Inadequate dietary Fe and Zn intake and consumption of poor-quality diets are major causes of iron and zinc deficiencies in most developing countries(Reference Muller and Krawinkel1). In most rural areas in developing countries households are generally characterised by limited financial resources for access to foods and rely on their own production. Agricultural activities are strongly dependent on rainfall. Food availability in these areas is dependent on harvest and storage conditions. Seasonal food shortage occurs when food production is not sufficient to cover the population needs in the period between two harvests. To adapt to such a situation, people may change their food pattern by modifying the types and quality of foods consumed and by changing their meal frequency and quantity(Reference Den Hartog, van Staveren and Brouwer2). In addition to environmental factors, socio-economic factors may also affect food pattern. This may result in different energy and nutrient intakes with consequences on nutritional status(Reference Ferro-Luzzi, Morris, Taffesse, Demissie and D’Amato3–Reference Savy, Martin-Prével, Traissac, Eymard-Duvernay and Delpeuch5). Furthermore, most rural areas in developing countries are characterised by cereals, roots and tubers as main staple foods. Antinutritional factors such as phytate and polyphenols in these foods might be high and therefore Fe and Zn bioavailability might be low. Unfortunately consumption of fruits, vegetables and animal foods is generally limited. In northern Benin, seasonality in food pattern has been reported in previous studies(6, 7). However, its impact on the energy and nutrient intake of children is not yet documented.
A recent study performed in northern Benin revealed that school-aged children were clearly stunted but not wasted and that children attending school were less stunted than children not attending school. Moreover, the iron status of the children was poor and school attendees were less anaemic than non-attendees (CES Mitchikpe, RAM Dossa, EAD Ategbo, JMA van Raaij and FJ Kok, unpublished results). These findings might be caused by inadequate dietary patterns, but food consumption data on school-aged children to support these findings are lacking.
Therefore, the objective of the present study was to analyse the actual food pattern and resulting energy and nutrient intakes of school-aged children in rural Benin, not only in relation to season but also in relation to school attendance.
Subjects and methods
Study area
The study was carried out in three villages in Atacora Province in northern Benin. The population density is low (21 inhabitants/km2). Economic activity is dominated by farming and is strongly dependent on rainfall. In the area, the unique rainy season lasts from May to October and is followed by the dry season lasting from November to April. Average annual rainfall is about 1300 mm. Cultivated food crops are maize, sorghum, millet, yam, cassava, bambara groundnuts, peanuts and beans. Early crops such as yam, sweet potato and early varieties of beans, maize and millet are harvested two to three months before the staple cereals are harvested in November and December(6). Food consumption in the household depends mainly on the size of the food stores until the next harvest. During the post-harvest season plenty of food is available. At the beginning of the rainy season the granaries get depleted (pre-harvest season). Cash crops like cotton, tobacco or cashew nuts are also cultivated. Small livestock and poultry are raised in some households but animals are mainly slaughtered when there is a ceremony or sold when there is an urgent need for money. A previous study performed in the same area showed that there were no socio-economic differences between households(6). The socio-economic status of the population is poor, mainly characterised by low income, poor sanitation, low school attendance and high illiteracy rate. The present study was carried out in 2002 and 2003. According to the national office in charge of food surveillance, agricultural production in these years was not atypical as far as harvest levels, rainfall, post-harvest losses and food prices are concerned.
Study design
Children aged 6 to 8 years were randomly selected and involved in a longitudinal study. Food intakes of the children were assessed by food consumption surveys carried out in November and December 2002, at the end of the harvest season, and six months later in June and July 2003, at the end of the pre-harvest season. Food intakes were measured during three consecutive days using an observed weighed records method. Data were collected by well-trained local assistants.
Subjects and sampling procedure
From the local register of births, a list with names of 302 children between the ages of 6 and 8 years was obtained. Out of those 302 children, 143 could be traced and served as the sampling population. Only 143 children could be traced mainly because of complicated naming practices and changing of names. Eighty out of the 143 children were randomly selected and this random selection resulted in a sample of forty-five boys and thirty-five girls. This random selection also resulted in a sample of fifty-seven children attending primary school (thirty-five boys and twenty-two girls) and twenty-three children not attending primary school (ten boys and thirteen girls). Informed consent was obtained from the parents of the children. Complete food consumption data sets became available on seventy-five children.
The baseline anthropometric characteristics (in the post-harvest season), including the prevalence rate of stunting and wasting of the randomly selected subjects, are presented in Table 1.
Table 1 Baseline anthropometric characteristics (post-harvest season; November/December 2002): school-aged children (6–8 years old), northern Benin
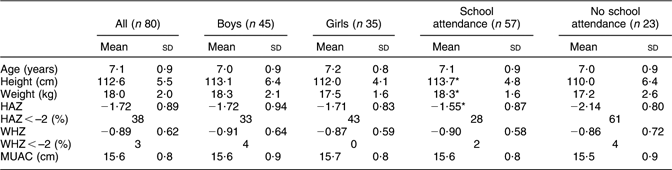
HAZ, height-for-age Z score; WHZ, weight-for-height Z score; MUAC, mid upper-arm circumference.
*Significantly higher than in children not attending school (P < 0·05).
Measurement of dietary intake
Food intake of the children was assessed using the observed weighed records method(Reference Cameron and Staveren8) during three consecutive days. Previous studies in the same area showed that food consumption is characterised by a monotonous diet and therefore increasing the number of days or assigning multiple days of intake for each individual randomly will not affect the within-person day-to-day variability(6, 7). All foods were weighed and recorded before and after cooking, as were served portions and leftovers, using a digital weighing scale (Tefal type Ovelys 3) for weights up to 3 kg and a spring weighing scale (Soehnle type 1203) for weights between 3 and 10 kg. Measurements were performed by well-trained local assistants every day from 07.00 h until subjects had eaten their last meal, usually between 19.00 and 21.00 hours. Foods consumed when local assistants were not present were assessed by recall using local household measures. For children who used to eat together with other members of the family, mothers were asked to serve their portion on a separate plate.
Data elaboration
Energy and nutrient intakes of the children were calculated using an updated version of the Benin food composition table. The previous version of this table as used in the University of Benin was derived mainly from the FAO food composition database. To update the food composition table, samples of foods consumed by the children were collected in two local markets in the study area. They were analysed for energy, macro- and micronutrients contents(Reference Mitchikpe, Dossa, Ategbo, van Raaij, Hulshof and Kok9). The amount of the various food categories consumed by the children (in grams) and the energy and nutrient contents of each food were computed using the computer programme Komeet 4·0 (B. Ware Nutrition software, Arnhem, The Netherlands). The contribution of each food to the daily energy and nutrient intakes of each child was calculated by dividing the energy and nutrient composition of the food consumed by the total intake. Subsequently, the contributions of a food category to the energy and nutrient intakes as obtained on the three measurement days were averaged.
Statistical analyses
Food, energy and nutrient intakes of the children consumed over three consecutive days were compared and checked for systematic day effects using ANOVA. Mean values were obtained by averaging intakes over the three consecutive days and these mean values were used for further analyses. Before statistical analyses, data were checked for normality. Data sets obtained in the post- and pre-harvest seasons were compared using the paired-samples t test or the Wilcoxon signed-rank test. Subgroups of children based on gender and school attendance were also compared using the independent-samples t test or Mann–Whitney test.
All statistical tests were two-tailed and P values less than 0·05 were considered statistically significant. Analyses were performed using the SPSS for Windows statistical software package version 11·0 (SPSS Inc., Chicago, IL, USA).
Results
The average daily diet for children in general provided three meals. The meal often consisted of a thick porridge of maize, sorghum or millet during the post-harvest season or a mixture of these foods and dried cassava flour during the pre-harvest season. The thick porridge, locally called dibou, is prepared from whole grains of cereal milled into fine flour. Pounded yam was also frequently consumed during the post-harvest season. Dibou was always served with sauce of green leafy vegetables (fresh or dried) or okra and condiments, or a spicy sauce of tomatoes or peanuts. Quantities of these accompanying sauces were relatively small. In addition, cooked beans were consumed, occasionally mixed with rice. Bambara groundnuts were cooked or grilled or used to prepare different types of cakes consumed with oil. The meal was prepared and distributed by mothers to all members of the household. Mothers were asked not to change the usual food habits of the household in order to avoid altering the subjects’ eating behaviour.
Daily intakes of foods of the children during the post- and pre-harvest seasons are given in Table 2. The median amount of maize consumed in the post-harvest season was not different from that consumed in the pre-harvest season (82 g). Intakes of millet, sorghum, cassava and vegetables in the post-harvest season were significantly lower than those in the pre-harvest season (P < 0·05). The amount of yam consumed in the post-harvest season (238 g) was substantially higher than that consumed in the pre-harvest season (0 g) (P < 0·05). Although the amount of animal products consumed in the post-harvest season (4 g) was two times the amount in the pre-harvest season (2 g), the difference did not reach statistical significance. For both seasons, intakes of the various foods were not different between boys and girls, except for beans in the post-harvest season (0 g v. 4 g respectively) (P < 0·05). Although for some foods there were differences between intakes of children attending and not attending school, none of these differences were statistically significant.
Table 2 Daily intakes of foods (g/d) in the post- (November/December 2002) and pre-harvest (June/July 2003) seasons: school-aged children (6–8 years old), northern Benin
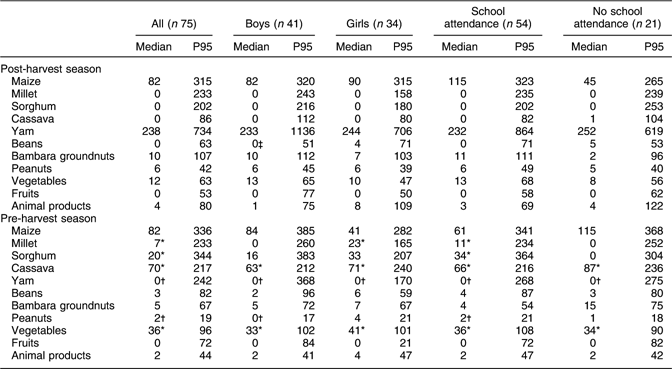
P95, 95th percentile.
*Significantly higher than in the post-harvest season (P < 0·05).
†Significantly lower than in the post-harvest season (P < 0·05).
‡Significantly different from girls (P < 0·05).
Daily intakes of energy, protein, carbohydrate, fibre, Ca, Fe and Zn in the post-harvest season were not different from those in the pre-harvest season (see Table 3). Median intakes of fat and vitamin C in the post-harvest season were respectively 23 % and 28 % lower than intakes in the pre-harvest season (P < 0·05). For both post- and pre-harvest seasons, boys showed higher intakes than girls for energy, protein, fibre, Fe and Zn (P < 0·05). Intakes of energy and nutrients of children attending school were not different from those of children not attending school.
Table 3 Daily energy and nutrient intakes in the post- (November/December 2002) and pre-harvest (June/July 2003) seasons: school-aged children (6–8 years old), northern Benin
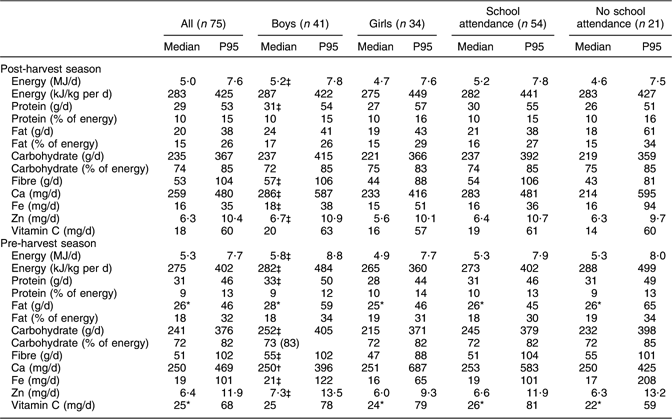
P95, 95th percentile.
*Significantly higher than in the post-harvest season (P < 0·05).
†Significantly lower than in the post-harvest season (P < 0·05).
‡Significantly different from girls (P < 0·05).
Figures 1–4 summarise the contributions of the various foods to daily energy, protein, Fe and Zn intake, respectively. Cereals (maize, millet and sorghum) contributed 43 % (post-harvest season) and 55 % (pre-harvest season) of the daily energy intake of all children (Fig. 1). Contributions to the daily energy intake from millet and cassava in the post-harvest season were significantly lower than those in the pre-harvest season (P < 0·05), whereas the contribution of yam was significantly higher (P < 0·05).
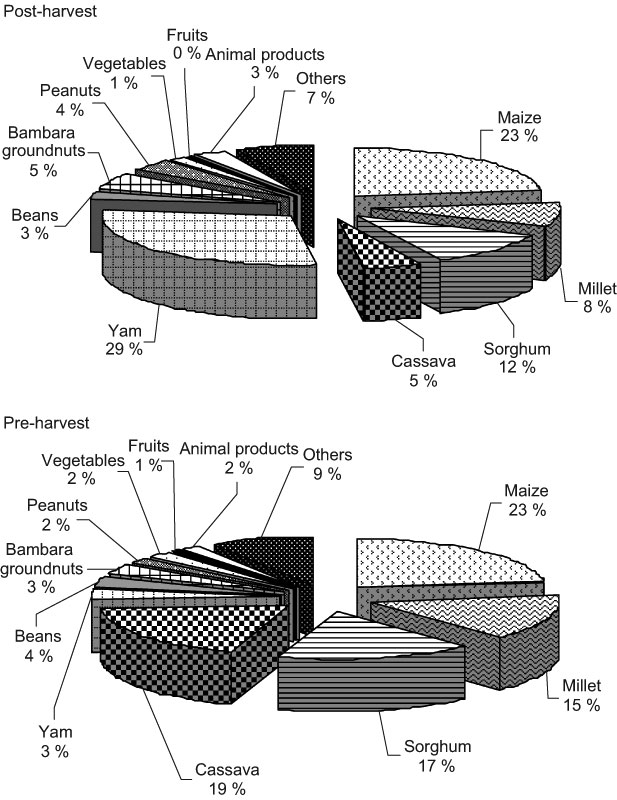
Fig. 1 Contribution of foods to the average daily energy intake of school-aged children (6–8 years old) in the post- (November/December 2002) and pre-harvest (June/July 2003) periods in northern Benin
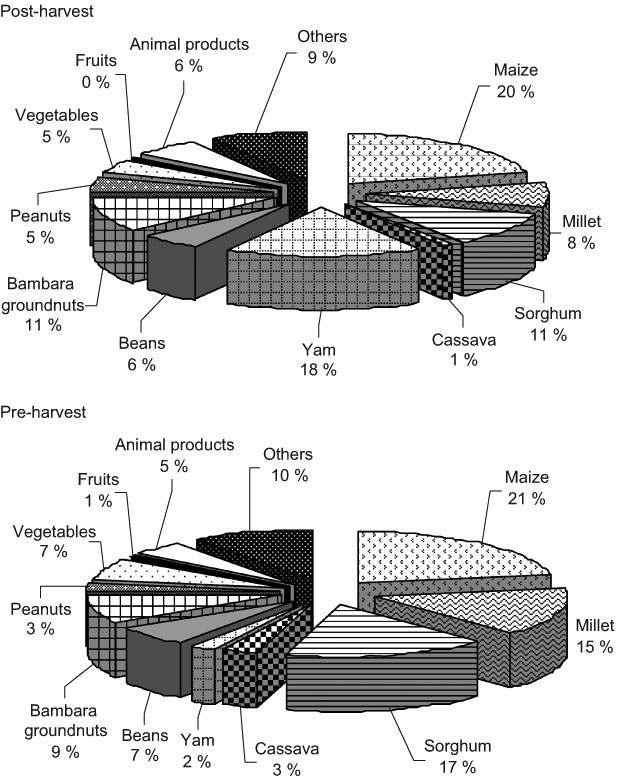
Fig. 2 Contribution of foods to average daily protein intake of school-aged children (6–8 years old) in the post- (November/December 2002) and pre-harvest (June/July 2003) periods in northern Benin

Fig. 3 Contribution of foods to average daily iron intake of school-aged children (6–8 years old) in the post- (November/December 2002) and pre-harvest (June/July 2003) periods in northern Benin
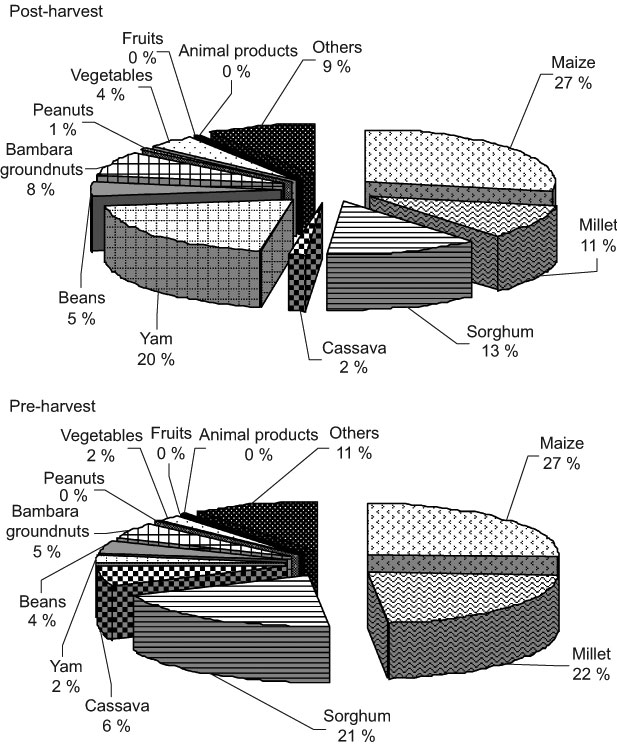
Fig. 4 Contribution of foods to average daily zinc intake of school-aged children (6–8 years old) in the post- (November/December 2002) and pre-harvest (June/July 2003) periods in northern Benin
Cereals contributed 39 % (post-harvest season) and 53 % (pre-harvest season) of the daily protein intake (Fig. 2). The contribution of millet in the post-harvest season was significantly lower than in the pre-harvest season (P < 0·05), whereas the contribution of yam was significantly higher (P < 0·05). Contributions of vegetables and animal products to daily protein intake fluctuated around 5 % and 7 %, respectively.
Cereals contributed 34 % (post-harvest season) and 50 % (pre-harvest season) of the daily intake of Fe (Fig. 3). The contribution of millet in the post-harvest season was significantly lower than in the pre-harvest season (P < 0·05), whereas contributions of yam and vegetables were significantly higher (P < 0·05). The contribution of animal products to the daily Fe intake was 1–2 %.
Cereals contributed 51 % (post-harvest season) and 70 % (pre-harvest season) of the average daily Zn intake (Fig. 4). Contributions of millet and cassava in the post-harvest season were significantly lower than those in the pre-harvest season (P < 0·05), whereas contributions of yam and vegetables were significantly higher (P < 0·05).
Table 4 provides the percentage of children not meeting their energy requirement. Data show that 49–59 % of boys and girls had an energy intake lower than their EAR (Estimated Average Requirement). The percentage of boys who had an intake more than 2sd below the EAR (7 % and 15 % in the post- and pre-harvest season, respectively) was lower than the percentage of girls (29 % and 27 % in the post- and pre-harvest season, respectively). Table 5 shows the percentages of children not meeting the protein, Fe and Zn requirements. For Zn intake, 24–32 % of the children were below the EAR and up to 79 % were below the RNI (Recommended Nutrient Intake).
Table 4 Percentage not meeting their energy requirement: school-aged children (6–8 years old), northern Benin

Post-harvest season, November/December 2002; pre-harvest season, June/July 2003; EAR, Estimated Average Requirement.
*EAR for energy was calculated based on the average body weight of the children and the recommended energy intake for children aged 7–8 years(10).
Table 5 Percentage not meeting their estimated average requirements and recommended intakes for protein, iron and zinc: school-aged children (6–8 years old), northern Benin

Post-harvest season, November/December 2002; pre-harvest season, June/July 2003; EAR, Estimated Average Requirement; RNI, Recommended Nutrient Intake for Fe and Zn and safe level of protein intake.
*Protein requirements were calculated based on the actual weight and age of the children and the FAO/WHO/UNU (1985) recommendations(11).
†Fe and Zn requirements were derived from the FAO/WHO (2002) recommendations(12).
‡Median basal requirement for a diet with intermediate bioavailability(12).
Discussion
The objective of the present study was to analyse the actual food pattern and the resulting energy and nutrient intakes of school-aged children in rural Benin, not only in relation to season but also in relation to school attendance.
The study was carried out in 6–8-year-old children in northern Benin. These children were clearly stunted but not wasted (Table 1). Prevalence rates of stunting (38 %) and wasting (3 %) are within reported ranges for the study area(7, 13). In northern Benin, not all school-aged children will attend school. The reasons for not attending school remain to be clarified. However, both school-attending and non-attending children are involved in the present study. With respect to the interpretation of our findings, it should be noticed that boys are over-represented in the school-attending group (thirty boys v. twenty-two girls).
All methods used to assess food consumption at individual level involve some biases. However, we did apply required survey techniques to reduce eventual discrepancies, mainly those related to observers. For example, the individual intake cannot be assessed unless the child’s food is served on a separate plate. This is not the common eating habit and might affect slightly the amount of foods served to the child. However, it might probably have a minor effect on the amount eaten. Unfortunately we were not able to assess such effect.
Food pattern
Foods were usually cooked by mothers who decide on portions to be served to family members. Boys from the same household eat together, sometimes with the father. Girls also eat together and sometimes with the mother. These habits were not affected by seasonality.
The availability of fruits and vegetables showed changes depending on the period of the year. For example, mangos and pulp of African locust pods are available only from March to July. Fresh green leafy vegetables are more available during the rainy season and just after the harvest (post-harvest season). During the dry season and at the beginning of the rainy season, dried green leafy vegetables are more available. The same trend applies for intake of these products. The same types of staple foods were consumed during both seasons. However the consumption showed seasonality.
The higher consumption of millet, sorghum, cassava and vegetables in the pre-harvest season and the lower consumption of yam and peanuts indicate that the food pattern of the children had changed. Such a change in food pattern was also reported in previous studies(6, 7) and might be related to the availability of foods. There were no differences between the food patterns of boys and girls, but this is not surprising as they came from the same area. The lack of difference between the food patterns of children attending and not attending school might be unexpected, but not if it is taken into account that both groups of children came from the same families. In the same area, no socio-economic differences between households have been reported(6, 7). Therefore no subsequent socio-economic effect on the food pattern of the children was expected.
In northern Benin, the seasonality in food pattern did not result in remarkable changes in the food groups eaten. Cereals were the main sources of energy, followed by yam in the post-harvest season and by cassava in the pre-harvest season. They all together contributed 75 % of the energy intake of the children (Figs 1–4). As a consequence, they were also the main sources of protein, Fe and Zn. Contributions of animal foods to protein and Fe intakes were marginal. Similar findings are reported in previous studies(6, 7). Because of missing values in the Benin food composition table, the Zn contribution from animal products was not taken into account in the calculation of Zn intake. However, underestimation of Zn intake is probably very small as the consumption of animal products was very limited (only 2–3 % of energy intake). Fe and Zn bioavailability from unrefined cereal foods has been shown to be low because of the phytate and polyphenols content of cereals(Reference Brune, Rossander-Hulten, Hallberg, Gleerup and Sandberg14–Reference Hurrell, Reddy, Juillerat and Cook16). The diet of the children was mainly based on cereals and, therefore, Fe and Zn bioavailability from such a diet might be low(12).
The consumption of a monotonous diet suggests that micronutrient intakes might be inadequate. Cereals, roots and tubers provided 58 % of protein intake and legumes provided about 20 % in each season (Fig. 2). These products contain several limiting amino acids (lysine, threonine and tryptophan for cereals; sulfur amino acids for legumes) and therefore children’s requirements might not be adequately fulfilled because of the monotonous diet. Protein quality of the diet might be improved by promoting appropriate combination of cereals and legumes.
Energy and nutrient intakes
In the post-harvest season, the median daily energy intake of the children was 5·0 MJ. This energy intake is higher than that reported for Kenyan school-aged children from a slum area (3·6 to 4·1 MJ/d)(17) but it is in line with the EAR, which is based on the actual weights of the children as shown in Table 4. By definition, 2–5 % of the children might be expected to have energy intakes more than 2sd below the requirement. However, especially for girls, the percentage of low intake was substantially higher (about 25 %; see Table 4). In addition, the actual available energy from the diet of the children might be lower because of the high fibre content. A correction factor of 0·95 is suggested for diets containing large amounts of fibre(11). Therefore the percentage of children not meeting the energy requirement might be higher. A long-term shortage of energy might be one of the explaining factors for the high prevalence of stunting among the children (Table 1).
The observed protein intake of the children is higher than that reported in Kenyan school-aged children (20·6 to 23·5 g/d)(17), but is in line with the requirements (Table 5). However, limiting amino acids in cereals may be a problem in the diets as consumed by the children(Reference McKevith18).
Contributions of protein and fat to the energy intake of the children (10 % and 15 %, respectively) were low and suggest that the diet is not well balanced. For example, low fat intake may result in an increased risk of inadequate absorption of fat-soluble vitamins such as vitamin A.
Because of the low contribution of animal products in the diet, the high fibre intake is not surprising. However, this high level certainly will have consequences on nutrient absorption such as reduced absorption of fats, fat-soluble vitamins and carotenoids(Reference Gibson, Perlas and Hotz19).
The Fe intake of the children is higher than that reported for Namibian schoolchildren although those children are older (11 years old on average)(Reference Vahatalo, Mikkila and Rasanen20); however it should be realised that the Namibian children were measured during the drought. Van’t Riet(17) has reported similar Fe intakes for children attending school but lower values for children not attending school. The Fe intake is in line with the requirement and suggests that it is probably adequate. By definition, half of the children with appropriate intakes will have their values below the EAR. This might mean that also the Zn intake of the children might have been adequate. However, the presence of antinutritional factors such as phytate and polyphenols in cereals might reduce Fe and Zn bioavailability(Reference Brune, Rossander-Hulten, Hallberg, Gleerup and Sandberg14, Reference Manary, Hotz, Krebs, Gibson, Westcott, Arnold, Broadhead and Hambidge15, Reference Hurrell21–Reference Towo, Matuschek and Svanberg23). The relationship between food intake and prevalence of iron and zinc deficiencies was shown in several studies(Reference Layrisse24–Reference Zimmermann, Chaouki and Hurell29).
Vitamin C is known to counteract the inhibiting effect of phytate in plant-based foods. The vitamin C intake of the children was lower than the recommended intake (35 mg/d)(12). The high percentage of children below the recommended intake (73–85 %) suggests that vitamin C intake might be inadequate. Therefore, the potential enhancing effect of vitamin C on Fe and Zn bioavailability probably is quite limited.
Seasonality
Although the food pattern was clearly different from post-harvest to pre-harvest season, seasonal differences with respect to energy and nutrient intakes were not large. The only significant differences were with respect to fat and vitamin C. The increased consumption of fat and vitamin C in the pre-harvest season is due to the availability of shea butter, fruits and fresh vegetables. Because of only small changes in fat and vitamin C intakes, the energy and nutrient intakes of the children were not really dependent on season. The lack of seasonality in energy and nutrient intakes may be explained by adaptation strategies to food shortage developed by households(Reference Den Hartog, van Staveren and Brouwer2).
School attendance
The lack of difference between the energy and nutrient intakes of school attendees and non-attendees was unexpected because of the difference in nutritional status (Table 1). The differences in stunting could not be explained by the present data, but is probably the result of past detrimental events in the life of the children.
In conclusion, the food pattern of school-aged children in northern Benin showed a seasonal variation. The diet was mainly plant-based with cereals, roots and tubers as main staple foods. Although there were changes in the food pattern, the energy and nutrient intakes of the children did not show seasonal variation. Energy and nutrient intakes seemed to be adequate for the whole group; however, some of the children may have inadequate intakes. Unexpectedly, the energy and nutrient intakes of school attendees and non-attendees were similar and did not differ between seasons. Because the diet contains only very small amounts of animal products, and is low in protein, fat and vitamin C and high in fibre, the absorption of nutrients such as fat, fat-soluble vitamins, carotenoids, Fe and Zn might be low. Therefore, although Fe and Zn intake might be adequate, their bioavailability from the diet as consumed by the children needs further investigation.
Acknowledgments
Conflicts of interest:There are no conflicts of interest to disclose.
Sources of funding:The study was funded by Wageningen University through the North–South Interdisciplinary Research and Education Fund (INREF).
Author contributions:All authors have contributed to the design of the study, data analysis and discussion of the results. In addition, the corresponding author has contributed to data collection.
Acknowledgments:Financial support provided by Wageningen University through the INREF is gratefully acknowledged.











