Since three-dimensional printing technology was first described by Charles Hull in 1986,Reference Hull1 technological advances have led to increased use in the medical field, including for education,Reference Jones and Seckeler2,Reference White, Sedler, Jones and Seckeler3 procedural planning,Reference Frolich, Spallek and Brehmer4,Reference Giannopoulos, Steigner and George5 and device development.Reference Dzian, Živčák, Penciak and Hudák6 Typical datasets for three-dimensional model generation come from computed tomography or magnetic resonance scans that are segmented to highlight the regions of interest, converted to stereolithographic format, and printed. Three-dimensional rotational angiography is a newer imaging modality performed in the cardiac catheterisation laboratory and produces a three-dimensional dataset that can be used for the virtual reconstruction of complex structures. It was initially used in neurovascular interventionsReference Thron and Voigt7 and was first used in a congenital cardiac catheterisation in 2007.Reference Rigatelli, Zamboni and Cardaioli8 With improved image processing and the ability to overlay three-dimensional reconstructions onto standard fluoroscopic imaging, rotational angiography use has continued to increase for diagnosticReference Panzer, Taeymans and De Wolf9–Reference Aldoss, Fonseca and Truong15 and interventionalReference Fagan, Kay, Carroll and Neubauer16–Reference van der Stelt, Krings, Molenschot and Breur25 congenital cardiac catheterisation.
Rotational angiography imaging provides datasets similar to other advanced imaging modalities for the creation of highly detailed, patient-specific three-dimensional virtual models. In addition, rotational angiography may be more advantageous than multi-slice CT scans, as the imaging is focused on the area of interest, generating less radiation exposure.Reference Struffert, Hauer, Banckwitz, Köhler, Royalty and Doerfler26 There are few publications detailing the use of rotational angiography datasets of CHD to generate three-dimensional-printed models.Reference Poterucha, Foley and Taggart19,Reference Parimi, Buelter and Thanugundla27 The purpose of this study was to create three-dimensional-printed models from rotational angiography datasets of CHD and assess the quality (both objectively and subjectively) of the models produced.
Materials and methods
After approval from the Institutional Review Boards of the University of Arizona and Nationwide Children’s Hospital, congenital catheterisations that used rotational angiography from January, 2017 to March, 2018 were retrospectively identified. Catheterisations were performed in Toshiba and Philips laboratories. Rapid ventricular pacing, contrast concentration, and contrast volume were based on standard practice and operator preference. Cases were chosen to reflect various CHD anatomies. Digital Imaging and Communications in Medicine data were segmented using a semi-automated process with Philips IntelliSpace Portal (Philips Medical Systems, The Netherlands), cleaned using Autodesk® Meshmixer™ (Autodesk, Inc., San Rafael, CA, USA), prepared for printing using Simplify3D® version 4.0.0 (Simplify3D Software, Cincinnati, OH, USA), and printed with polylactic acid on a Dremel® 3D Idea Builder (Dremel, Mount Prospect, IL, USA, list price $1299; Fig 1a–d). All segmentations, model generations, and printings were performed by a single user (MDS) who has personally generated over 250 three-dimensional-printed models of CHD. Data collected included patient demographics, diagnosis, and contrast concentration and volume used for the rotational angiography. Three-dimensional printing time and materials’ costs were also collected.
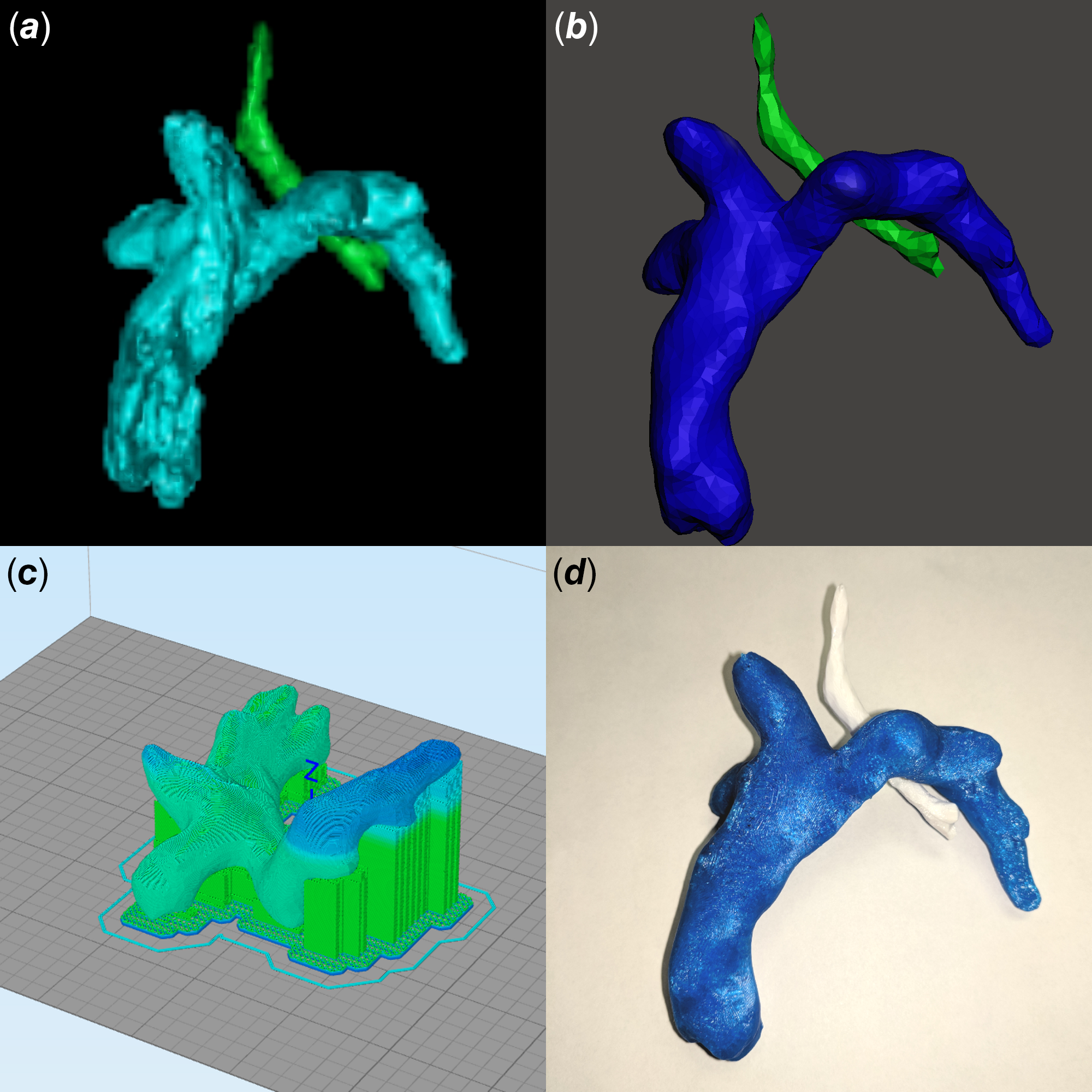
Figure 1. Generation of a three-dimensional-printed model from three-dimensional rotational angiography data. (a) Digital Imaging and Communications in Medicine data are first segmented to define the anatomy of interest (Fontan circuit in cyan, trachea in green). (b) The model is cleaned (Fontan circuit in blue, trachea in green). (c) The model is prepared for printing. (d) Final three-dimensional-printed model (Fontan circuit in blue, trachea in white).
Printed three-dimensional models were objectively assessed using a method described for three-dimensional printing of livers.Reference Witowski, Wake and Grochowska28 The printed models were imaged using a Siemens Inveon uCT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA, USA). A total of 360 projections were acquired in a full rotation, using low magnification with a 4×4 binning. The camera was set in rat mode with a transaxial field of view of 3072 px (81.02 mm), and axial scan length was set at 128 mm, which requires three bed positions and uses a 31.52% field of view overlap for reconstruction. Exposure settings were 80 kV, with 500 µA current and an acquisition time of 250 ms. Image reconstruction was done using a Feldkamp algorithm, a Shepp–Logan filter, slight noise reduction, and twofold downsampling. Digital Imaging and Communications in Medicine images from the CT were segmented using Philips IntelliSpace Portal and were compared to the original digital models using MeshLab version 2016.12 (Visual Computing Laboratory, Pisa, Italy). Hausdorff distances were calculated with vertex samples, using at least 100,000 samples, and the root mean square of the difference was calculated for each model comparison.Reference Cignoni, Rocchini and Scopigno29 Distances were reported as “mesh units”, not absolute measures, and the closer the root mean square was to 0, the more similar the models.
For a subjective assessment of the printed models, two blinded, independent, non-interventional paediatric cardiologists provided descriptions of the anatomy and any interventions (e.g., hypoplastic left heart syndrome after Norwood reconstruction with a modified Blalock–Taussig–Thomas shunt) based on the three-dimensional-printed models. In addition, they provided a subjective rating (1–5) of quality and accuracy of the three-dimensional-printed models as compared to the original digital models.
Data were compared using Mann–Whitney U test, χ2, and κ for agreement, as appropriate. A p-value <0.05 was considered statistically significant.
Results
Data from 15 rotational angiograms were three-dimensional printed. Patient characteristics and diagnoses are shown in Table 1. Details of image acquisition and three-dimensional printing are shown in Table 2. Because of the focal application of contrast to the structure(s) of interest, initial segmentation time was less than 10 minutes per model, which was half the typical time for segmentation of similar anatomy from CT or MR dataset (MDS, personal experience). The median time to print the three-dimensional models was 3.83 hours (IQR 3.35, 7.02 hours), with a material cost of $2.84 per model. Print time and material cost were affected by model size and complexity. All models were of vascular structures (aorta, main/branch pulmonary arteries, superior/total cavopulmonary connections, coronary artery fistula, superior caval vein). There was very high agreement between the original digital reconstructions and those generated from CT scans of the printed models (root mean square for Hausdorff distance 0.013 ± 0.003 mesh units). Model descriptions by the independent reviewers are shown in Table 3. They correctly described the anatomy and interventions for 80 and 87% of the models (p = 0.334) and rated the quality and accuracy of the models high with a substantial agreement (5 versus 5, p = NS; κ = 0.615). Two models were incorrectly described by both reviewers.
Table 1. Patient demographics and cardiac diagnoses. Data are shown as n (%) or median (interquartile range)
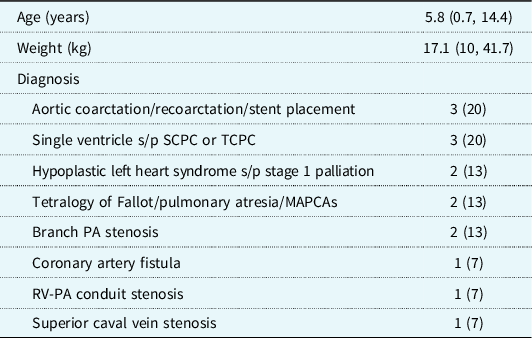
MAPCAs = major aortopulmonary collateral arteries; PA = pulmonary artery; RV-PA = right ventricle to pulmonary artery; SCPC = superior cavopulmonary connection; TCPC = total cavopulmonary connection
Table 2. Image acquisition details and costs and times for three-dimensional model printing. Data are shown as n (%) or median (interquartile range)
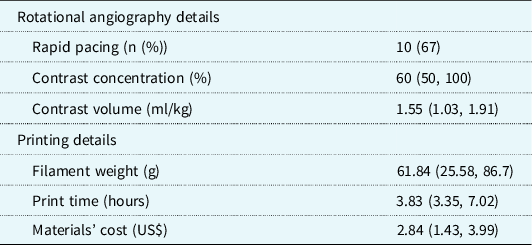
Table 3. Summary of anatomic descriptions of underlying cardiac defects and interventions along with descriptions provided by the independent reviewers. There were two models of complex, abnormal branch pulmonary arteries that were incorrectly identified by both reviewers. Of note, the full cardiac diagnoses are provided for completeness, but the majority of the three-dimensional-printed models did not include intracardiac anatomy, so the description of this was not expected of the reviewers
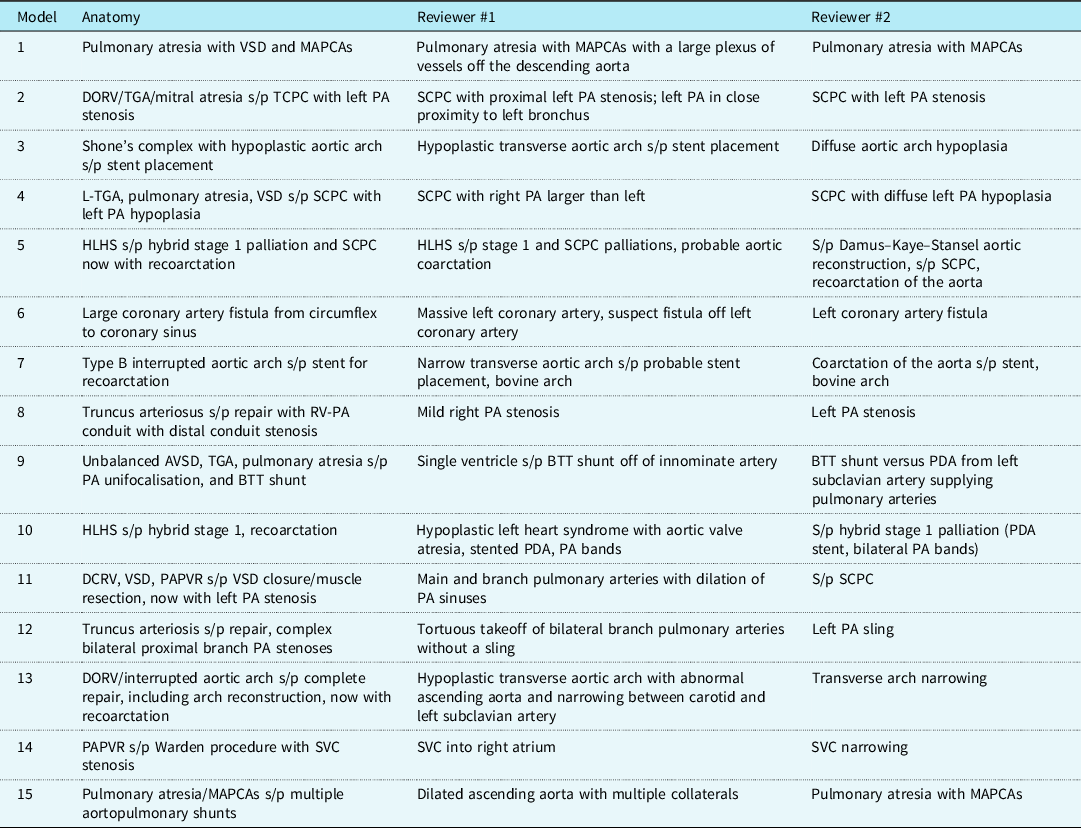
AVSD = atrioventricular septal defect; BTT = Blalock–Taussig–Thomas; DCRV = double chamber right ventricle; DORV = double outlet right ventricle; HLHS = hypoplastic left heart syndrome; MAPCAs = major aortopulmonary collateral arteries; PA = pulmonary artery; PAPVR = partial anomalous pulmonary venous return; PDA = patent ductus arteriosus; RV-PA = right ventricle to pulmonary artery; SCPC = superior cavopulmonary connection; SVC = superior caval vein; TCPC = total cavopulmonary connection; TGA = transposition of the great arteries; VSD = ventricular septal defect
Discussion
In this series, rotational angiography data from congenital cardiac catheterisations of a variety of defects and surgical palliations were successfully and accurately printed as three-dimensional models. While there are two small prior reports of this technique,Reference Poterucha, Foley and Taggart19,Reference Parimi, Buelter and Thanugundla27 we generated models from a wide variety of CHD anomalies and provided objective measures to assess the accuracy of the printed models. There are several potential advantages of using three-dimensional datasets from rotational angiography to generate three-dimensional-printed models.
A valuable clinical application of three-dimensional-printed models from rotational angiography is for surgical planning. The utility of three-dimensional-printed models from CT and MR datasets for planning the surgical approach for complex CHD has been well documented.Reference Batteux, Haidar and Bonnet30 Many patients with CHD undergo a pre-operative diagnostic cardiac catheterisation to obtain haemodynamic data. Utilisation of rotational angiography data for three-dimensional models minimises the need for additional procedures, radiation, and general anaesthetic exposure. Our findings show that the three-dimensional-printed models from rotational angiography datasets are high quality, accurate, and should be acceptable for surgical planning.
Another use for three-dimensional-printed models generated from rotational angiography is for educational purposes. Additional advanced imaging is not always obtained for patients who undergo cardiac catheterisations, but there is a value in understanding their complex anatomy. If rotational angiography datasets are being obtained as part of routine care, then these existing datasets can be used to generate libraries of CHD for trainees, such as has been done for interventional radiology, neuroradiology, and structural heart disease.Reference Giannopoulos, Steigner and George5,Reference Kerrien, Yureidini, Dequidt, Duriez, Anxionnat and Cotin31 Many interventionalists use the 2D angiography performed during procedures to educate patients and families, but recent studies have shown three-dimensional models to be more effective for family understanding in pre-procedure meetings for cardiac and neurosurgical procedures.Reference Guo, He and Duan32,Reference Boyer, Yell, Andrews and Seckeler33 The additional time needed to generate three-dimensional virtual models from rotational angiography for this purpose is minimal, and it is feasible to three-dimensional print a small model after the procedure and before hospital discharge. As three-dimensional printing technology becomes faster, it is conceivable that printed models of pre- and post-intervention anatomy could be generated during the catheterisation procedure. These models would provide families a better understanding of the indications and outcomes of an intervention.
Looking beyond the current limitations of our technology, there is a much more practical and exciting potential use of real-time three-dimensional printing. A major challenge for transcatheter interventions in infants and children with CHD is the need to modify existing equipment, such as stents, to fit their unique anatomy.Reference Sullivan, Liou and Takao34 The use of three-dimensional printing to create custom stents for a patient has been proposed.Reference Zablah and Morgan35 As three-dimensional printing of metals becomes faster, it is conceivable that a custom stent could be “designed” using a rotational angiography dataset and three-dimensional printed for implantation during the same catheterisation procedure. This would obviate the need for additional imaging and invasive procedures in this fragile population. The application of real-time three-dimensional printing is still futuristic, due to the high complexity and expense of three-dimensional printing metals, in addition to regulatory and safety challenges. Through continued research and development, however, this technology should be obtainable in the future.
While some are hesitant to adopt the use of rotational angiography due to the radiation exposure involved, this concern has not been borne out in studies. In fact, rotational angiography has been found to have a lower radiation dose than standard two-dimensional angiographyReference Haddad, Waller and Johnson36 and two-dimensional digital subtraction angiography,Reference Struffert, Hauer, Banckwitz, Köhler, Royalty and Doerfler26 and the ability to focus the imaging only on the specific anatomy of interest also allow a lower dose than multi-slice CT scans.Reference Struffert, Hauer, Banckwitz, Köhler, Royalty and Doerfler26 A complete understanding of fluoroscopy equipment and optimisation of the settings to minimise radiation exposure remains key. The combination of cardiac motion and the relatively slow acquisition time of rotational angiography, compared to multi-slice CT scans, limits the ability to visualise intracardiac structures with current rotational angiography technology. Currently, rotational angiography offers excellent visualisation of vascular structures. Since the majority of angiography for congenital cardiac interventions is for vascular structures (angioplasty, stenting, and vessel embolisation), this is of minimal concern. Further development of rotational angiography technology and techniques will lead to improved imaging of intracardiac structures.
This study is limited by the small numbers and retrospective design. In addition, depending on the institutional experience and available equipment, the model creation and three-dimensional printing times and costs may vary. While there were some limitations in the ability of the reviewers to describe the exact underlying CHD for several of the models, this was not an expected goal of the study, particularly in light of the fact that only part of the anatomy was visualised with the rotational angiography and physically printed for most of the patients. Regardless of these limitations, the data presented provide insight into an intriguing potential future application to further integrate three-dimensional printing into clinical is for patients with CHD.
This proof of concept study has shown that Digital Imaging and Communications in Medicine data from standard rotational angiography can be successfully converted into three-dimensional-printed models for a variety of CHD with excellent accuracy for defining anatomy. The cost of printing the models was negligible, but the time to print is still too long to allow for real-time use of the models. As the speed of three-dimensional printing technology increases, a novel future application of this technique could allow for the printing of patient-specific stents and devices in the catheterisation lab based on rotational angiography datasets.
Acknowledgements
The authors wish to acknowledge and thank Brenda Baggett for her help performing the CT scans of the three-dimensional-printed models. Dr S. received a grant from the Sarver Heart Center Congenital Heart Disease Education Grant and the University of Arizona College of Medicine Vernon, and Virginia Furrow Award for Medical Education Research to establish a three-dimensional printing programme for congenital heart disease at the University of Arizona, but not to directly support the current study.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees of the University of Arizona and Nationwide Children’s Hospital.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121000287.







