Parkinson’s disease (PD) is a degenerative disorder of the central nervous system mainly affecting the locomotor activity. It has a higher incidence among the elderly, and incidence increases with age.Reference Pihlstrom and Toft 1 The motor symptoms of PD include resting tremor, bradykinesia, rigidity, and postural reflex impairment.Reference Nussbaum and Ellis 2 The typical pathophysiological features of PD are progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and subsequent accumulation of Lewy bodies in the remaining dopaminergic neurons.Reference Hirsch, Graybiel, Duyckaerts and Javoy-Agid 3 , Reference Ou, Yang, Cao, Wei, Chen and Chen 4 PD is caused by complicated factors, including age,Reference Ou, Yang, Cao, Wei, Chen and Chen 4 environment,Reference Bellou, Belbasis, Tzoulaki, Evangelou and Ioannidis 5 genetics,Reference Lesage and Brice 6 oxidative stress,Reference Sharma and Nehru 7 mitochondrial dysfunction,Reference Bolisetty and Jaimes 8 and inflammation,Reference Tian, Zhang, Zhang, Qiao and Fan 9 among others.
Presently, the main therapeutic strategy for PD is drug therapy. The most widely used medicines for PD include levodopa (L-dopa),Reference LeWitt 10 , Reference LeWitt, Huff, Hauser, Chen, Lissin and Zomorodi 11 dopamine receptor agonists,Reference Bonuccelli, Del Dotto and Rascol 12 catechol-O-methyltransferase inhibitors,Reference Muller, Kolf, Ander, Woitalla and Muhlack 13 and monoamine oxidase inhibitors.Reference Degli Esposti, Piccinni, Sangiorgi, Nobili and Buda 14 Nevertheless, drugs including -L-dopa cause serious side effects and fail to inhibit disease progressionReference Jalloul, Poree, Viardot, L’Hostis and Carrault 15 ; therefore, more effective and safe PD drugs are urgently needed. Previous studies showed that traditional Chinese medicine such as salidroside and green tea polyphenols had protective effects in a rat model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).Reference Guo, Yan, Yang, Yang, Bezard and Zhao 16 , Reference Wang, He, Chen, Zhang, Zhang and Chen 17 Similar effects were found with ginkgo biloba extract (GBE) treatment.Reference Rojas, Serrano-Garcia, Mares-Samano, Medina-Campos, Pedraza-Chaverri and Ogren 18 Whether GBE would have protected effects in α-synuclein A53T transgenic mice, a mouse model of PD, and its mechanism is remains unclear. In the present study, the effects of GBE on the α-synuclein A53T PD model were studied.
Materials and Methods
Animals Feed and Treatment
Fifty α-synuclein A53T transgenic mice were purchased by the Model Animal Research Center of Nanjing University (Nanjing, China) under laboratory animal production license number XCYK (Su) 2010-0001. Mice were housed in an specific pathogen-free (SPF)-grade laboratory in Guangdong Medical Laboratory Animal Center (Guangdong, China) under experimental animal license SYXK (Yue) 2013-0002. All experiments were approved by the Guangdong Medical Laboratory Animal Center ethical boards. The mice were exposed to a 10:14-hour light/dark cycle, 20% to 26% humidity at 21 ± 2°C and had ad libitum access to food and water for 8 months. The mice were randomly divided into five groups of ten mice each: group 1 was treated with normal saline (normal saline group); group 2 was treated with a high dose of GBE (60 mg/kg, high-dose group; batch 1990813; acquired from Dr. Willmar Schwabe); group 3 was treated with an intermediate dose of GBE (40 mg/kg, intermediate-dose group); group 4 was treated with a low dose of GBE (40 mg/kg, low-dose group); and group 5 was treated with Madopa (75 mg/kg, Madopa group; batch 1990813; Roche Pharmaceuticals, Basel, Switzerland). After 21 days of treatment, locomotor activity was evaluated by using a forced swim test, a pole-climbing test, and a wire-hang test. Further, mice were sacrificed by cervical dislocation. Brains were dissected out and divided into two parts. One part was used to detect superoxide dismutase (SOD), glutathione peroxidase (GSH-PX), and malondialdehyde (MDA), and the other was used to detect the expression of tyrosine hydroxylase (TH) and dopamine transporters (DAT).
Spontaneous Locomotor Activity
Pole Test
A 25-mm diameter cork ball was attached a top a wooden pole (diameter, 1 cm; height, 50 cm) with gauze to prevent slippage. The mouse was positioned at the top of the cork ball, head up; the time taken by the mouse to descend from the pole was measured. This was divided into two parts: duration of descent from the cork ball to the wooden pole and duration of descent from the pole to the floor. Trials completed within 3 seconds were assigned 3 points; 3 to 6 seconds, 2 points; and >6 seconds, 1 point. During a habituation period 1 day before the beginning of the testing phase, each mouse was underwent one practice run. The pole test was administered twice per day for 3 consecutive days (total, six trials).
Forced Swim Test
A previously described, the forced swim test protocol was adapted for mice using a glass cylinder (15-cm diameter) filled with water 13-cm deep at room temperature.Reference Chen, Xie, Turkson and Zhuang 19 Mice were pretested for 5 minutes 24 hours before the test. During the 2-minute test period, mice that swam continuously were assigned 3 points, those that mostly swam and occasionally floated, 2.5 points; those that floated for >50% of the trial, 2 points; those that mostly floated and occasionally swam, 1.5 points; and those that floated continuously, 1 point. The forced swim test was administered twice per day for 3 consecutive days (total, six trials).
Wire-Hang Test
The wire-hang test was conducted to assess grip strength and endurance. First, mice were placed on the lid of a wire cage, which was then inverted. The mice were induced to maintain a grip on the wires, by gently vibrating the cage lid. The cage was filled with spongy material to prevent injury from falling. Trials in which the mouse’s two hind paws grasped the lid were assigned 3 points; those in which only one hind paw grasped, 2 points; and those that did not grasp the lid, 1 point. The wire-hang test was conducted twice per day over 3 consecutively days (total, six trials).
Immunohistochemistry (IHC)
The number of neurons expressing TH in the SNpc and those expressing DAT in the striatum were detected using IHC. Following sacrifice, the mice’s SNpc and striatum were dissected from the brain, transferred to 4% paraformaldehyde, and embedded in paraffin. The tissue was cut into 3-μm serial sections using a Leica microtome (RM2235; Leica Microsystems GmbH, Wetzlar, Germany). IHC was performed according to a previously published protocol.Reference Rojas, Serrano-Garcia, Mares-Samano, Medina-Campos, Pedraza-Chaverri and Ogren 18 Briefly, sections were incubated in 3% H2O2 in phosphate-buffered saline (PBS; 0.1 mol/L, pH 7.4) for 7 minutes, washed with PBS (0.1 mol/L, pH 7.4) three times, and treated with a solution containing 1% bovine serum albumin and 0.3% Triton X-100 for 2 hours. Further, the sections were incubated overnight at 4°C with rabbit anti-TH antibodies (1:750; ab75875; Abcam, Cambridge, UK) or rabbit anti-DAT antibodies (1:500; ab111468; Abcam). After washing twice with PBS (0.1 mol/L, pH 7.4), sections were treated for 40 minutes with biotinylated anti-rabbit immunoglobulin G, washed three times in 0.1 mol/L PBS, and processed using streptavidin-biotinylated horseradish peroxidase complex (Santa Cruz Biotechnology, Dallas, TX). The reaction was visualized using DAB (DakoCytomation, Carpinteria, CA) and cresyl violet counterstaining (Sigma-Aldrich, St. Louis, MO). Images were obtained using a microscope (BX41, Olympus Corporation, Tokyo, Japan). Mean optical density was quantified using Image ProPlus 6.0 (Media Cybernetics, Silver Spring, MD).
Analysis of SOD and GSH-PX Activity and MDA Expression
SOD activity, GSH-PX activity, and MDA expression were detected using an SOD Assay Kit, GSH-PX Assay Kit, and MDA Assay Kit, respectively (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China), according to the manufacturers’ instructions.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 19.0; IBM Corporation, Armonk, NY). The data, which fit the normal distribution, are expressed as mean±standard deviation. The data were analyzed using a one-way analysis of variance followed by a post hoc LSD test. All p values <0.05 were considered significant.
Results
GBE Recovered the Locomotor Activity of α-Synuclein A53T Transgenic Mice
The ultimate therapeutic goal of GBE treatment is to ameliorate the functional impairments of PD. Accordingly, we tested for locomotor activity impairment using the pole test, forced swim test, and wire-hang test (Figure 1). The mice in the high-dose, intermediate-dose, low-dose, and Madopa groups showed significantly better locomotor performance compared with the control group (p<0.05). GBE treatment improved locomotor activity in A53T α-synuclein transgenic mice in a dose-dependent manner. The therapeutic effectiveness of intermediate- and high-dose GBE was similar with Madopa.
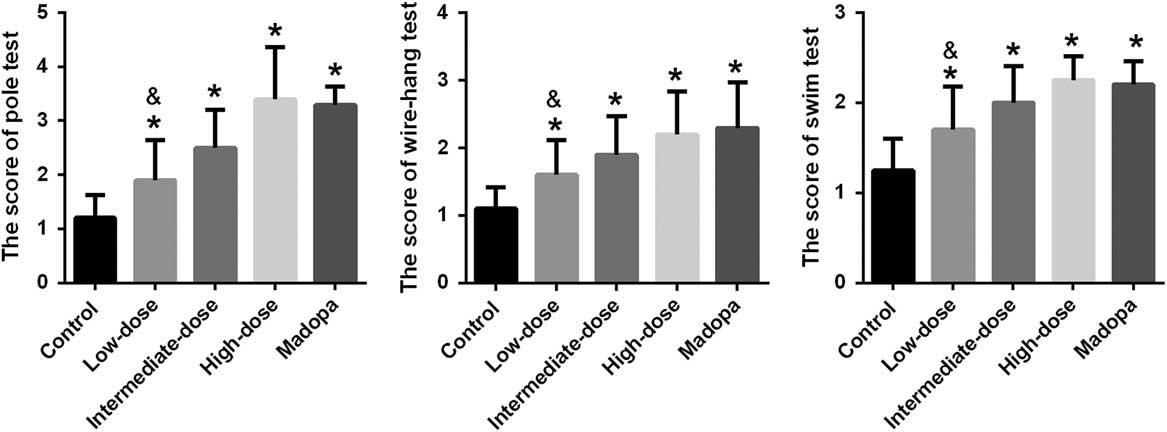
Figure 1 Scores were achieved by the pole test, forced swim test, and wire-hang test in α-synuclein A53T transgenic mice. *p<0.05 compared with the control group; &p<0.05 compared with the Madopa group.
GBE Improved the Activity of Different Antioxidant Enzymes in α-Synuclein A53T Transgenic Mice
The neurological damage seen in patients with PD involves free radical synthesis and oxidative stress. To explore the effects of GBE treatment, we determined whether it was associated with changes in the activity of antioxidant enzymes such as SOD and GSH-PX and in the expression of MDA. The results are shown in Figure 2. SOD activity improved significantly in the high-dose, intermediate-dose, low-dose, and Madopa groups compared with the control group (p<0.05). GBE treatment improved SOD activity in A53T α-synuclein transgenic mice in a dose-dependent manner. The therapeutic effectiveness of intermediate- and high-dose GBE was similar with Madopa. GSH-PX activity showed a trend similar to the SOD activity. In contrast, MDA expression was significantly reduced in the high-dose, intermediate-dose, low-dose, and Madopa groups compared with the control group (p<0.05). GBE treatment reduced MDA expression in A53T α-synuclein transgenic mice by a dose-dependent manner. The therapeutic effectiveness of intermediate- and high-dose GBE was similar with Madopa.
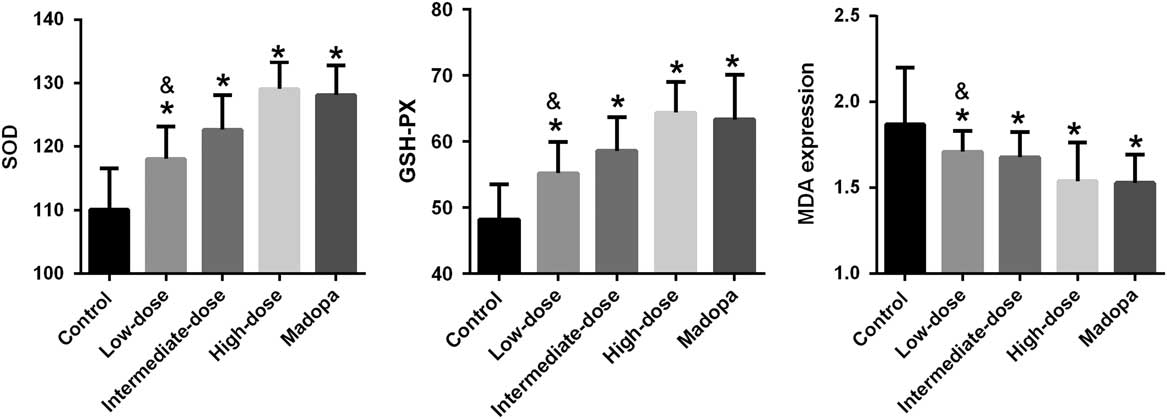
Figure 2 SOD activity, GSH-PX activity, and the expression of MDA were measured in α-synuclein A53T transgenic mice. *p<0.05 compared with the control group; &p<0.05 compared with the Madopa group.
GBE Recovered the Expression of TH and DAT in the A53T α-Synuclein Transgenic Mice
In the brain of A53T α-synuclein transgenic mice, the expression of TH was detected by IHC (Figure 3). TH expression was significantly higher in the high-dose, intermediate-dose, low-dose, and Madopa groups than in the control group (p<0.05). GBE treatment enhanced TH expression in A53T α-synuclein transgenic mice in a dose-dependent manner. The therapeutic effectiveness of intermediate- and high-dose GBE was similar to Madopa. In the brain of A53T α-synuclein transgenic mice, the expression of DAT was detected by IHC (Figure 4). DAT expression was significantly higher in the high-dose, intermediate-dose, low-dose, and Madopa groups compared with the control group (p < 0.05). GBE treatment enhanced DAT expression in A53T α-synuclein transgenic mice in a dose-dependent manner. The therapeutic effectiveness of intermediate- and high-dose GBE was similar with Madopa.
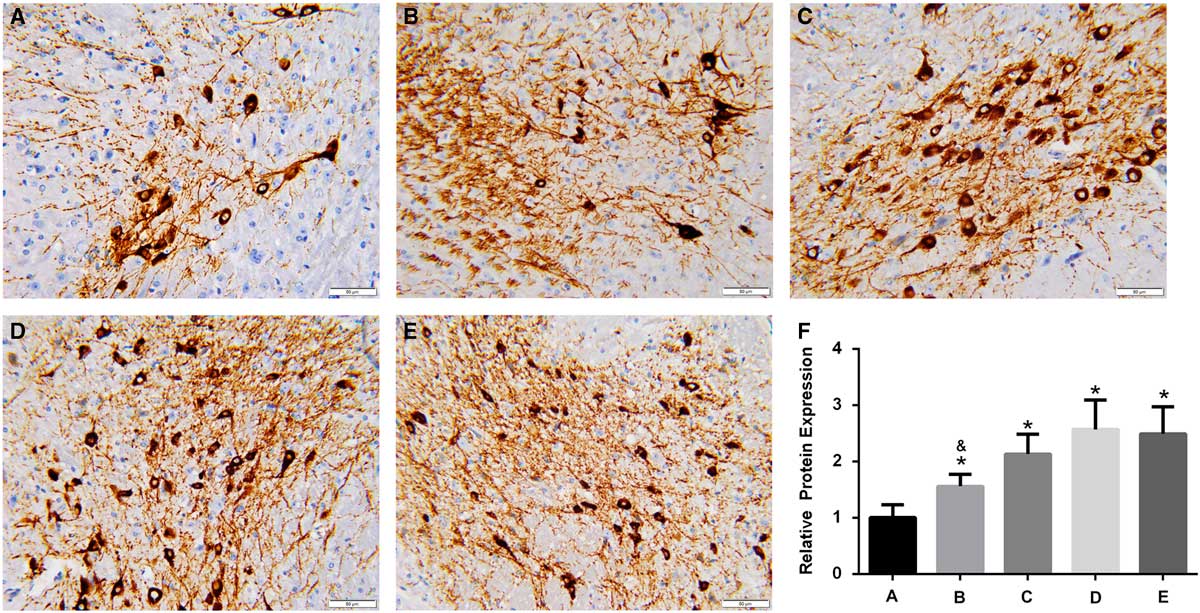
Figure 3 Expression of TH in the brain of A53T α-synuclein transgenic mice. (A-E) Representative images of IHC staining of TH in the control, low-dose, intermediate-dose, high-dose, and Madopa groups, respectively (400×) (F) The results of TH expression are presented as mean ± standard deviation; bars indicate TH protein expression levels. *p<0.05 compared with the control group, &p<0.05, compared to Madopa group.
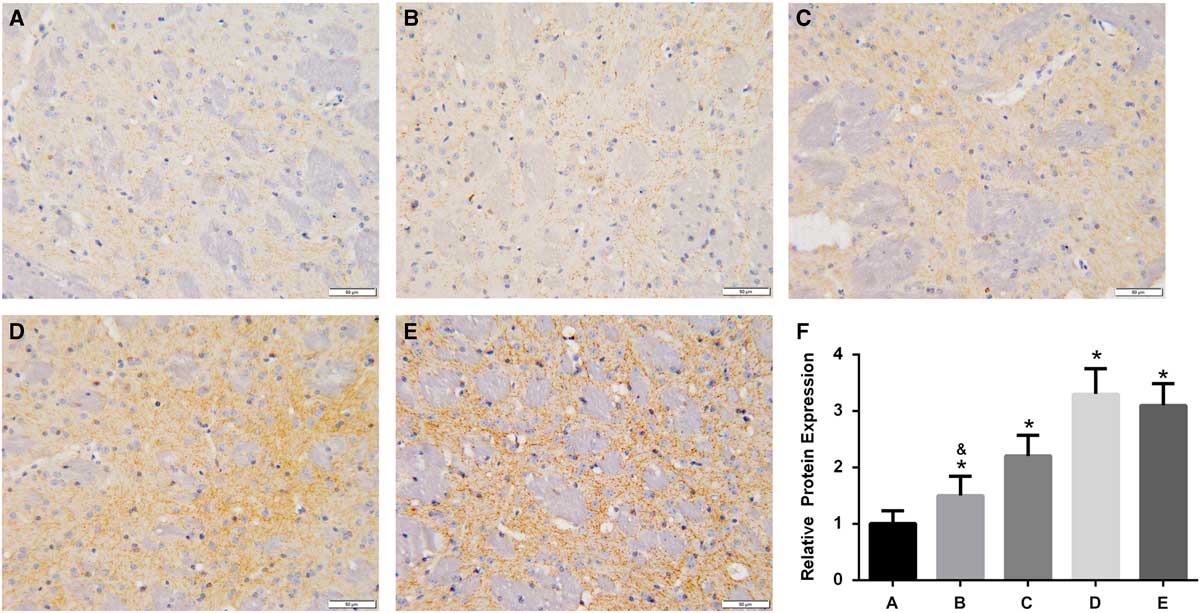
Figure 4 Expression of DAT in the brain of A53T α-synuclein transgenic mice. (A-E) Representative images of IHC staining of DAT in the control, low-dose, middle-dose, high-dose, and Madopa groups, respectively (400×). (F) The results of DAT expression are presented as mean ± standard deviation; bars indicate DAT protein expression levels. *p<0.05 compared with the control group, &p<0.05 compared with the Madopa group.
Discussion
Animal models of PD are mainly two types: transgenic mouse models and chemically induced models. The chemically induced models, such as the MPTP-induced PD model, are acute in the course of disease progression and fail to replicate all the features of PD. The transgenic mouse models, such as the A53T α-synuclein transgenic mice, have the ability to replicate all of the features of PD.Reference Dawson, Ko and Dawson 20 In this study, A53T α-synuclein transgenic mice were used as the PD model. The level of α-synuclein gradually increases in dopaminergic neurons in humans during normal aging. In patients with PD, α-synuclein is a major component of Lewy bodies, which accumulate in the dopaminergic neurons and lead to PD progression. Missense mutations of α-synuclein, including the A53T mutant, have been causally linked to familial PD.Reference Chartier-Harlin, Kachergus and Roumier 21 A53T α-synuclein overexpression induces pervasive mitochondria macroautophagy defects and promotes dopamine neuron degeneration.Reference Chen, Xie, Turkson and Zhuang 19 The previous study found that locomotor activity and dopaminergic neuron function in A53T α-synuclein transgenic mice were impaired with aging, similar to disease progression in patients with PD.Reference Oaks, Frankfurt, Finkelstein and Sidhu 22 A53T α-synuclein transgenic mice have better simulated disease progression in patients with PD than other models, such as the MPTP-induced mouse model of PD.
In the present study, we investigated the effects of GBE on A53T α-synuclein transgenic mice. The results showed that the GBE treatment improved locomotor activity and the activity of SOD and GSH-PX, inhibited MDA expression, and recovered the expression of TH and DAT. According to therapeutic effectiveness, the groups can be ranked from low to high as low-dose, intermediate-dose, Madopa, and high-dose groups. GBE inhibited PD progression in α-synuclein A53T transgenic mice by a dose-dependent manner. The therapeutic effectiveness in the intermediate-dose, Madopa, and high-dose groups was similar.
GBE is obtained from the leaves of the Ginkgo biloba tree and is a complex mixture of ingredients. It mainly contains two active compounds: flavonoids (24%) and terpenoids (6%). The relative molecular weights of these GBE weights are low and can potentially penetrate the blood-brain barrier; hence, GBE has a broad spectrum of pharmacological actions in the central nervous system.Reference DeFeudis and Drieu 23 In the present study, GBE treatment improved locomotor activity in A53T α-synuclein transgenic mice as measured by a pole test, forced swim test, and wire-hang test. The results suggest that GBE is an effective drug to treat patients with PD. These results are similar to those of Rojas et al,Reference Rojas, Serrano-Garcia, Mares-Samano, Medina-Campos, Pedraza-Chaverri and Ogren 18 which demonstrated that GBE protects the locomotor activity in MTPT-induced PD in mice.
The pathomechanisms of PD remain unknown; however, the presence of ongoing oxidative stress and the generation of reactive oxygen species are known to play an important role in PD development.Reference Sharma and Nehru 7 During PD development, oxidative stress markers, such as reactive oxygen species, lipid peroxidation, nitric oxide formation, nicotinamide adenine dinucleotide phosphate-oxidase activity, glutathione system, SOD, and MDH, have been shown to be significantly changed compared with healthy controls.Reference Sharma and Nehru 7 GSH was reduced by up to 40%, and the activity of SOD and GSH-PX declined.Reference Hedrich, Winkler and Hagenah 24 The GBE-active compounds, flavonoids and terpenoids, have the ability to eliminate oxygen-free radicals and act as antioxidants.Reference Yallapragada and Velaga 25 Saini et alReference Saini, Taliyan and Sharma 26 have demonstrated that in streptozotocin-induced diabetic cardiomyopathy in rats, EGb 761 attenuated oxido-nitrosative stress, improved antioxidant enzyme levels, and led to diabetic cardiac dysfunction. In aged female rats, GBE treatment inhibited the expression of MDA and 8-oxo-2′-deoxyguaniosine, improved the expression of brain-derived neurotrophic factor in plasma, decreased oxidative damage, and improved cognitive functions.Reference Belviranli and Okudan 27 In myocardial ischemia-reperfusion injury rabbits, GBE reduced the generation of oxygen free radicals and increased the antioxidant capacity of the myocardial cells.Reference Ran, Yang and Chang 28 In the present study, we found that GBE treatment improved SOD and GSH-PX activity, inhibited MDA expression, and decreased oxidative damage in A53T α-synuclein transgenic mice. The current evidence shows that GBE treatment increased SOD and GSH-PX activity and decreased MDA expression, reduced the generation of oxygen-free radicals, and decreased oxidative damage shown in other PD models, similar to the results of the present study.Reference Rojas, Serrano-Garcia, Mares-Samano, Medina-Campos, Pedraza-Chaverri and Ogren 18
DAT is a transmembrane Na+/Cl– transporter and controls the reuptake of extracellular dopamine into presynaptic neurons, playing an important role in maintaining normal dopamine (DA) homeostasis.Reference Torres 29 Tyrosine hydroxylase converts l-tyrosine to L-3,4-dihydroxyphelylalanine (L-dopa), which is the precursor for dopamine.Reference Feve 30 Decreased levels of DAT and TH are positively associated with the development of PD in clinical cases.Reference Torres 29 , Reference Johnson, Lim, Senthilkumaran, Zhou and Bobrovskaya 31 In the present study, we found that GBE treatment improved the activity levels of DAT and TH, which may maintain normal DA homeostasis and improve dopamine expression, ultimately inhibiting the development of PD. Such results are similar to those of Rojas et al, which demonstrated that GBE protects in MTPT-induced PD mice.Reference Rojas, Serrano-Garcia, Mares-Samano, Medina-Campos, Pedraza-Chaverri and Ogren 18
In conclusion, GBE treatment improved the locomotor activity and stopped the development of PD in A53T α-synuclein transgenic mouse model of PD by a dose-dependent manner, suggesting that GEB is effective drug for PD treatment. The neuroprotective effects may be partly responsible for improving the activity of SOD and GSH-PX, inhibiting the expression of MDA, decreasing oxidative damage, and recovering the expression of TH and DAT to maintain normal DA homeostasis.
Acknowledgments and Funding
SK and XT received support to complete this research from Science and Technology Planning Project of Guangdong Province, China (2013B060300033).
Disclosures
SK, LY, ZR, ZZ, JL, HZ, LD, and XT are employees of the Guangdong Medical Laboratory Animal Center.
Statement of Authorship
SK and XT participated in the design of the study and drafted the manuscript. SK and LY performed the statistical analysis. SK, LY, ZR, ZZ, JL, HZ, and LD carried out mice feeding and treated. SK and LD undertook all of the experiments. All authors read and approved the final manuscript.






