Introduction
Obstructive sleep apnoea hypopnoea syndrome (OSAHS) is characterised by recurrent episodes of complete or partial obstruction of the upper airway during sleep. This leads to oxygen desaturation, fragmented sleep and daytime somnolence.Reference Feng, Li, Zhou, Jia and Kang1 For people with sleep apnoea, the combination of disturbed sleep and oxygen starvation may lead to hypertension, heart disease, and mood and memory problems.Reference Chandola, Ferrie, Perski, Akbaraly and Marmot2 Sleep apnoea also increases the risk of automobile crashes.Reference George3 It is therefore important to assess the risk of OSAHS early.
There is evidence that OSAHS is associated with a group of proinflammatory cytokines. Prior studies have shown that biomarkers of inflammation are raised in apnoeic patients.Reference Yokoe, Minoguchi, Matsuo, Oda, Minoguchi and Yoshino4 These inflammatory changes are postulated to occur, in part, due to snoring that evokes vibration frequencies associated with soft-tissue damage (local inflammation).Reference Yokoe, Minoguchi, Matsuo, Oda, Minoguchi and Yoshino4 In addition, there is evidence of systemic inflammation in patients with OSAHS.
Tumour necrosis factor-α (TNF-α) is a proinflammatory cytokine chiefly produced by activated macrophages, although it can be produced by many other cell types, such as cluster of differentiation 4+ lymphocytes, natural killer cells and neurons.Reference MacKenzie, Planas and Goetz5 It is associated with a variety of immunological functions such as the regulation of cellular immune responses and modulation of the expression of many other cytokines.Reference MacKenzie, Planas and Goetz5
The production of TNF-α, which can be attributed to obstructive sleep apnoea (OSA)-induced hypoxic stress, is mediated by nuclear factor κβ activation.Reference Tamaki, Yamauchi, Fukuoka, Makinodan, Koyama and Tomoda6 The prevailing T-helper type 1 (Th1) cytokine pattern of the immune cells in the peripheral blood of OSAHS patients can partially be explained by hypoxia. In fact, experimental evidence supports the role of hypoxia in inducing the expression of the proinflammatory cytokines, in particular TNF-α, but also interleukin (IL)-1b and IL-6.Reference Naldini, Carraro, Silvestri and Bocci7
Interleukin-10 is one of the most important anti-inflammatory cytokines. It is produced mainly by T-helper type 2 (Th2) lymphocytes, macrophages, B cells and monocytes. Convincing evidence suggests that IL-10 is crucial for atherosclerotic plaque stability, whereby IL-10 blocks the synthesis of proinflammatory cytokines.Reference Grütz8
It has been revealed that positive airway pressure treatment significantly lessens the symptoms of OSAHS and related diseases; it is a gold standard therapy for moderate and severe OSAHS.Reference Koseoglu, Ikinciogullari, Cetin, Uysal, Kum and Arli9
The present study aimed to explore the significance of the TNF-α/IL-10 ratio and the effect of continuous positive airway pressure (CPAP) in patients with different degrees of OSAHS severity.
Materials and methods
Study population
The study included 135 adult OSAHS patients, all of whom had visited the chest or otolaryngology clinics at the Xiangyang Central Hospital Affiliated to Hubei University of Arts and Science, from January 2008 to January 2012, for the evaluation of sleep problems. All patients underwent diagnostic polysomnography in the sleep laboratory to confirm OSAHS and determine the severity of the condition. We also enrolled 94 control volunteers, matched in terms of age and body mass index (BMI), who were undergoing routine physical examination in our hospital during the same period as the patients.
Participants were excluded from the present study if they had: chronic obstructive pulmonary disease, active inflammatory disease or autoimmune disorders, severe heart failure, advanced renal or hepatic disease, personal or family history of psychiatric disorders, or previously documented coronary artery disease. All patients gave written informed consent before serum sample collection. The study protocol was approved by the ethics committee of our hospital.
Polysomnography
All subjects underwent overnight polysomnography using the Sandman Elite® sleep diagnostic system. The subjects were advised not to consume caffeine-containing beverages or take medication that might affect their sleeping pattern. Polysomnographic monitoring was performed using a standard technique.
Apnoeas were defined as the complete cessation of airflow for 10 seconds or longer. Hypopnoea was defined as a reduction in airflow with a 50 per cent fall from baseline for at least 10 seconds, accompanied by a 3 per cent drop in oxygen saturation from the preceding stable saturation level or event-associated arousal. The apnoea–hypopnoea index (AHI) reflected the number of apnoeas and hypopnoeas per hour of sleep. The diagnosis of OSAHS was based on an AHI of more than 5. Patients were subdivided into mild (AHI = 5–14), moderate (AHI = 15–29) and severe (AHI = 30 or higher) OSAHS groups.
Tumour necrosis factor-α and interleukin-10 measurement
All subjects went to bed at 21.00 and were awakened at 06.00. Serum samples were obtained at 07.00. Samples were collected after the initial polysomnography, and after one month of CPAP therapy in OSAHS patients who underwent this treatment.
Following centrifugation, the sera were stored at −80°C until analysed. Tumour necrosis factor-α (Biovision, Milpitas, California, USA) and interleukin (IL)-10 (Biovision) were measured by enzyme-linked immunosorbent assay methods.
Continuous positive airway pressure treatment
Ninety suitable patients with moderate to severe OSAHS, who had agreed to possible CPAP therapy before undergoing diagnostic sleep study, began nasal CPAP therapy within one month after polysomnography. All were evaluated for symptoms and side effects, and objective compliance data were downloaded from the CPAP devices.
Statistical analysis
Data normality was analysed using the Kolmogorov–Smirnov test. Differences between the two groups were analysed using the unpaired t-test, Mann–Whitney U test or chi-square test, as indicated. Differences among the groups were analysed by one-way analysis of variance followed by the Tukey post-hoc test or the Kruskal–Wallis analysis when appropriate. Pearson's correlation analysis was used to assess correlations between the tumour necrosis factor-α/IL-10 ratio and baseline and polysomnography variables. Analyses were performed using the Statistical Package for the Social Sciences (SPSS®) software (version 13.0). A value of p < 0.05 (two-tailed) was considered statistically significant.
Continuous, normally distributed variables are presented as means ± standard deviations. Categorical data are presented as numbers and percentages.
Results
Baseline characteristics
A total of 135 OSAHS patients and 94 control subjects were enrolled in the present study. The baseline characteristics and clinical profiles of both groups are shown in Table I.
Table I Baseline characteristics and clinical profiles

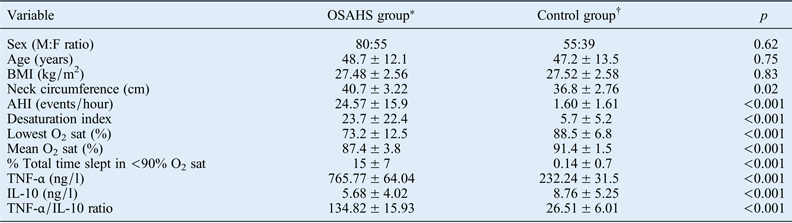
Values represent means ± standard deviations, unless indicated otherwise. Significance was set at p < 0.05. *n = 135; †n = 94. OSAHS = obstructive sleep apnoea hypopnoea syndrome; M = male; F = female; BMI = body mass index; AHI = apnoea–hypopnoea index; O2 sat = oxygen saturation; TNF-α = tumour necrosis factor-α; IL-10 = interleukin 10
There were no differences in age, sex or BMI between the OSAHS patients and controls. The mean age was 48.7 ± 12.1 years in the OSAHS group and 47.2 ± 13.5 years in the control group. Of the 135 OSAHS patients, 80 were male and 55 were female. In the control group, 55 of the 94 subjects were male and 39 were female. The mean BMI values for the control group and OSAHS group were 27.52 ± 2.58 and 27.48 ± 2.56, respectively (p = 0.83).
The mean AHI was 24.57 ± 15.9 events per hour in OSAHS patients versus 1.60 ± 1.61 events per hour in the control group (p < 0.001). There were also significant differences between the groups in terms of desaturation index, lowest oxygen saturation percentage, mean oxygen saturation percentage and percentage of the total time slept in which oxygen saturation levels were lower than 90 per cent (all p < 0.001).
The plasma level of tumour necrosis factor-α (TNF-α) was significantly higher in the OSAHS group than in the control group (765.77 ± 64.04 ng/l vs 232.24 ± 31.5 ng/l; p < 0.001). However, the interleukin (IL)-10 level was significantly lower in the OSAHS group than in the control group (5.68 ± 4.02 ng/l vs 8.76 ± 5.25 ng/l; p < 0.001). The TNF-α/IL-10 ratio was significantly higher in the OSAHS group than in the control group (134.82 ± 15.93 vs 26.51 ± 6.01; p < 0.001).
Biochemical characteristics according to severity
As shown in Table II, the TNF-α level was significantly higher in the severe OSAHS group (836.72 ± 71.06 ng/l) than in the moderate OSAHS group (764.48 ± 63.28 ng/l) or mild OSAHS group (545.36 ± 54.06 ng/l). Thus, the TNF-α level was elevated with increased OSAHS severity. In contrast, the IL-10 level was reduced with increased OSAHS severity (mild group, 6.68 ± 4.74 ng/l; moderate group, 5.77 ± 4.06 ng/l; severe group, 4.87 ± 3.84 ng/l). When the three groups were compared, the severe OSAHS group had the highest TNF-α/IL-10 ratio value (severe group, 171.81 ± 18.51; moderate group, 132.49 ± 15.59; mild group, 81.64 ± 11.41; p < 0.001).
Table II Biochemical characteristics by osahs severity

Values represent means ± standard deviations, unless indicated otherwise. Significance was set at p < 0.05. *n = 45; †n = 44; ‡n = 46. OSAHS = obstructive sleep apnoea hypopnoea syndrome; AHI = apnoea–hypopnoea index; TNF-α = tumour necrosis factor-α; IL-10 = interleukin 10
Correlation data
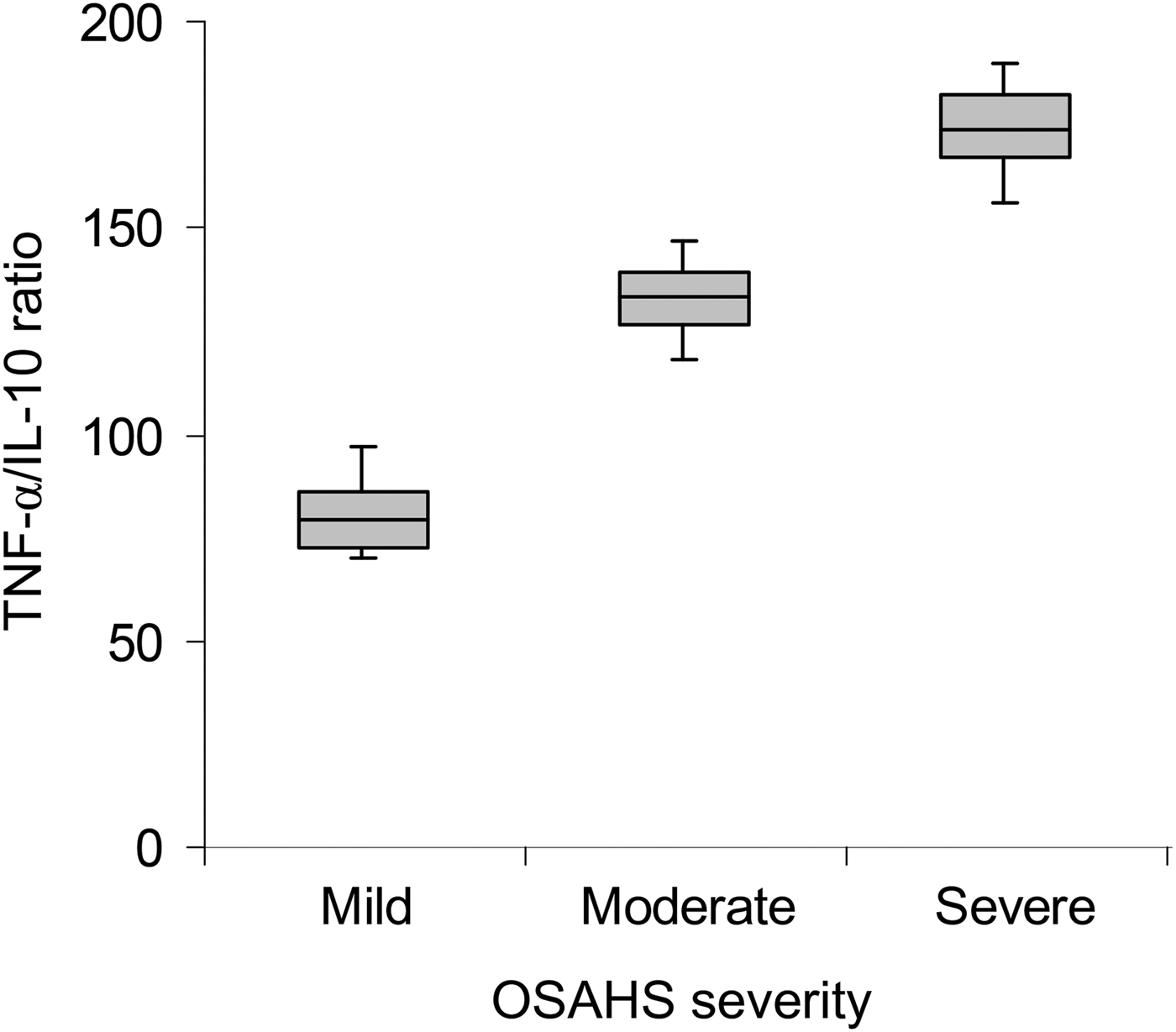
A correlation was performed to investigate the relationship between the TNF-α/IL-10 ratio and the severity of OSAHS. As shown in Figure 1, a significant positive correlation was found between increments in TNF-α/IL-10 ratio and severity of OSAHS (ratio value was 81.64 ± 11.41 in the mild OSAHS group, 132.49 ± 15.59 in the moderate OSAHS group and 171.81 ± 18.51 in the severe OSAHS group; p < 0.001).

Fig. 1 Box-and-whisker plot showing tumour necrosis factor-α (TNF-α)/interleukin-10 (IL-10) ratio in mild, moderate and severe obstructive sleep apnoea hypopnoea syndrome (OSAHS) patients. Boxes and error bars represent means and standard deviations respectively.
Following log transformations of the TNF-α/IL-10 ratio values, we performed multiple comparisons among the three subgroups. The results showed that the TNF-α/IL-10 ratio values were significantly higher in the moderate OSAHS group (p < 0.01) and severe OSAHS group (p < 0.001) when compared with those of the mild OSAHS group.
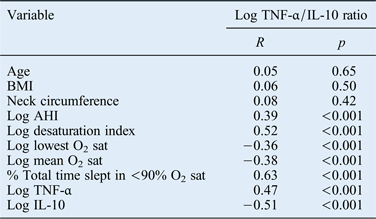
The associations between the log TNF-α/IL-10 ratio and other variables are summarised in Table III. Pearson's correlation analysis showed that the TNF-α/IL-10 ratio correlated positively with AHI and all indices of OSAHS except for age, BMI and neck circumference (all p < 0.001).
Table III Correlations between log tnf-α/il-10 ratio and clinical characteristics*

Significance was set at p < 0.05.
* Using Spearman's rank correlation coefficients. TNF-α = tumour necrosis factor-α; IL-10 = interleukin 10; BMI = body mass index; AHI = apnoea–hypopnoea index; O2 sat = oxygen saturation
Effect of continuous positive airway pressure therapy
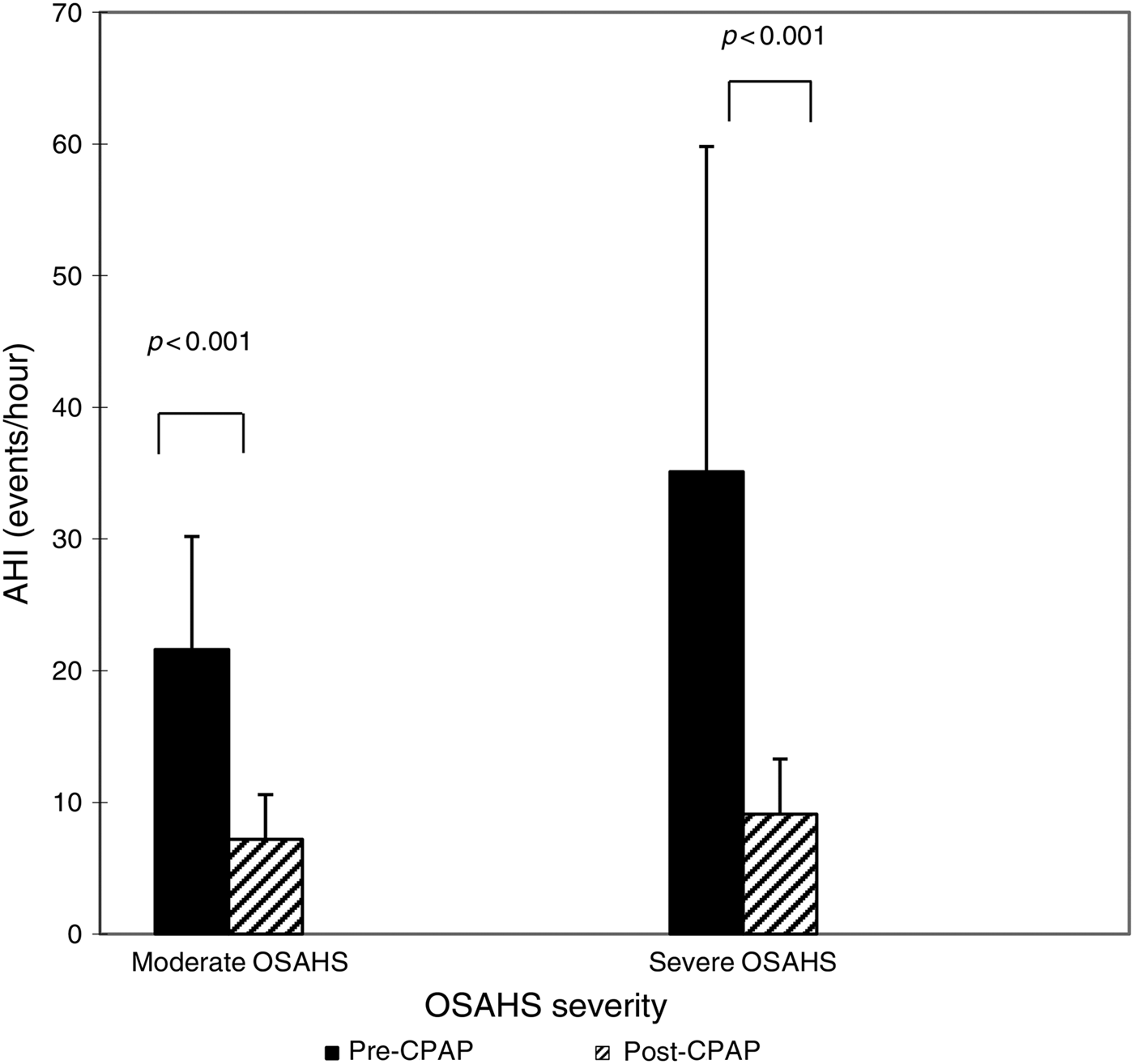
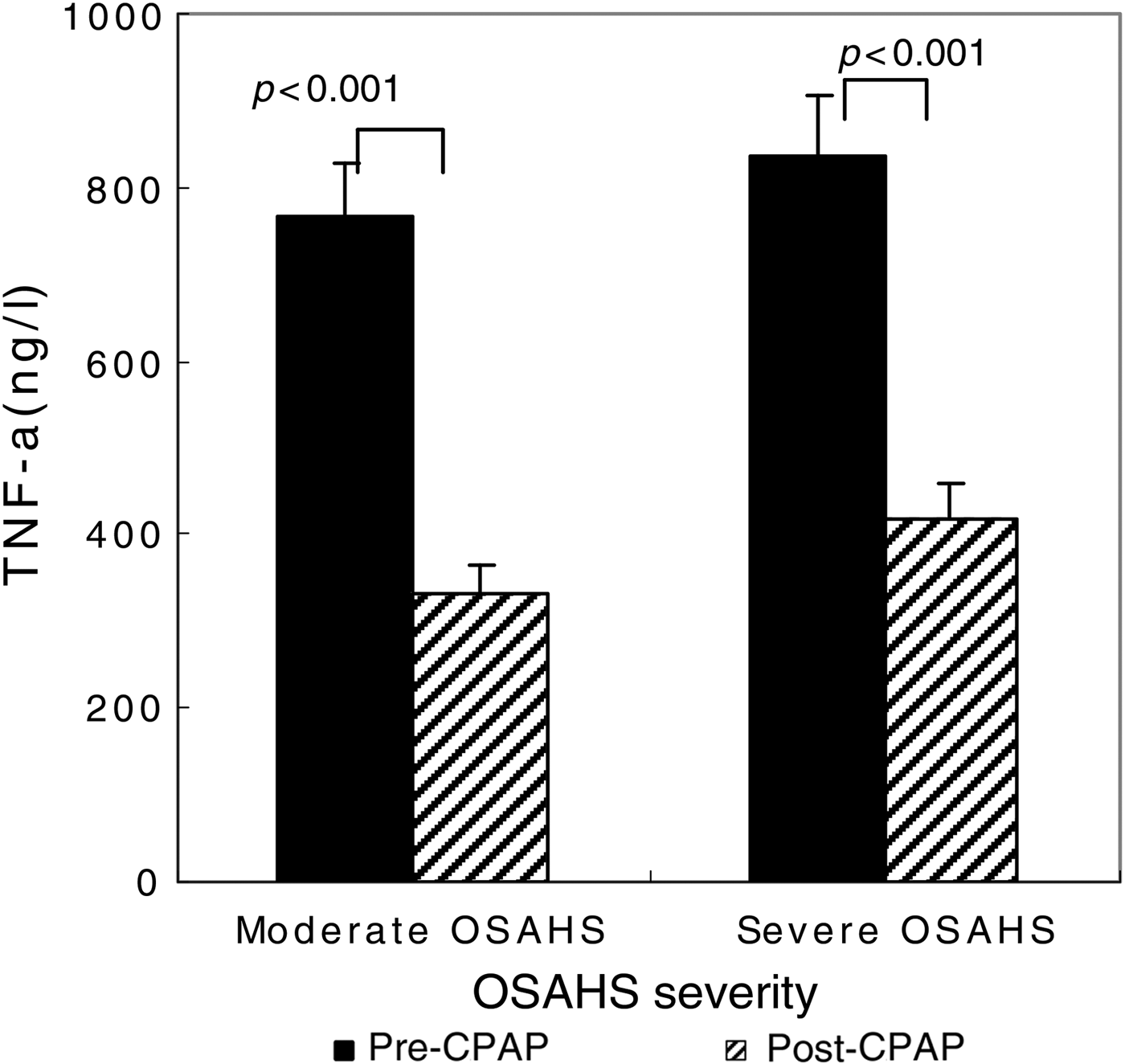
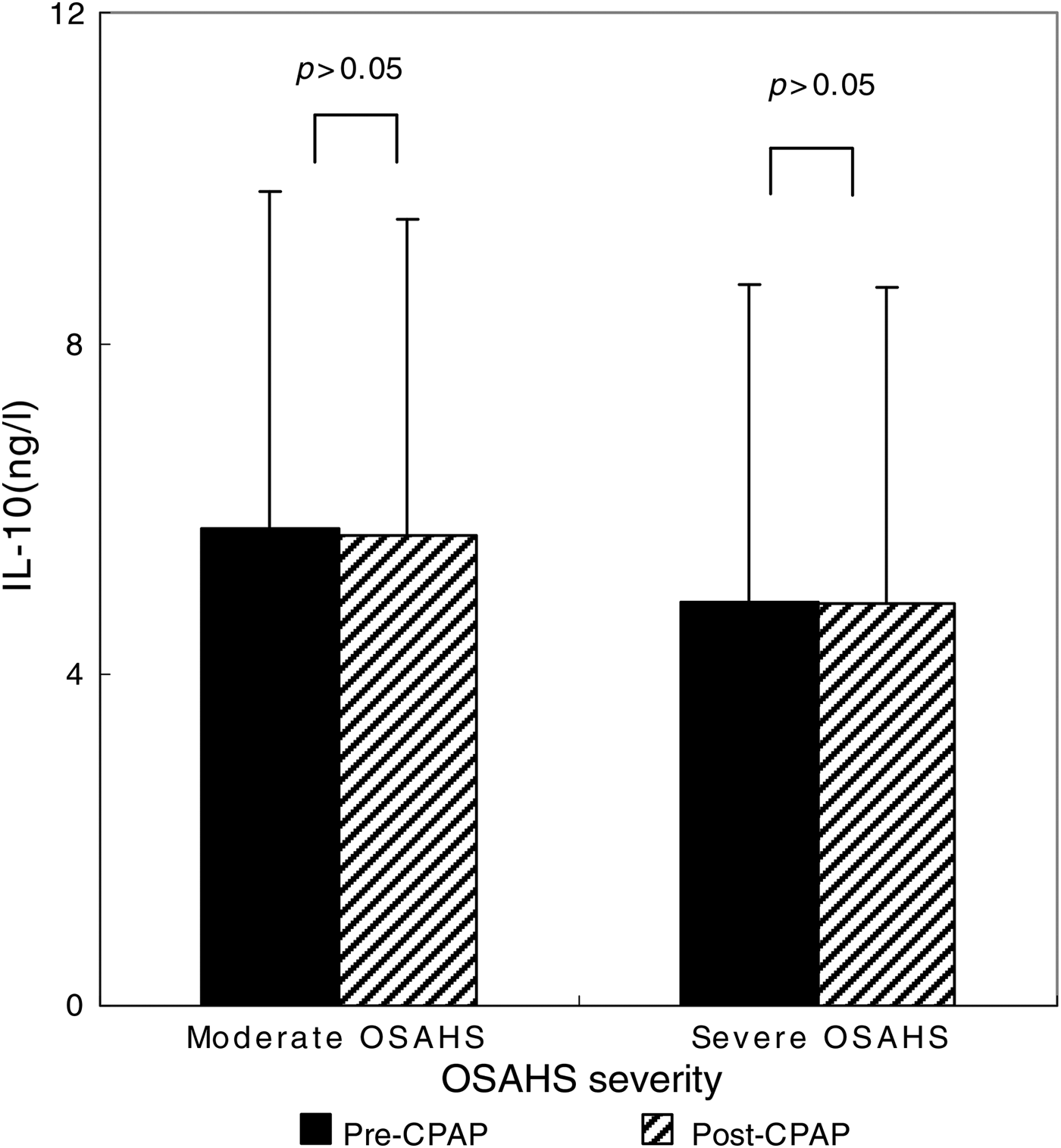
After the 90 CPAP-treated patients with severe OSAHS had received 1 month of CPAP therapy, AHI fell from 35.1 ± 24.7 to 9.1 ± 4.2 (p < 0.001 compared with pre-CPAP levels; Figure 2). The CPAP therapy led to a significant decrease in TNF-α levels (from 836.72 ± 71.06 to 418.2 ± 42.1 ng/l; p < 0.001) (Figure 3), but this same course of CPAP therapy had no significant effect on IL-10 levels (p > 0.05) (Figure 4).

Fig. 2 Impact of one month of continuous positive airway pressure (CPAP) therapy on apnoea–hypopnoea index (AHI) in obstructive sleep apnoea hypopnoea syndrome (OSAHS) patients. Boxes and error bars represent means and standard deviations respectively.

Fig. 3 Impact of one month of continuous positive airway pressure (CPAP) therapy on tumour necrosis factor-α (TNF-α) levels in obstructive sleep apnoea hypopnoea syndrome (OSAHS) patients. Boxes and error bars represent means and standard deviations respectively.

Fig. 4 Impact of one month of continuous positive airway pressure (CPAP) therapy on interleukin-10 (IL-10) levels in obstructive sleep apnoea hypopnoea syndrome (OSAHS) patients. Boxes and error bars represent means and standard deviations respectively.
Discussion
Obstructive sleep apnoea hypopnoea syndrome (OSAHS) should be considered a systemic disease rather than a local abnormality. Although the exact mechanism of OSAHS is not yet fully elucidated, inflammation is now thought to play a crucial role in the pathogenesis of the disease, and there is evidence that patients with OSAHS have increased interleukin (IL)-6, tumour necrosis factor-α (TNF-α) and C-reactive protein levels.Reference Steiropoulos, Papanas, Nena, Antoniadou, Serasli and Papoti10–Reference Guven, Turkkani, Ciftci, Ciftci and Erdogan12
One study concluded that the neutralising of TNF-α with etanercept, which is a TNF-α antagonist, is associated with a significant reduction in objective sleepiness in obese patients with OSA.Reference Vgontzas, Zoumakis, Lin, Bixler, Trakada and Chrousos13 Another report showed that circulating levels of IL-6 and TNF-α were significantly elevated in OSA patients, and the levels of these cytokines were related to the severity of OSA.Reference Ciftci, Kokturk, Bukan and Bilgihan14 In the present study, we found that the TNF-α/IL-10 ratio and TNF-α levels were significantly higher in OSAHS patients compared with controls. We also investigated the correlation between increments in TNF-α/IL-10 ratio and OSAHS severity. Among the three subgroups of OSAHS patients, TNF-α/IL-10 ratio and TNF-α levels increased with the severity of the disease.
The relationship between the TNF-α/IL-10 ratio and OSAHS suggests a crucial role of activated inflammation in the pathogenesis of OSAHS. Therefore, inflammatory cytokines represent the potential to be assessed as biomarkers for OSAHS, owing to the inflammatory nature of the disease. These disease biomarkers could possibly provide information related to diagnosis, severity, prognosis and response to treatment. In fact, there has been a plethora of work conducted on inflammatory markers in subjects with OSA.
Tumour necrosis factor-α, a Th1 cytokine, acted as a proinflammatory cytokine, whereas IL-10, one of the most important Th2 cytokines, acted as an anti-inflammatory cytokine. In the OSAHS group, TNF-α was significantly higher but IL-10 was lower than in the control group. Our findings suggest a superior Th1-biased inflammatory response but suppressive Th2 anti-inflammatory response in OSAHS patients.
The mechanisms by which inflammation contributes to OSAHS are not known. Re-oxygenation after a brief period of hypoxia, as experienced repetitively and systematically by OSAHS patients, may predispose these individuals to cell stress. It has been suggested that such events favour the activation of a proinflammatory response as mediated through the nuclear transcription factor nuclear factor κβ, a master regulator of inflammatory gene expression. The findings of the current study suggest either that: a more substantial or a different pattern of hypoxaemia might be necessary to activate systemic inflammation; the system may need to be primed before hypoxic exposure; or increases in inflammatory markers in OSAHS patients may be more related to other factors such as obesity or nocturnal arousal.Reference Querido, Sheel, Cheema, Van Eeden, Mulgrew and Ayas15
Studies from various laboratories and clinical environments suggest that OSAHS is an inflammatory disorder. The increase in proinflammatory cytokine production in OSAHS patients can have important consequences for patient outcomes, especially with regard to the increased risk for developing atherosclerosis, and cardiovascular and cerebrovascular diseases.Reference Aihara, Oga, Chihara, Harada, Tanizawa and Handa16 In a meta-analysis performed to investigate the effect of CPAP therapy on systemic inflammation in patients with OSA, it was found that CPAP therapy could partially suppress systemic inflammation in OSA patients; furthermore, substantial differences were present among the various inflammatory markers.Reference Xie, Pan, Ren, Du and Guo17
• Local and systemic inflammation is present in obstructive sleep apnoea hypopnoea syndrome (OSAHS)
• Inflammation was activated and anti-inflammatory cytokines were decreased in OSAHS patients
• Continuous positive airway pressure therapy is an effective treatment to lower inflammatory response
Polysomnography is considered the gold standard diagnostic test for OSA.Reference Epstein, Kristo, Strollo, Friedman, Malhotra and Patil18 In-laboratory polysomnography is unsuitable for screening because of complexity, expense and inaccessibility.Reference Gurubhagavatula, Fields, Morales, Hurley, Pien and Wick19 In our study, the severity of OSAHS was assessed using diagnostic polysomnography in a sleep laboratory. A Pearson's correlation analysis was then conducted to assess the correlations between the TNF-α/IL-10 ratio and baseline and polysomnography variables. Clinical data can also be useful in identifying OSAHS. Furthermore, a previous study showed that a CPAP trial could predict sleep apnoea with a sensitivity of 80 per cent, a specificity of 97 per cent, and positive and negative predictive values of 97 per cent and 78 per cent, respectively.Reference Senn, Brack, Russi and Bloch20 The findings suggested that polysomnography could have been avoided in 35 (46 per cent) of the 76 sleep apnoea patients with positive CPAP trial results. In our study, one month of CPAP therapy in the patients with severe OSAHS resulted in decreased AHI, with a corresponding significant decrease in TNF-α levels; however, this same course of CPAP therapy had no significant effect on IL-10 levels. It is concluded that one month of CPAP therapy had an appreciable effect on TNF-α and AHI parameters, but not on IL-10 levels.
The present study has some limitations. First, the number of participants was small; further studies with a larger population will elucidate the relationship between the TNF-α/IL-10 ratio and OSAHS. Second, only serum TNF-α and IL-10 levels were detected in the present study. Additional studies investigating other cytokines, such as Th1 and Th2 cytokines, might provide additional information on the disease.
Acknowledgements
This study was supported by grants from: the Hubei Natural Science Foundation of China (grant number 2012FFA071), the Xiangyang Science Foundation (grant number 2012[40]10) and the Sun Research Foundation (grant number 201202).