and Verona were considered.
Lung function and allergological tests
All outcome variables were assessed following standard procedures that included previously validated spirometric tests. Individuals underwent spirometry for forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC), measured according to the American Thoracic Society reproducibility criteria(24). FEV1 % predicted and the lower limit of normal (LLN) for the FEV1/FEC were calculated on the basis of Quanjer et al.’s equations(Reference Quanjer, Stanojevic and Cole25). Individuals with a FEV1/FVC ≥ 70 % and ≥LLN underwent a methacholine challenge test, which followed a protocol described elsewhere(Reference Chinn, Burney and Jarvis26). Individuals with a FEV1/FVC < 70 % or <LLN underwent a bronchodilator challenge test and were invited (if eligible) to undergo a methacholine challenge test on a second occasion. Atopy was assessed by SPT to common allergens(27).
Dietary exposure assessment: flavonoid intake estimates
All exposure measurements were assessed following standard procedures that included previously validated questionnaires. Information on dietary intake was collected using the Italian version of the validated European Prospective Investigation into Cancer and Nutrition (EPIC) FFQ, which was based on Italian dietary habits and developed in the frame of an international survey(Reference Pisani, Faggiano and Krogh28). The NAF (Nutritional Analysis of Food Frequency Questionnaires, National Cancer Institute, Milan, Italy) software(Reference Pala, Sieri and Palli29) was used to compute the daily intakes of food items, energy, macro- and micronutrients. Nutrient data for specific foods were obtained from the food composition database for epidemiological studies in Italy(Reference Salvini30). Information on flavonoid intake was derived from the foods contained in the FFQ (mostly plant-derived). Estimates for intakes of total flavonoids and seven major subclasses (flavanones, anthocyanins, flavan-3-ols, flavonols, flavones, polymers and proanthocyanidins), expressed as aglycones (mg/d), were derived from sixty-five foods (see online supplementary material, Supplemental Table S1) using the US Department of Agriculture’s updated and expanded flavonoid content of foods and proanthocyanidin databases(Reference Bhagwat, Gebhardt and Haytowitz31,Reference Bhagwat, Haytowitz and Prior32) .
Overall, out of the 2749 individuals participating in the clinical stage of the survey, 1182 (43 %) filled in the FFQ. Forty-three individuals with incomplete FFQ (less than 80 % of the 434 questions and nested questions of the FFQ filled in) were excluded from the analyses. We estimated BMR using sex-specific equations for adults (≤60 years old)(Reference Harris and Benedict33) and the elderly (>60 years old)(Reference Fredrix, Soeters and Deerenberg34). Twenty-four individuals were excluded as BMR could not be computed due to missing data. Individuals were also excluded if they had extreme values of total energy intake (EI), which might suggest an unrealistic response: we excluded those with a ratio of EI to expected BMR below the 0·5th sample centile or above the 99·5th sample centile. Eight individuals were excluded according to the cut-off points on the top (four persons) and bottom (four persons) 0·5th percentile of the distribution of EI:BMR. Ten individuals (five women and five men) with extremely low EI levels (<2510 kJ (<600 kcal) for women and 3347 kJ (<800 kcal) for men) were excluded from the analyses(Reference Slattery, Jacobs and Dyer35) (Fig. 1).

Fig. 1 Flowchart of participant selection (EPIC, European Prospective Investigation into Cancer and Nutrition; COPD, chronic obstructive pulmonary disease; CA, current asthma; PA, past asthma; CB, chronic bronchitis; AR, allergic rhinitis; NAR, non-allergic rhinitis)
Identification of cases and controls in clinics
The sample for the current analysis included 1097 individuals (Fig. 1) with information on the clinical visit and on food and nutrient intakes, who were hierarchically classified as follows.
1. One hundred and fifty-nine cases of current asthma (CA):
a. he/she reported a history of asthma AND asthma-like symptoms/medicines in the last 12 months;
b. he/she reported a history of asthma OR asthma-like symptoms/medicines in the last 12 months plus one of the following conditions: (i) he/she had a positive methacholine challenge test with a provocative dose of methacholine causing a 20 % drop in FEV1 (PD20) of <1 mg; (ii) he/she had pre-bronchodilator FEV1/FEC < 70 % or <LLN(Reference Quanjer, Stanojevic and Cole25) with a positive reversibility test (i.e. FEV1 > 12 % and >200 ml after the administration of 400 μg salbutamol); (iii) he/she had pre-bronchodilator FEV1/FEC < 70 % or <LLN with a post-bronchodilator FEV1/FVC > LLN and >70 % and a post-bronchodilator FEV1 > 80 % predicted(Reference Quanjer, Stanojevic and Cole25).
2. Seventy-eight cases of past asthma (PA): he/she reported a history of asthma but did not fulfil the criteria for CA.
3. Ten cases of COPD: post-bronchodilator FEV1/FEC < 70 % or <LLN without asthma.
4. Forty-seven cases of CB: he/she was not a COPD or asthma case and he/she reported chronic cough or phlegm (>3 months/year for at least 2 years).
5. One hundred and sixty-seven cases of allergic rhinitis (AR) and 125 cases of non-allergic rhinitis (NAR): he/she had nasal allergies or nasal problems in the presence of animal(s), pollens and/or dust, plus a negative SPT (NAR) or a positive SPT to at least one allergen (AR).
6. Three hundred and ninety-seven controls: individuals without any nasal/respiratory symptoms/conditions reported in the clinical questionnaire neither in the clinic nor in the screening questionnaire, who were not cases and had both (i) pre-bronchodilator FEV1/FVC > LLN and >70 %; and (ii) FEV1 > 70 % predicted.
Ninety-seven individuals were unclassified due to missing data.
Individuals with COPD and unclassified individuals were excluded from the present analyses, therefore the final sample included 990 participants. Due to the hierarchical classification, cases of CA and PA could also present COPD/CB/AR/NAR; cases of CB could present AR/NAR.
Statistical analyses
Participant characteristics were summarized as percentages, or as medians and interquartile ranges (IQR), or means and standard deviations; flavonoid and food intakes were summarized as medians and IQR. The χ 2 test, Kruskal–Wallis test and Student’s t test were used to test differences (α = 0·05), where appropriate. The main exposures of interest (total flavonoids and each subclass) were considered as continuous variables and analysed for a variation of one standard deviation. Exposures were also categorized into quartiles based on the distribution of the exposure in controls. Quartiles allow the comparison of extreme groups, such as those with highest and lowest intakes of nutrients or foods, and are less sensitive to the effects of outliers than continuous variables. On the other hand, the estimates of measures of disease risk related to dietary exposures expressed as continuous variables make easier the comparison of evidence obtained in different studies.
To analyse the associations between each flavonoid class and case–control status, several multinomial logistic regression models were fitted to the data, using a six-level variable (CA, PA, CB, AR, NAR, control) as outcome. Multivariable associations of exposures with case–control status were expressed by relative risk ratios (RRR; using control as the reference category) and their 95 % confidence intervals. Analyses were adjusted for study sample/cohort (ISAYA, ECRHS-Italy, new random sample), centre (Verona, Pavia, Torino and Sassari), gender, age, BMI, education (low = completed before the age of 16 years; high = completed after the age of 16 years) as a proxy of socio-economic status, smoking habit (never smoker; past smoker, i.e. not smoking in the last month; current smoker), alcohol intake (g/d), total energy intake (kcal/d), vitamin C intake (mg/d) and total fruit intake (g/d). The analyses were performed using the statistical software package Stata version 14.2.
Results
Among the groups of cases and controls, we found no differences in terms of age (overall mean: 50·7 (sd 12·3)years), gender (overall proportion of males: 48·79 %), BMI (overall median: 24·7 (IQR 22·3–27·7) kg/m2), physical activity and educational level (Table 1).
Table 1 General characteristics of the participants: adults from the GEIRD (Genes Environment Interaction in Respiratory Diseases) population-based multi-case–control study in Pavia, Torino, Sassari and Verona, Italy, 2007–2010

CA, current asthma; PA, past asthma; CB, chronic bronchitis; AR, allergic rhinitis; NAR, non-allergic rhinitis; IQR, interquartile range.
Comparing the characteristics of the individuals who fulfilled the EPIC FFQ (n 1182) and those who did not (n 1567), we found that mean age was significantly higher in the groups of controls, CA and AR among individuals who fulfilled the questionnaire (51·0 (sd 12·0), 50·5 (sd 12·6) and 49·7 (sd 12·5) years, respectively) compared with the individuals who did not (49·7 (sd 12·9), 45·2 (sd 11·8) and 46·9 (sd 12·7) years, respectively; see online supplementary material, Supplemental Table S2).
Among participants included in the analysis, we found that smoking habits, drinking habits and alcohol intake varied significantly across the groups (P = 0·013, 0·018 and 0·023, respectively), with the highest proportion of drinkers and current smokers in the group affected by CB (57·45 and 34·04 %, respectively). There were no statistically significant differences in the intakes of total or subclasses of flavonoids across the case groups (overall median total flavonoids intake: 382·1 (IQR 248·8–529·8) mg) and in the intakes of foods rich in flavonoids (Table 2). Overall median fruit and vegetable intakes were 289·1 (IQR 191·9–425·5) g/d and 130·2 (IQR 86·7–194·5) g/d, respectively. There were no statistically significant differences in the intakes of total energy intake and vitamin C across the groups (overall median energy intake: 7988·5 (IQR 6299·0–10 037·0) kJ/d (1909·3 (IQR 1505·5–2398·9) kcal/d); overall median vitamin C intake: 117·6 (IQR 82·6–161·9) mg/d).
Table 2 Distribution of the dietary flavonoid, food and nutrient intakes studied, according to case or control status, among adults from the GEIRD (Genes Environment Interaction in Respiratory Diseases) population-based multi-case–control study in Pavia, Torino, Sassari and Verona, Italy, 2007–2010

CA, current asthma; PA, past asthma; CB, chronic bronchitis; AR, allergic rhinitis; NAR, non-allergic rhinitis; IQR, interquartile range.
In the unadjusted continuous regression analyses, there were no significant associations between any of the flavonoid subclasses and the outcomes considered (Table 3). After adjustment, we found that an increase of 1 sd in intake of flavanones, equivalent to 26·1 mg/d, was associated with a reduced risk of NAR (RRR = 0·68, 95 % CI 0·47, 0·97). Similarly, adjusted analyses exploring the exposures per quartile showed that those in the highest v. lowest quartile of flavanone intake had a reduced risk of NAR (RRR = 0·24, 95 % CI 0·10, 0·59). We found no consistent evidence of an association between a high intake of other subclasses of flavonoids and the risk of any of the other outcomes.
Table 3 Unadjusted and adjusted* relative risk ratios (RRR) and 95 % confidence intervals of being a respiratory disease case rather than a control (n 397), according to the intake of flavonoids†, among adults from the GEIRD (Genes Environment Interaction in Respiratory Diseases) population-based multi-case–control study in Pavia, Torino, Sassari and Verona, Italy, 2007–2010
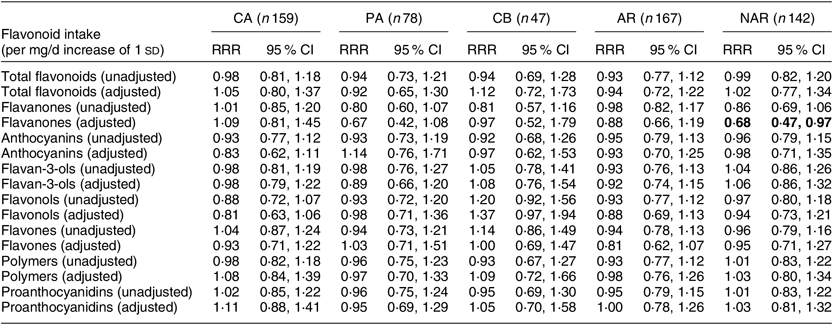
CA, current asthma; PA, past asthma; CB, chronic bronchitis; AR, allergic rhinitis; NAR, non-allergic rhinitis.
* The estimates were adjusted for age, gender, centre, study cohort, BMI, smoking habits, alcohol intake, educational level, total fruit intake, vitamin C intake and total energy intake.
† Significant results are shown in bold.
Discussion
In this multi-case–control study of Italian adults from the general population, we found that having a higher intake of flavanones was associated with a reduced risk of NAR. The association was statistically significant when the groups were examined as the highest v. lowest quartile of intake and when the intake was analysed as a continuous exposure.
Flavonoids have been described to have anti-inflammatory and antioxidant properties(Reference Ferk, Misik and Nersesyan7–Reference Rice-Evans, Miller and Paganga13). Airway inflammation, which is exacerbated by oxidative stress(Reference Lago, Toledo-Arruda and Mernak14), is known to be related to the pathogenesis and worsening of pulmonary diseases(Reference MacNee2,Reference Rebuck and Chapman36) . In our study, we considered asthma, CB and rhinitis, diseases in which airway inflammation plays a key role(Reference Eifan and Durham37). Depending on the severity of asthma, inflammation can affect the large and small airways(Reference MacNee2). Similarly, in CB, inflammation affects the lumen and the wall of the airways(Reference Braman38). Experimental studies show that flavonoids can counteract the oxidative stress and inflammation caused by environmental insult to the airways. Quercetin, one of the most investigated flavonoids, has been shown to reduce superoxides and nitric oxide radicals, which are mediators of inflammation(Reference Huk, Brovkovych and Nanobash Vili39,Reference van Acker, Tromp and Haenen40) . Flavonoids can inhibit the metabolism of arachidonic acid, which is involved in the production of reactive oxygen species(Reference Ferrandiz and Alcaraz10,Reference Middleton and Kandaswami41) . Moreover, they seem to reduce the adhesion of inflammatory cells(Reference Lago, Toledo-Arruda and Mernak14).
In spite of the large body of experimental evidence supporting an association of flavonoid intake with respiratory diseases, epidemiological studies are still scant and have reported mixed results, which might partly be explained by the different types of flavonoids studied(Reference Tanaka and Takahashi42). Several population-based studies have suggested that higher intakes of foods rich in flavonoids (mainly fruits, vegetables, wine and tea) were associated with a lower risk of asthma(Reference Hosseini, Berthon and Wark1,Reference Shaheen, Sterne and Thompson43) , although this association has not been confirmed in other studies(Reference Garcia-Larsen, Arthur and Potts44). The few population-based studies that have examined the association of flavonoid intake and airways disease are limited and the evidence is inconclusive. Tabak et al. reported that a higher intake of catechins was associated with a lower risk of COPD and asthma in a population of Dutch adults(Reference Tabak, Arts and Smit45). Garcia et al. found no evidence of an association of three subclasses of flavonoids with asthma or CB in a population-based case–control study in London(Reference Garcia, Arts and Sterne20). Moreover, a recent clinical trial on both adults and children with poor asthma control revealed no association between the supplementary use of soya isoflavone and lung function(Reference Smith, Kalhan and Wise46).
The recent hypothesis that microbiota might play a role to protect against inflammation-mediated airways diseases was investigated in a small sample of twenty-three allergic individuals(Reference Cuervo, Hevia and Lopez47). The study showed that a higher intake of a diet rich in phenolic compounds was associated with a more stable gut microbiota, which might contribute to the management of allergic conditions through a reduced inflammation. A recent cohort study reported that a higher intake of soya genistein was associated with improved lung function and asthma control in individuals with stable asthma(Reference Bime, Wei and Holbrook48). Evidence from human trials is also limited. A recent randomized controlled trial found no evidence that supplementation with soya genistein had any effect on outcomes of asthma or lung function in adults with unstable asthma(Reference Smith, Kalhan and Wise46), while in an intervention in forty-two asthmatic adults, a passion fruit puree extract rich in bioflavonoids reduced the severity of asthma symptoms(Reference Watson, Zibadi and Rafatpanah49).
In our study, we found limited evidence to suggest that flavonoids are associated with airways disease. The individuals in our population-based study had stable asthma, CB or AR. It might be possible that the beneficial effect of flavonoids occurs in cases of greater chronic airway inflammation, which is usually reduced in stable patients. We found a statistically significant negative association of flavanones with non-atopic rhinitis. The association with atopic rhinitis was also negative in both approaches used, but not statistically significant. Atopy in those affected by rhinitis is defined on the basis of an SPT, which measures the systemic presence of serum allergen-specific IgE in allergic individuals. However, it has also been found in individuals affected by non-atopic rhinitis that allergen-specific IgE can be found locally in the nasal mucosa, which is not detectable through an SPT(Reference Hamizan, Rimmer and Alvarado50). Flavonoids are thought to inhibit the formation of IgE(Reference Kimata, Shichijo and Miura9,Reference Santana, Pinheiro and Mernak18) and this might explain the positive effect found on individuals with non-atopic rhinitis in the present study.
Our study did not confirm an association of any of the flavonoid subclasses studied with CB, which is in line with the results reported in an earlier case–control study in the general population(Reference Garcia, Arts and Sterne20). Anthocyanin intake was associated with a reduced risk of CB, but this did not reach statistical significance. Although inflammation is a central feature in CB, it might be possible that avoidance of environmental pollutants and related environmental insult might be the best effective way to reduce the risk of this disease(Reference Braman38).
Some observational studies have analysed the association between flavonoids and atopic rhinitis. The extract of French maritime bark, which is rich in flavonoids, appeared to reduce eye and nasal symptoms, but the results were limited by the small sample of participants(Reference Ross51). In another clinical trial, a preventive effect of enzymatically modified isoquercitrin was found on ocular symptoms, but not on nasal symptoms(Reference Hirano, Kawai and Arimitsu52).
Our study has several strengths. We considered several chronic respiratory diseases, giving a wide view of the possible role of flavonoids in different pulmonary health conditions. Case and control status was assessed in a two-stage process; first by a screening questionnaire on symptoms and use of medicines, then through a clinical visit(Reference de Marco, Accordini and Antonicelli21). The EPIC FFQ was validated through comparison with multiple interviews and the analysis of nitrogen in urine samples(Reference Pisani, Faggiano and Krogh28). We analysed the associations between flavonoid intakes and chronic respiratory diseases considering seven subclasses of flavonoids, computed using data from the US Department of Agriculture database, which provides updated information of flavonoid contents of foods.
We acknowledge some limitations to the study. Having five case groups and only one control group might have reduced the power to detect statistically significant differences between the participants. Moreover, we defined the potential confounders a priori based on pre-existing knowledge on their relationship with flavonoids and with the outcomes evaluated; however, residual confounding may still exist. The Italian version of the EPIC FFQ provides an extensive list of foods with high content of flavonoids, which allowed us to derive estimates for all major subclasses. However, the earlier validation study of the Italian version of the EPIC FFQ showed modest levels of agreement in the intakes of macronutrients and some foods, and has not been validated specifically for flavonoid intake(Reference Pisani, Faggiano and Krogh28). Finally, we used data from a single FFQ and assumed that adults maintained relatively stable dietary habits to investigate disease risk(Reference Macdonald, New and Reid53).
Overall, the results of the present population-based, multi-case–control study suggest that intake of flavanones is negatively associated with the risk of having NAR. No associations were found between other flavonoids and the considered outcomes.
Acknowledgements
Acknowledgements: The authors are indebted to all the individuals of the GEIRD study for their participation. Financial support: The GEIRD project was funded by the Cariverona Foundation; the Italian Ministry of Health; Chiesi Farmaceutici; and the Italian Medicines Agency (AIFA). The funders had no role in the design; in the collection, analysis and interpretation of the data; in the writing of the manuscript; and in the decision to submit the manuscript for publication. Conflict of interest: The authors declare no conflict of interest. Authorship: V.G.-L., M.E.Z. and L.C. conceived and designed the study. L.C., M.E.Z., R.B., I.C., P.P. and M.F. contributed to the data collection. V.M. performed the statistical analysis. L.C., M.E.Z. and V.M. drafted the manuscript. L.C., M.E.Z., V.G.-L., A.G., V.M., P.P. and M.F. contributed to the interpretation of data, revised the paper critically for important intellectual content and approved the version to be published. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committee of Azienda Ospedaliera of Verona, as coordinating centre. Written informed consent was obtained from all subjects.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019003562






