Introduction
Astrobiology and the search for life beyond Earth are necessarily informed by the study of environments, and organisms that reside within them, on our own planet. We grow our knowledge base of what might be considered a habitable environment on other worlds by reference to extreme terrestrial environments and the survival strategies employed by the biota indigenous to these locales; different categories of extremophile life. ‘Habitability’ is defined as the set of physical and chemical environmental parameters that permit the persistence of life, and these are in turn delineated by biological constraints and the survival envelope exhibited by terrestrial extremophiles (Dartnell Reference Dartnell2011). It should also be made clear, however, that many environments that cellular life can adapt to tolerate may not be appropriate for the progression of prebiotic chemistry; extremophiles may well be irrelevant to the origin of life in the first place (Cleaves & Chalmers Reference Cleaves and Chalmers2004). Nonetheless, a major component of current astrobiology is engaged with the exploration of other planetary bodies in the Solar system and comparison of their environments, past or present, to the habitable envelope of terrestrial life, in order to determine the prospects for extraterrestrial life.
Beyond the orbital distance, a planet's habitability is affected by many astrophysical, geological and geochemical factors. In its astrobiology roadmap, NASA defined the principal habitability criteria as ‘extended regions of liquid water, conditions favourable for the assembly of complex organic molecules’ and arguably most important, ‘energy sources to sustain metabolism’. When we apply these criteria to the Earth, we observe an atmosphere that generates a sufficient greenhouse effect to allow liquid water across the surface; an ozone layer that provides protection from solar ultraviolet radiation; a global magnetic field to protect the atmosphere from the solar wind and provide shielding from cosmic radiation; and a planetary mass that can maintain plate tectonics (e.g. Kasting et al. Reference Kasting, Whitmire and Reynolds1993), which are all crucial for the long-term maintenance of life. An accessible liquid medium is deemed essential for life as terrestrial organisms are intricately linked to liquid water. However, fluids other than water might be found on other planetary bodies therefore leading to an environment in which non-terrestrial biochemistries could evolve (e.g. Bains Reference Bains2004). We have not observed such non-water biochemistries at present so our current search for extraterrestrial habitable environments favours those that would support terrestrial biochemistry.
Terrestrial analogues
Specific locations on Earth that are similar in some important respects to extraterrestrial locales, such as the Martian surface or Europan ocean, are used as field-sites for exploration-related activities, and are known as ‘analogue sites’ (Fig. 1). Not only do such analogue environments inform researchers on habitability, but also the potential for the long-term preservation of signs of past life, and thus guide the selection of which potential biosignatures to target and the most appropriate analytical methods. A third function offered by terrestrial analogues is in the field-testing and in situ validation of biosignature detection instrumentation or even testing the navigation or manoeuvrability and traction of rover designs on representative terrains.

Fig. 1. Global map of the Earth with 33 analogue sites identified. All sites are described in Tables 1–3. Site 1 broadly corresponds to high-altitude atmospheric analogues for Venus.
Terrestrial planets and moons composed predominantly of silicate rocks with the potential for supporting complex organic chemistry are a primary focus of astrobiological research. Through studying diverse environments on Earth we know that once life originates, it has the ability to adapt to a range of extreme environmental conditions that might previously have been deemed unsuitable. Prokaryotic and eukaryotic life on Earth can exist over an extraordinary range of pH, temperature, pressure, salinity and radiation levels (Rothschild & Mancinelli Reference Rothschild and Mancinelli2001). This observation combined with analogue research guides our assessment of the habitability of other planetary bodies and as such has considerably widened the number of potential habitable environments that could be explored on other terrestrial worlds. Analogue studies therefore now underpin almost all planetary exploration missions and are an essential first step in the search for habitable environments in the Solar system. Exploration of these areas allows comparisons and conclusions to be made regarding extraterrestrial observations of materials and features, and hypotheses formed regarding their origins and evolution. These include environmental, mineralogical, geomorphological, geochemical and biological conditions that resemble those found at present or at some point in the past on another planetary body. While each individual analogue site is not a perfect representation of the changing conditions through time of another world, the combination of analogue research with laboratory simulations and data obtained from orbiters and landers allows steps to be taken to advance our knowledge (Léveillé Reference Léveillé2010).
At present, terrestrial analogue studies are the best way for us to study the habitability potential of other planets and moons, and to help us design and develop tools and technologies for their exploration. That said, they have their limitations, no analogue site is a perfect representation of another planet or moon and there are no clearly defined and followed criteria with which to evaluate them. Soare et al. (Reference Soare, Pollard and Green2001) attempted to redress this with a three-tiered model which in this review we apply specifically to habitability.
The first tier or ‘analogues of the first order’ are based upon direct, empirical evidence. For example, we have data and images to certify that Mars has volcanoes, is composed of volcanic rocks, is covered in impact craters and has ice caps, all possible sites for habitable niches. This means that volcanoes, basalts, impact craters and ice-covered environments on Earth are analogues of the first-order albeit never perfect given the drastically different environment of both planets. ‘Analogues of the second order’ are based on indirect or highly suggestive evidence that is awaiting data to provide confirmation. Research into the conditions and biota within Lake Vostok is an example of an analogue of the second order as it is used to assist studies into the habitability potential of a brine ocean on Europa for which there is abundant indirect evidence supporting its presence (e.g. Kargel et al. Reference Kargel, Kaye, Head, Marion, Sassen, Crowley, Ballesteros, Grant and Hogenboom2000; Pappalardo Reference Pappalardo2010; Keszthelyi Reference Keszthelyi2011) and its possible composition. Another example, especially relevant for the search for habitable environments on Mars, is the lack of observed liquid water on its surface. Evidence of past water action is present as landforms that look like artefacts of fluvial action (e.g. Malin & Edgett Reference Malin and Edgett2000) and to some extent even mineral deposits that require water for their formation. While the connection between these features and water seems plausible or even undeniable, direct evidence for running water is still elusive. Studying analogues for these features, however, is important; they are simply second-order observations that have the potential to be proven false. Analogues of the third order are those that are not sustained by direct or indirect target evidence. There is no direct or indirect evidence that life exists anywhere other than on Earth; however, terrestrial analogues for this life are incredibly important and are integral to the development of scenarios for planetary habitability. Extremophiles are, therefore, an example of third-order analogues.
The purpose of this review is not to provide an exhaustive list of all terrestrial analogue sites that have been used to study the habitability potential of Venus, Mars and the outer icy moons. Instead, its aim is to evaluate the number and scope of key sites that are contributing most to our understanding of potential extraterrestrial habitable environments in the Solar system, and to assess our ability to access and utilize the information. Through this review of these analogue sites we are able to better understand: (1) the processes that have led to a habitable environment, or the loss of one, on Earth and other planetary bodies; (2) the diversity of environments that can inform our knowledge about multiple locations in the Solar system; (3) the potential for life, its preservation and identification over geological time; and (4) identify where research is being duplicated, where there are gaps in our knowledge of certain sites and what new analogue sites are needed. The analogue sites covered in this review are shown on the map in Fig. 1 and are numerically correlated to the analogues sites listed in Tables 1–3.
Table 1. Selected analogue sites for Mars

Table 2. Selected Analogue Sites for Europa and Enceladus

Table 3. Selected Analogue Sites for Titan

Terrestrial planets
Venus
Venus has the highest surface temperature of any planet in the Solar system, due to its proximity to the Sun and the powerful greenhouse effect produced by its thick 90 bar atmosphere of >96% CO2. Venus is at the inner edge of the stellar HZ and has undergone a runaway greenhouse effect (Walker Reference Walker1975; Kasting Reference Kasting1988) that vaporized any past oceans (Hasimoto et al. Reference Hasimoto, Roos-Serote, Sugita, Gilmore, Kamp, Carlson and Baines2008). The surface temperature is around 460 °C, preventing the possibility of liquid water or even stable organic molecules (although the possibility of supercritical water in the subsurface has been discussed by Schulze-Makuch & Irwin Reference Schulze-Makuch and Irwin2002). The Venusian surface, therefore, is not a habitable environment for life as we know it, at least not today, and as such there are limited analogue sites that exist on Earth.
High above the surface, however, there is a potential habitable zone where temperatures lie in the range between freezing and 120 °C and liquid water is available. The lower and middle cloud deck within this high-altitude habitable region may therefore support an aerial biosphere (Cockell Reference Cockell1999). These clouds offer long-lasting droplets of water, although they are highly acidic with dissolved H2SO4, and metabolic energy sources are available through sulphate reduction (Cockell Reference Cockell1999) or photosynthesis (Schulze-Makuch et al. Reference Schulze-Makuch, Grinspoon, Abbas, Irwin and Bullock2004). Hyperthermophilic acidophiles found in hot acidic waters, such as Acidianus infernus (Segerer et al. Reference Segerer, Neuner, Kristjansson and Stetter1986), which exhibit optimal growth at 88 °C and in acidic conditions as low as pH 0.5 are therefore key analogue organisms for Venus.
While analogue sites on Earth do not offer long-lived aerosols with comparable temperatures or acidities, the presence of life at high altitudes in the terrestrial atmosphere is well-known (Rothschild & Mancinelli Reference Rothschild and Mancinelli2001). Womack et al. (Reference Womack, Bohannan and Green2010) summarizes the argument that the atmosphere represents a genuine niche for micro-organisms rather than simply a transient transport phase between terrestrial surface locations. Evidence shows that bacteria may be actively metabolizing in clouds above the Earth driving biogeochemical cycling, as well as reproducing. The limiting factor on a terrestrial aerial biosphere is likely to not be nutrient availability or environmental extremes, but residence time in the atmosphere before precipitating back down (see review in Womack et al. Reference Womack, Bohannan and Green2010).
Unlike the surface-based analogue sites discussed elsewhere in this review, the exact locations for Venusian atmospheric analogue habitable environments are transient and simply require access to the high-altitude atmosphere. For example, Sattler et al. (Reference Sattler, Puxbaum and Psenner2001) demonstrate growth of bacteria in super cooled cloud droplets sampled from a meteorological station on a mountain top in the Alps, whereas Temkiv et al. (Reference Temkiv, Finster, Hansen, Nielsen and Karlson2012) used hail stone fall as a natural contamination-proof means for sampling within storm clouds. Viable bacteria have also been found at much greater altitudes, beyond the tropopause boundary in the stratosphere, through sampling by stratospheric balloon flight (Harris et al. Reference Harris, Wickramasinghe, Lloyd, Narlikar, Rajaratnam, Turner, Al-Mufti, Wallis, Ramadurai and Hoyle2001; Wainwright et al. Reference Wainwright, Wickramasinghe, Narlikar and Rajaratnam2003) and high-altitude research aircraft (Smith et al. Reference Smith, Griffin and Schuerger2010). As we learn more about the limits to life and the conditions that might have existed on Venus in the past and are present today in the clouds, there is a growing need for more terrestrial analogue sites to inform understanding of how life might be able to tolerate the potential Venusian atmospheric niche; in particular acidic and nutrient-poor conditions, and permanent residence within aerosolized droplets.
Mars
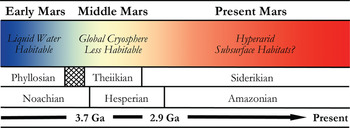
In many respects, Mars is the most Earth-like planet in the Solar system and is believed to have once provided a much warmer and wetter environment: habitable conditions for the emergence and persistence of life. This planetary similarity is reflected in the number and diversity of Martian analogue sites available for study on Earth. Mars, however, has undergone global environmental change in its past (shown in Fig. 2), and so various terrestrial analogue sites are taken not just as emulations of the conditions prevalent in different regions on Mars, but also at different points in its evolutionary history. One of the main steps in assessing habitability on Mars is the evidence of past or present water. The selected analogue sites listed in Table 1 are, therefore, organized into rough epochs of Early Mars (Fig. 3), Middle Mars (Fig. 4) and Present Mars (Fig. 5), and focus on the evolving climate of Mars and the habitable analogues that can be applied. The sites listed in this table have been selected not only to include areas that are highly utilized and so have been well-characterized, but also highlight those less well-known areas that hold valuable information and require greater study to guide our understanding of the range of habitable environments possible on Mars. Mars is a planetary analogue success story. Observations and investigations on Mars have driven analogue research of hundreds of sites that have focused our attention on areas of Mars we might have originally overlooked, and ultimately helped us to better understand the limits to life on Earth and its preservation over geological time.
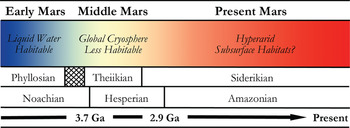
Fig. 2. Sketch of the mineralogical and climate history of Mars, with Early, Middle and Present Mars from this review included. The changing habitable conditions on the planet are also shown adapted from Bibring et al. (Reference Bibring2006).

Fig. 3. Early Mars. (A) Site 2 – Stromatolites from the Pilbara Region, Western Australia (courtesy of F. Westall). (B) Site 3 – Iron-rich acidic river at Berrocal, Rio Tinto (L.J. Preston and Preston et al. Reference Preston, Shuster, Fernández-Remolar, Banerjee, Osinski and Southam2011). (C) Site 4 – Polygons at The Golden Deposit, Canadian High Arctic (adapted from Battler et al. Reference Battler2012). (D) Site 5 – Grand Prismatic Spring, Yellowstone National Park (courtesy of M. Parenteau). (E) Site 6 – Radar image of the Haughton Impact Structure, Canadian High Arctic. (F) Site 7 – Geological map of the Dongwanzi Ophiolite Complex region, China (Adapted from Kusky et al. Reference Kusky, Li and Tucker2001).

Fig. 4. Middle Mars. (A) Site 8 – Drainage patterns at Axel Heiberg Island, Canadian High Arctic (courtesy of A. Singleton). (B) Site 9 – Beacon Valley, Dry Valleys, East Antarctica (adapted from Fairén et al. Reference Fairén, Davila, Lim, Bramall, Bonaccorsi, Zavaleta, Uceda, Stoker, Wierzchos, Dohm, Amils, Andersen and McKay2010). (C) Site 10 – Fimmvörðuháls volcanic crater and lava field, Iceland (L.J. Preston). (D) Site 11 – Koriaksky volcano, Kamchatka, Russian Federation (courtesy of C. Souness). (E) Site 12 – View of the Bockfjord Volcanic Complex, Svalbard (courtesy of C.Cousins/Arctic Mars Analog Svalbard Expedition 2010). (F) Site 13 – Mount Kilimanjaro, Tanzania (Credit: Muhammad Mahdi Karim).

Fig. 5. Present Mars. (A) Site 14 – The desert environment of the Atacama, South America (courtesy of J. DiRuggiero). (B) Site 15 – The Antarctic Dry Valleys, Antarctica (courtesy of M. McLeod). (C) Site 16 – The Mojave Desert, USA. (D) Site 17 – Landscape of the Moon, Namib Desert, Africa (Credit: Harald Süffle, CC-BY-SA-2.5). (E) Site 18 – The Kess Kess carbonate mounds near the Ibn Battuta Centre, Morocco (Credit: Andres Rueggeberg). (F) Site 19 – The hyperarid Qaidam Basin, Tibetan Plateau (Credit: NASA/Ames).
The persistence of life on the Martian surface today seems unlikely, given the extremely cold and desiccating conditions, high UV radiation flux, and the lack of magnetospheric shielding against ionizing radiation (Dartnell et al. Reference Dartnell, Desorgher, Ward and Coates2007). Localities on Earth exhibiting a subset of Mars-like conditions, however, have been shown to support thriving microbial communities (Table 1; Fig. 5). During the earliest epoch, when Mars was probably warmer with abundant surface liquid water and a global hydrological cycle (Andrews-Hanna et al. Reference Andrews-Hanna, Phillips and Zuber2007; McEwen et al. Reference McEwen2007), the situation might have been much different. The evidence shows that ‘Early Mars’ had an environment similar to the Earth (Fig. 3), supporting the hypothesis that life may have had the chance to flourish (Schulze-Makuch et al. Reference Schulze-Makuch, Dohm, Fairén, Baker, Fink and Strom2005) as it did on Earth during this time. However, after the first few hundred million years, the environmental histories of these two planets diverged drastically. Mars underwent a global climate shift resulting in a drop in surface temperatures and loss of liquid water (e.g. Fairén et al. Reference Fairén, Dohm, Baker, de Pablo, Ruiz, Ferris and Anderson2003; Bibring et al. Reference Bibring2006). This resulted in a global cryosphere with ice located throughout the soil, in glaciers and in the polar caps (as observed in the analogue sites within Fig. 4); and led to the creation of the Mars we observe today.
Detailed mineral compositions of Mars, obtained by landers and orbiters, have revealed that Mars presented a geochemically active environment (Gendrin et al. Reference Gendrin2005; Bibring et al. Reference Bibring2007). Mineralogical surface alteration products give an insight into the conditions on the surface throughout these climatic shifts and are described in detail by Bibring et al. (Reference Bibring2006). Three eras are proposed and shown in Fig. 2: (a) the Phyllosian era – abundant phyllosilicates created through non-acidic aqueous alteration; (b) the Theiikian era – sulphate deposits formed by acidic aqueous alteration; and (c) the Siderikian era – global ferric oxides produced through atmospheric water-free alteration. A number of analogue sites in Table 1 have been selected to represent these eras.
Mars has transitioned through three climatic stages: from relatively wet to semi-arid to hyper-arid conditions, and consequently the surface habitability has deteriorated greatly over its planetary history (Fig. 2). Analogues for habitability during these time frames are, therefore, required to mimic the geological and as far as possible the environmental conditions present, as well as the microorganisms that could survive in these places. These provide valuable insights into the range of habitable environments possible on Mars in the primordial past, at present, and as the conditions deteriorated; and how life could have survived and adapted as it is found to do in extreme environments on Earth.
Icy moons
The terrestrial planets do not present very habitable conditions on their surfaces today, but Mars may contain preserved biosignatures within surface materials, or active communities deep in the subsurface, and Venus may offer habitable conditions high in the atmosphere. As such, the traditional formulation of the habitable zone around a star is perhaps too restrictive, especially when we consider the potential for habitable environments on terrestrial planets other than Earth, and subsurface aqueous environments within the icy moons of the gas giant planets, physically located far beyond the proposed habitable zone and thawed by a heat budget provided not by insolation but tidal heating (e.g. Sotin et al. Reference Sotin, Head and Tobie2002; Roberts & Nimmo Reference Roberts and Nimmo2008).
Some of the most important astrobiological targets in the Solar system are found on moons. The most promising icy satellites of Jupiter and Saturn, namely Europa, Enceladus and Titan, have been revealed by recent space missions to be geologically active bodies hosting a wealth of potentially habitable environments. The problem for astrobiological ground-truthing, however, is that the environments on these moons are so extreme compared with those on Earth that suitable terrestrial analogues are fewer in number, and our knowledge of the conditions actually present on the Ice moons are mainly based on inferences, rather than definitive data.
Europa
Europa is constantly bombarded with ionizing radiation (Baumstark-Khan & Facius Reference Baumstark-Khan, Facius, Horneck and Baumstark-Khan2002) as it lies within Jupiter's magnetosphere and temperatures at the surface range from 86 to 132 K (−187 to −141 °C) (Spencer et al. Reference Spencer, Tamppari, Martin and Travis1999) far below the lowest limits for microbial growth. As such the ice itself is not a habitable environment that any currently known terrestrial life could withstand, however, the ice could provide enough protection from the radiation to allow preservation of organics and any life forms within it (Gilichinsky et al. Reference Gilichinsky, Soina and Petrova1993; Hoover & Gilichinsky Reference Hoover, Gilichinsky, Paepe and Melnikov2001; Gilichinsky Reference Gilichinsky, Horneck and Baumstark-Khan2002). The low temperatures will preclude biological activity anywhere near the Europan surface but perhaps near the base of the ice layer at the interface with an ocean below, temperatures might prove to be more favourable. A number of ice-dominated habitats on Earth could provide analogues for habitable niches here (Fig. 6).

Fig. 6. Europa and Enceladus Surface Ice. (A) Site 20 – Radar image of Lake Vostok, Antarctica (Credit: NASA Goddard). This site can also be used as an analogue for the Brine Ocean and ocean floor environments. (B) Site 21 – Permafrost site from the Canadian Arctic (L.J. Preston). (C) Site 22 – Aerial photograph of sulphur-on-ice deposits, Borup Fiord Pass, Ellesmere Island (Credit: Damhait Gleeson, NASA/JPL).
There is abundant indirect evidence supporting the presence of a brine ocean beneath the ice of Europa (Kargel et al. Reference Kargel, Kaye, Head, Marion, Sassen, Crowley, Ballesteros, Grant and Hogenboom2000; Pappalardo Reference Pappalardo2010) and suggestions that its composition consists of hydrated minerals (Carlson et al. Reference Carlson, Johnson and Anderson1999; McCord et al. Reference McCord1999); but whether these are sulphate salts, acid or alkaline conditions remains unknown. This putative ocean has been the focus of much attention surrounding possible habitable environments (e.g. Reynolds et al. Reference Reynolds, Squyres, Colburn and McKay1983; Jakosky & Shock Reference Jakosky and Shock1998; Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999; McCollom Reference McCollom1999; Chyba Reference Chyba2000; Kargel et al. Reference Kargel, Kaye, Head, Marion, Sassen, Crowley, Ballesteros, Grant and Hogenboom2000; Chyba & Hand Reference Chyba and Hand2001; Chyba & Phillips Reference Chyba and Phillips2001; Navarro-Gonzalez et al. Reference Navarro-Gonzalez, Montoya, Davis, McKay and Greeley2002; Pierazzo & Chyba Reference Pierazzo and Chyba2002; Schulze-Makuch & Irwin Reference Schulze-Makuch and Irwin2002; Marion et al. Reference Marion, Fritsen, Eicken and Payne2003) and the impact on life of the range of salinity, acidity, temperature and pressure conditions that might be found there (see Fig. 7 for potential terrestrial analogue sites).

Fig. 7. Europa and Enceladus Brine Ocean. (A) Site 23 – Salt deposits at Lake Tirez, Spain (courtesy of O. Prieto-Ballesteros). (B) Site 25 – The saline soda lake of Mono Lake, California (L.J. Preston). (C) Site 26 – Halite deposits off the western coast of the Dead Sea, Israel (Credit: Wilson44691). Note: No image available for site 24 – The Orca Basin.
Finally, a habitable seafloor environment has been postulated for Europa due to extensive evidence from the Earth's subsurface and ocean floor environments; particularly in deep sea hydrothermal vent fields, of vast thriving habitats (Fig. 8). Analogues for these potentially habitable environments are important to consider, even if at present any actual search for them on the moon is restricted to indicators of their activity on the surface and in the near-subsurface. Analogues for all these scenarios, and their potential for habitability, are documented in Table 2.

Fig. 8. Europa and Enceladus Ocean Floor. (A) Site 27 – Hydrothermal vent field, Lost City, Mid-Atlantic Ridge (Credit: National Science Foundation (University of Washington/Woods Hole Oceanographic Institution)). (B) Site 28 – Location of the Marianna Trench, Pacific Ocean (Credit: Google Earth 2013). (C) Site 29 – Aerial photograph of the Lidy Hot Springs, USA (courtesy of the USGS). (D) Site 30 – Columnar jointing of the Columbia River Basalts, USA (L.J. Preston).
Enceladus
Saturn's small moon drew astrobiological interest with the sighting of present-day geological activity occurring at its surface. Jets of fine icy particles and water vapour were observed emerging from the south polar terrain by the Cassini orbiter and are documented by Porco et al. (Reference Porco2006). Dozens of these jets feed into a large plume that reaches several thousand kilometres into space (Porco et al. Reference Porco2006) and contains water vapour, simple organic compounds and notable levels of volatile species (e.g. N2, CO2, CH4) (Waite et al. Reference Waite2006). The south polar terrain surrounding the source regions of the plume was analysed and found to be surprisingly warm (Spencer et al. Reference Spencer, Pearl, Segura, Flasar, Mamoutkine, Romani, Buratti, Hendrix, Spilker and Lopes2006).
Analysis of icy particles within Enceladus’ plume strongly suggest the presence of a subsurface alkaline ocean composed of NaCl, NaHCO3, Na2CO3 and K+ (Postberg et al. Reference Postberg, Kempf, Schmidt, Brilliantov, Beinsen, Abel, Buck and Srama2009). Reviews into how this plume might form are provided by Kieffer et al. (Reference Kieffer, Lu, Bethke, Spencer, Marshak and Navrotsky2006), Spencer et al. (Reference Spencer, Pearl, Segura, Flasar, Mamoutkine, Romani, Buratti, Hendrix, Spilker and Lopes2006); Castillo-Rogez et al. (Reference Castillo-Rogez, Matson, Vance, Davies and Johnson2007); Collins & Goodman (Reference Collins and Goodman2007); Meyer & Wisdom (Reference Meyer and Wisdom2007); Nimmo et al. (Reference Nimmo, Spencer, Pappalardo and Mullen2007) and McKay et al. (Reference McKay, Porco, Altheide, Davis and Kral2008). Most models regarding the origin of this plume include a subsurface liquid water aquifer, and it is this aquifer, with its potential to support the origin and evolution of life (McKay et al. Reference McKay, Porco, Altheide, Davis and Kral2008), that has particular interest for habitability. A plausible subsurface ecosystem on Enceladus would be unlike many terrestrial biomes as they would be independent of O2 and organic materials produced by photosynthesis. Therefore, terrestrial analogues that do not rely on sunlight, oxygen or organics produced at the surface need to be investigated.
Habitable environments and sites where biosignatures of life might be preserved on Enceladus are probably similar to those postulated for Europa and so terrestrial analogues for both have been grouped into Table 2 and show in Figs. 6–8.
Titan
Titan is the only moon in the Solar system to possess an atmosphere and, also like Earth; the surface is now known to have many dynamic processes. A dense N2-rich atmosphere, evidence of dendritic networks resembling fluvial systems (e.g. Tomasko et al. Reference Tomasko2005), as well as lakes and seas (e.g. Stofan et al. Reference Stofan2007; Hayes et al. Reference Hayes2008; Paillou et al. Reference Paillou, Lunine, Ruffie, Encrenaz, Wall, Lorenz and Janssen2008), composed of a mixture of methane and ethane with heavier hydrocarbons, dissolved nitriles and/or atmospheric gases (Flasar Reference Flasar1983; Lunine et al. Reference Lunine, Stevenson and Yung1983; Lorenz & Lunine Reference Lorenz and Lunine2005; Mitri et al. Reference Mitri, Showman, Lunine and Lorenz2007), combined with free H2 near the surface creates a very dynamic environment. If such methane–ethane mixtures are suitable to serve as a biosolvent, then the Titan surface environment might be considered to be habitable, although for exotic life with a very different biochemistry to our own. In addition, Sotin et al. (Reference Sotin2005) and Lopes et al. (Reference Lopes2007) have inferred the existence of cryovolcanism, and so heating of some of Titan's hydrocarbon reservoirs from below (e.g., Schulze-Makuch & Grinspoon Reference Schulze-Makuch and Grinspoon2005) would further promote prebiotic organic reactions (Schulze-Makuch et al. Reference Schulze-Makuch2011) and increases the number of habitable environments. Baross et al. (Reference Baross, Benner, Cody, Copley, Pace, Scott, Shapiro, Sogin, Stein, Summons and Szostak2007) noted that Titan's environment meets the absolute requirements for life, including thermodynamic disequilibrium, an abundant carbon inventory and a fluid environment.
In addition to the hydrocarbon-drenched surface environment, there is indirect evidence from radar measurements of a deep subsurface ocean (Lorenz et al. Reference Lorenz2008), probably composed of a water–ammonia mixture. Such an environment may therefore also be considered habitable for organisms with a polar solvent biochemistry closer to that of terrestrial life. In this way, it is possible that Titan could harbour two biospheres: one on the surface using hydrocarbons as the biosolvent and another, water-based, deep in the interior, but entirely ecologically segregated from each other as they would have to be based on fundamentally different biochemistries. Considering both potential habitats, the range of habitable environments on Titan extends from the moon's surface to a few kilometres depth within the silicate core, which yields a total potential biosphere volume of ∼4×1010 km3: more than twice that of the Earth's (Norman & Fortes Reference Norman and Fortes2011).
The environment on Titan is so different to that on Earth, in terms of physical characteristics such as the temperature regime as well as chemical composition of the fluid reservoirs and lack of liquid water and free oxygen, that terrestrial analogue sites are poor matches. Life as we know it may not be found on Titan, but we have not yet been able to rule-out the possibility of viable biochemical systems based on very different solvents, informational storage or structural molecules. Nonetheless, a few terrestrial locales do replicate some features of the Titanic environment (detailed in Table 3 and Fig. 9) and have been used to inform discussion on the habitability of the moon. Additional analogue sites are greatly needed for potential habitable environments on Titan to enable us to better understand the moon and where to send future probes; however, this is a difficult mission. We need to learn more about Titan to recognize the valuable terrestrial analogues, but in the search for habitability, analogues commonly drive our exploration.

Fig. 9. Titan. (A) Site 31 – Liquid asphalt of Pitch Lake, Trinidad and Tobago (courtesy of D. Schulze-Makuch). (B) Site 32 – Emerging gas bubble at Rancho La Brea Tar Pits, California (Credit: Daniel Schwen).
Conclusions
We have collated information on a selection of first, second and third tier analogue sites on Earth to enable the study of potential habitable environments on Venus, Mars, Europa, Enceladus and Titan. Atmospheric habitable zones on Venus are discussed in comparison with high-altitude terrestrial biospheres. The habitability and biosignature preservation potential of 18 analogue sites has been described to address the three climatic and mineralogical eras of Mars. These range from the deserts of the Atacama, to perennial springs in the Canadian High Arctic, and volcanoes in Russia and Africa. A total of 11 analogue sites are reviewed that are helping to predict the habitability potential of the surface ice layers, brine oceans and ocean floors of the moons Europa and Enceladus. Aspects of one analogue site in particular, Lake Vostok and other Antarctic subglacial lakes can be used to represent many aspects of these icy moons. Finally the habitability of Titan, with its extreme environment, can be to some extent investigated using three natural asphalt and petroleum reservoir analogue sites. The terrestrial analogues described in this review have been assigned in Tables 1–3 to a particular planetary body; however, many have features that are applicable to more than one. This wider applicability is not always apparent from the primary literature, perhaps as authors are focused on one particular target, and so we have made these opportunities clear in this review. Permafrost sites throughout the Earth are good analogues for Europa, Enceladus, Mars and potentially any celestial body that is unable to sustain liquid water on its surface. Other examples include the deep subsurface habitats at Lidy Hot Springs and within the Columbia River Flood Basalts. These are important analogues for potential subsurface habitats on any rocky planetary body, especially Mars and the icy moons.
Aside from the extraterrestrial applicability of a site, we can see from this review that the value of a terrestrial analogue for habitability does not solely rely on the similarity of the analogue to its target, or the level to which we are confident in its fidelity. A much simpler value can be assigned: accessibility. All the analogue sites described in this review are of value, however, only half of them are readily accessible without specialized equipment or excessive financial contributions. It is of no surprise, therefore, that analogue sites most often cited in the literature are those that are easy to get to and can be revisited if needed; are large enough to sustain multiple sampling excursions and teams; and permissions regarding visitation and sampling under most circumstances are obtainable. How many scientifically valuable sites are being understudied or simply overlooked due to a lack of accessibility or available resources?
A large and some would say key, area of research into habitability involves extremophiles and the types of life found in these planetary analogue environments. Habitability does not necessitate the presence of life, just the conditions which could allow for it, and although this review is focused on the analogue sites themselves, it has found that more often than not they contain some form of extremophilic life, and may indeed only be an analogue site due to this biota. As such a compilation of the range and diversity of both prokaryotic and eukaryotic extremophilic life would be invaluable to researchers. An extremophile database has been started called ExtremeDB (http://extrem.igib.res.in/) and is explained by Majhi et al. (Reference Majhi, Behera, Kulshreshtha, Mahmooduzafar, Kumar and Kumar2013) but is currently early in development focused primarily on prokaryotes. The completion of this database is a huge undertaking and will need support and commitment from the scientific community to make it work.
It goes without saying that this review is not able to document every analogue site that has been used in the past, or is appropriate to be used in the future, for planetary habitability studies. What is required is a repository for this information that is open access, and populated and curated by scientists and specialists working in these areas.
A number of attempts have been made to create an analogue database such as NASA's Analogs Database (http://external.jsc.nasa.gov/analogs/index.cfm) initially compiled to support NASAs Vision for Space Exploration activities. The number of sites currently in the catalogue stands at 37 (only 4 more than this review). However, there are a number of important ways in which this original effort can be improved upon. The NASA Analogs Database provides only scant information on many of the listed sites, there is little critical assessment of the fidelity of the correspondence with extraterrestrial targets, and there is poor global coverage of appropriate analogue sites, the vast majority are based in North America. Furthermore, the site appears to not have been updated since 2011. This discussion is not intended to be critical of the volunteers who created this initial database – it is no small order to set-up then curate and maintain a database such as this – but as a rallying cry for the extremophile and analogue communities as a whole to come together to pool knowledge and effort to generate a resource of great utility for all: a database which is more comprehensively detailed, broader in its geographical coverage, and consistently kept up-to-date.
A ‘Catalogue for Planetary Analogues’ has been compiled by the CAFE Study (Preston et al. Reference Preston, Barber and Grady2012) commissioned by the European Space Agency. This catalogue contains 93 analogue sites from all seven continents and includes information on the geology, biology and chemistry of the sites, accessibility, costs, logistics and previous campaigns. Currently this catalogue only documents analogue sites for Mars and the Moon and is at present not publically available. It was clear during the compilation of this catalogue, and indeed this review, how difficult it is to curate such a huge volume of information, to make sure there is an equal global spread of sites and to gain access to the data. However, a catalogue or database of these analogue sites is greatly needed to share first-hand knowledge of field sites, to reduce accidental duplication of research and to promote greater international cooperation.
Acknowledgements
The authors would like to thank all individuals, named in the captions for each figure, for their generous contribution of images. LJP is supported by funding from the Science and Technology Facilities Council (STFC) and LRD is supported by a UK Space Agency Aurora Fellowship.