Anaemia affects 36 % of pregnancies globally, presenting a major public health problem across low, middle and high-income populations(Reference Stevens, Paciorek and Flores-Urrutia1). Given the global burden of the condition, and the inadequacy of public health efforts to substantially reduce prevalence rates globally for more than three decades, a better understanding regarding the role of nutritional factors other than iron is required, with the potential to offer new and innovative strategies to manage anaemia in pregnancy. The B vitamin riboflavin is involved in iron metabolism, although its role in the prevention of anaemia is largely overlooked. This review aims to explore riboflavin intakes and status during pregnancy in different populations, to evaluate the evidence for the under-recognised role of riboflavin in the maintenance of haemoglobin (Hb) concentrations and its potential to protect against the development of anaemia during pregnancy.
Burden of anaemia in pregnancy
Anaemia in pregnancy is associated with maternal morbidity and impaired quality of life, an increased risk of post-partum haemorrhage, greater need for blood transfusion and a high rate of maternal mortality(Reference Harrison, Lauhon and Colvin2–Reference Randall, Patterson and Gallimore4). Anaemia is also associated with adverse offspring outcomes including preterm birth, low birthweight, small for gestational age, stillbirth and a higher risk of perinatal death(Reference Nair, Churchill and Robinson3,Reference Randall, Patterson and Gallimore4) . In addition, there is evidence of impaired growth and neurodevelopmental outcomes in children born to anaemic mothers(Reference Quezada-Pinedo, Cassel and Duijts5). Furthermore, iron deficiency, even within high-resource settings, is associated with poorer cognitive performance in children(Reference McCarthy, Kiely and Hannon6). Apart from the adverse health impacts for the mother and baby, anaemia in pregnancy may also pose a significant economic burden, with evidence that it is associated with reduced work capacity and productivity, and increased economic loss(Reference Blakstad, Nevins and Venkatramanan7,Reference Haas and Brownlie8) . Iron deficiency is responsible for the majority of anaemia cases globally, and is the most common micronutrient deficiency worldwide(Reference De Benoist, McLean and Cogswell9).
The double burden of malnutrition continues to be a major global health problem, whereby the presence of micronutrient deficiencies exist even among seemingly over-nourished populations. Iron deficiency is common among women of reproductive age in the UK. Some 49 % of British girls (11–18 years) and 25 % of women (19–64 years) report iron intakes below the lower reference nutrient intake, with anaemia and low iron stores presenting in 9 % and 5 % of girls and women, respectively(10). The average daily iron intake among women in the UK is 10 mg/d, 10–15 % of which is estimated to be absorbed(Reference Pavord, Daru and Prasannan11). Although iron absorption is enhanced during pregnancy, requirements also increase to 27 mg/d, almost double that of a non-pregnant woman(Reference Pavord, Daru and Prasannan11). The late foetal and early postnatal periods are critical stages of rapid brain development, increased neural plasticity and high nutritional requirements(Reference Georgieff, Brunette and Tran12). As pregnancies progress, the risk of developing anaemia increases, owing to higher iron requirements, increased maternal erythropoiesis and haemodilution(Reference Scholl13). Plasma volume and red blood cell mass are both known to expand during pregnancy, but as plasma volume expansion exceeds the rate of red blood cell mass production, haemodilution occurs(Reference Perry and Lowndes14). This leads to the characteristic fall in Hb concentrations that commences during the first trimester, reaching the lowest levels at the end of the second trimester(Reference Perry and Lowndes14).
Increased iron requirements during pregnancy, coupled with poor dietary intake, makes pregnancy a vulnerable time for the development of anaemia. In particular, vegans, vegetarians, those with short interpregnancy intervals, multiparous women, teenagers and women from lower socio-economic backgrounds are all at a higher risk for developing anaemia(Reference Pavord, Daru and Prasannan11). In low- and middle-income populations, hereditary blood disorders and infections such as hookworm and malaria also contribute substantially to the overall prevalence(Reference Petry, Olofin and Hurrell15). Apart from iron deficiency, which is the most common and well-known nutritional cause of anaemia, other nutrients are also implicated; namely, folate, vitamin B12, vitamin B6 and riboflavin, with the latter two B vitamins typically receiving less research and public health attention.
Riboflavin intake and status
Riboflavin, also known as vitamin B2, is an essential nutrient which exists in two co-factor forms, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). As reviewed extensively elsewhere(Reference McNulty, Pentieva and Ward16), these co-factors play crucial roles in oxidation-reduction reactions involved in energy metabolism, intermediary metabolism, the maintenance of antioxidant status, as well as the metabolism of iron. Riboflavin co-factors are also involved in the metabolism of other B vitamins including folate, vitamin B12, vitamin B6 and niacin, which are important for the normal functioning of one-carbon metabolism(Reference McNulty, Pentieva and Ward16). Riboflavin status is determined using the functional biomarker assay, erythrocyte glutathione reductase activation coefficient (EGRac), which is considered the gold standard measurement of status. However, due to the rather laborious and time-consuming nature of this assay, including the requirement for thrice-washing of cells at the blood processing stage, this assay is rarely performed in human studies or as part of national nutrition surveys. An EGRac value ≥1·40 is the most commonly accepted cut-off to indicate a riboflavin deficiency(Reference McNulty, Pentieva and Ward16).
Riboflavin status during pregnancy is very rarely reported in human studies. In fact, even among non-pregnant populations, very little is known about the global prevalence of riboflavin deficiency, with most studies (including population-based surveys) relying on reported dietary intake data only, without corresponding biomarker assessment. Clinical riboflavin deficiency has been described in low- and middle-income countries(Reference McNulty, Pentieva and Ward16). Using convenience sampling, riboflavin deficiency has been reported among an estimated 90 % of adults in China(Reference Campbell, Brun and Junshi17), 76 % of adults in Guatemala(Reference Boisvert, Castaneda and Mendoza18), 71 % of reproductive-aged women in Malaysia(Reference Aljaadi, How and Loh19), and up to 92 % of reproductive-aged women in Cambodia(Reference Whitfield, Karakochuk and Liu20). In high-income populations, whilst such severe deficiency is rare, a growing body of evidence suggests that sub-clinical (functional) deficiency is more widespread than generally recognised, particularly among reproductive-aged women(Reference McNulty, Pentieva and Ward16,Reference McNulty, Ward and Hoey21) . In the most recent UK National Diet and Nutrition Survey, 67 % of girls and 58 % of women were found to have a suboptimal or deficient riboflavin status(Reference Bates, Cox and Nicholson22).
Clinical signs and symptoms of riboflavin deficiency (ariboflavinosis) may occur following prolonged periods of having dietary intakes below 0·5 mg/d, and typically present in tissues with rapid cellular turnover such as the epithelia. A sore throat, hyperaemia and oedema of the mouth and tongue, cheilosis, angular stomatitis, glossitis and normochromic, normocytic anaemia are among the reported signs of deficiency(23). Riboflavin deficiency rarely occurs in isolation and is often accompanied by other nutritional deficiencies. While there are no clinically obvious signs and symptoms associated with a biochemical riboflavin deficiency, these are common in many high-income populations and have been associated with an increased risk of hypertension, disturbed metabolism of other B vitamins within the one-carbon cycle, and impaired iron metabolism(Reference McNulty, Pentieva and Ward16).
In typical Western diets, milk and dairy products make the largest contributions to riboflavin intakes, accounting for 22 % and 27 % of total riboflavin intakes in Irish and UK diets respectively(24,Reference Kehoe, Walton and Hopkins25) . Other rich dietary sources of riboflavin have been summarised in Table 1 and include fortified cereal products. Riboflavin is highly bioavailable, with an estimated absorption of up to 95 %, and a maximal uptake of up to 27 mg per meal, with little or no absorption at doses higher than this(Reference Zempleni, Galloway and McCormick26). Furthermore, although certain bacteria within the gut microbiome have been shown to synthesise riboflavin, it remains unclear whether this can make a meaningful contribution to help meet dietary needs in the host(Reference Bedani, Cucick and Albuquerque27). In the US and Canada, mandatory riboflavin enrichment policies have been in place over many years, at levels of 4 mg/kg for flour, with the aim of replacing the riboflavin lost from grain during milling(28). Population-based data from the US suggests dietary riboflavin intakes among reproductive-aged women are sufficient to meet requirements, and are higher than estimates from similar populations in the UK and Ireland (Table 2). In the absence of nationally representative biomarker data, however, the adequacy of riboflavin status in the US and Canada remains unclear, though analysis of a convenience sample of reproductive-aged women from Canada recently revealed functional riboflavin deficiency among 40 %(Reference Aljaadi, Wiedeman and Barr29).
Table 1. Food sources of riboflavin in Irish adults*

* Source: Data from the National Adult Nutrition Survey (NANS); 2008–2010.
Table 2. Official dietary riboflavin recommendations (mg/d) in pregnant and non-pregnant women globally

* Nordic: Denmark, Finland, Iceland, Norway, Sweden.
† D-A-CH: Germany, Austria, Switzerland.
‡ EFSA: European Food Safety Authority.
Metabolic roles of riboflavin in pregnancy
Pregnancy is associated with a progressive fall in riboflavin status as maternal riboflavin is accreted for the placenta and developing foetus(23). In fact, the status of most B vitamins has been shown to fall over the course of pregnancy due to increased requirements for the developing foetus, accretion of vitamins by the placenta, hormonal changes and haemodilution, as reviewed elsewhere(Reference Pentieva, Caffrey and Duffy30) (Fig. 1). Infants born to deficient mothers in The Gambia were found to be riboflavin deficient at birth, and to remain so throughout the first two years of their life in the absence of any intervention(Reference Bates, Prentice and Paul31). Given the higher requirements for riboflavin in pregnancy and poor status among women of reproductive age generally, riboflavin deficiency during pregnancy is likely to be a significant but largely unrecognised problem, with consequences for both maternal and offspring health; the extent of which have not been fully investigated.
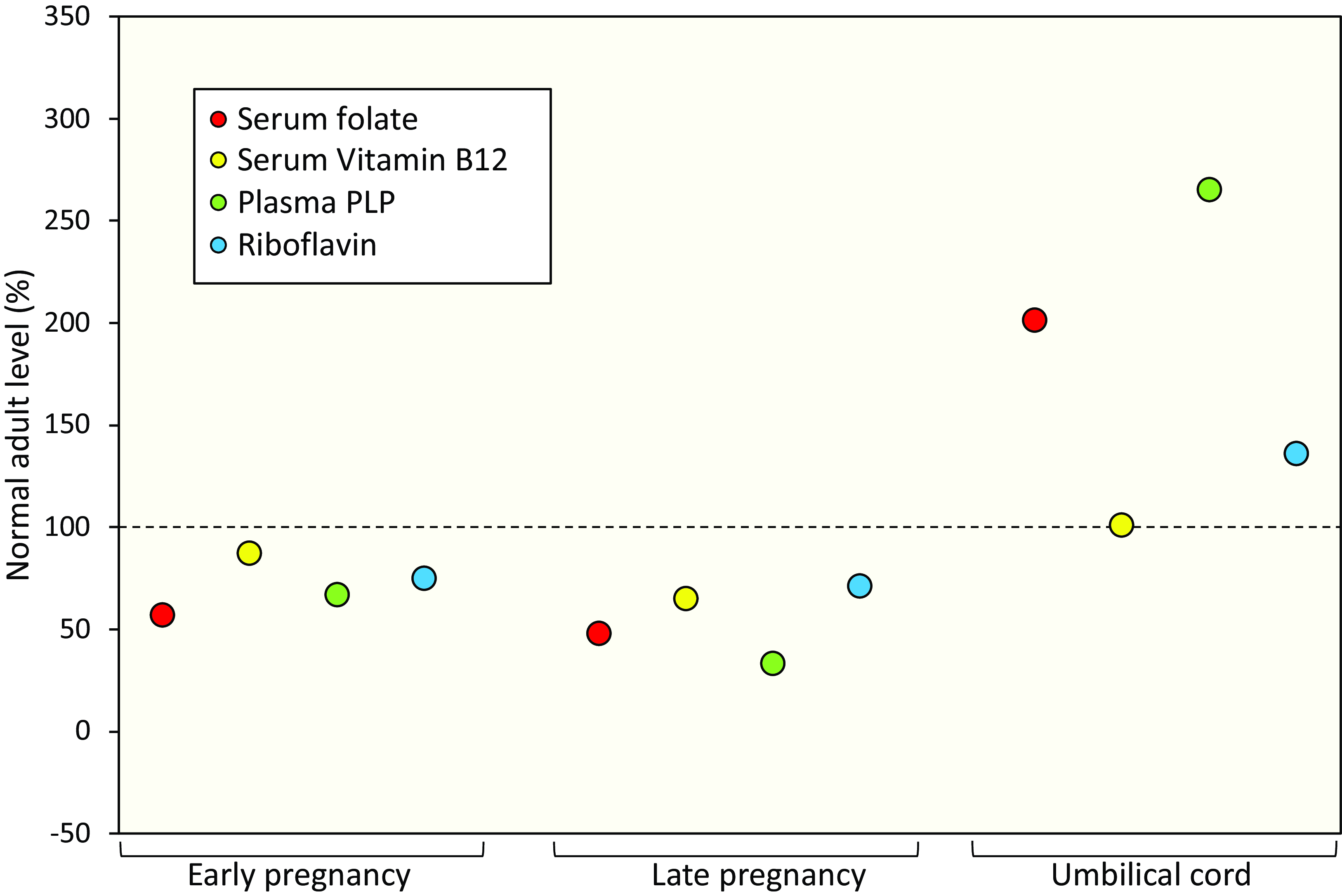
Figure 1. B vitamin biomarkers during pregnancy in unsupplemented mothers and in umbilical cord blood. B-vitamin biomarker values are represented as a percentage of the biomarker values in non-pregnant, non-lactating unsupplemented women (punctured line). Early pregnancy and Late pregnancy are defined as <14 and >36 gestational weeks, respectively. Adapted from Pentieva et al. (2024)(Reference Pentieva, Caffrey and Duffy30).
Very few studies have considered the impact of maternal riboflavin deficiency in pregnancy on maternal or offspring outcomes. However, in our recent observational analysis of the OptiPREG study, involving more than 2000 pregnancies, we provide new evidence that riboflavin may be important for the maintenance of blood pressure during pregnancy(Reference Duffy, McNulty and Pentieva32). A small study of 372 pregnant women from the Netherlands reported that dietary riboflavin intake was positively associated with foetal growth, though in the absence of biomarker measurements, the nature of this association remains unconfirmed(Reference Badart-Smook, Houwelingen and Monique33). Maternal riboflavin intake around the time of conception was previously associated with alternations in the DNA methylation of offspring(Reference Dominguez-Salas, Moore and Baker34), although further research would be required to elucidate the phenotypic consequences of such alterations. Historical studies from pregnant animals showed skeletal and soft tissue abnormalities among offspring born to riboflavin-deficient rats and mice(Reference Warkany and Nelson35). Other animal studies documented impaired gastrointestinal development in the presence of a riboflavin-deficient diet during weaning(Reference Williams and Powers36), suggesting that riboflavin in early life, possibly including the in-utero period, may play a role in gastrointestinal maturation(Reference Powers37). Impairments in the gastrointestinal tract may lead to altered nutrient absorption and thus could explain the possible relationship between riboflavin deficiency and foetal growth.
Dietary riboflavin recommendations during pregnancy
The recommendations for riboflavin intake are increased during pregnancy compared to non-pregnant women, albeit with significant variations in estimated requirements from different countries/regions (ranging from 1·3 to 1·9 mg/d, as summarised in Table 3). Recommendations for riboflavin have often been based on limited, outdated evidence, using extrapolations from adult data rather than evidence from pregnancy cohorts, making this an area clearly deserving more research(23). In a sample of 156 Gambian pregnant women with low riboflavin intakes (0·5 mg/d), riboflavin deficiency was reported (mean EGRac of 1·78), with a further marked deterioration of status near parturition, suggesting that late pregnancy may be associated with especially high riboflavin requirements(Reference Bates, Prentice and Paul38). Interestingly, when a fortified dietary supplement (which increased mean riboflavin intakes to 1·5 mg/d) was provided to these women for 8 months during lactation, riboflavin status improved (mean EGRac of 1·42), albeit biomarker values remained within the deficient range. It could be speculated, therefore, that intakes greater than the current UK recommendations (1·4 mg/d) would be needed to achieve biochemical and functional normality in these women(Reference Bates, Prentice and Paul38). Moreover, a small, non-randomised intervention from the Philippines reported higher riboflavin requirements among pregnant compared to non-pregnant women(Reference Kuizon39). In support of these earlier findings, we recently reported biochemical riboflavin deficiency among 31 % of Irish pregnant women, despite 64 % reporting to take a riboflavin supplement (mean dose of 2 mg/d, predominantly in the form of a prenatal multivitamin)(Reference Duffy, McNulty and Pentieva32). A noteworthy randomised trial of 1729 women from the UK, Singapore and New Zealand showed modest reductions in plasma concentrations of riboflavin from preconception to early pregnancy, and again from early to late pregnancy. In this study, intervention with 1·8 mg/d of riboflavin from preconception through to delivery led to substantial improvements in status compared to the control group, though some 67 % and 82 % of participants were still deemed to have insufficient status in early and late pregnancy, respectively(Reference Godfrey, Titcombe and El-Heis40). Overall, these findings call into question the adequacy of current riboflavin intake recommendations in meeting the higher requirements during pregnancy, particularly so for countries such as the UK and USA where requirements are set at only 1·4 mg/d, suggesting that official dietary riboflavin recommendations may need to be reconsidered.
Table 3. Dietary intakes and status of riboflavin among reproductive-aged women from population-based cohorts

* Women aged 20 years or above.
† Women aged 19–64 years.
‡ Women aged 18–64 years.
Abbreviations: EGRac, erythrocyte glutathione reductase activation coefficient; ROI, Republic of Ireland; UK, United Kingdom; USA, United States of America.
Riboflavin and anaemia
Biochemically confirmed riboflavin deficiency has been implicated in the development of anaemia, and the observational data linking riboflavin with anaemia are summarised in Table 4. Preliminary findings from our OptiPREG study showed for the first time within a high-income population of over 2000 pregnancies, that riboflavin status was a predictor of Hb concentrations and risk of anaemia(Reference Duffy, Pentieva and Ward42). Notably, riboflavin status was reported to be a significant predictor of anaemia risk (Hb < 120 g/l) in a sample of over 400 non-pregnant women from The Lao People’s Democratic Republic, where poor riboflavin status (EGRac > 1·30) was found among 97 % of women sampled(Reference Hess, Smith and Sitthideth41). Likewise, in a cohort of reproductive-aged women in Canada and Malaysia, a deficient status of riboflavin was found to be a significant predictor of Hb concentrations and associated with a 2-fold greater risk of anaemia(Reference Aljaadi, How and Loh44). Moreover, evidence from over 700 Chinese women showed than inadequate dietary riboflavin intakes were associated with an increased risk of anaemia after 5 years of follow-up(Reference Shi, Zhen and Wittert43).
Table 4. Observational studies investigating riboflavin in relation to haematological status or anaemia in women

Abbreviations: EAR, estimated average requirement; EGRac, erythrocyte glutathione reductase activation coefficient; FFQ, food frequency questionnaire; Hb, haemoglobin.
Randomised controlled trials (RCTs) from pregnant populations in low- and middle-income countries have demonstrated favourable effects on haematological status when riboflavin was administered alone or in combination with other nutrients (such as iron, folate or vitamin A; summarised in Table 5). An RCT of Indonesian pregnant women found that the greatest improvements in Hb concentrations were observed among those supplemented with riboflavin (5 mg) in combination with iron, compared with those supplemented with iron alone, iron and folate, or iron and vitamin A(Reference Suprapto, Widardo and Suhanantyo48). Similarly, Ma et al. (2008), found additional improvements in Hb concentrations and a reduced prevalence of anaemia among pregnant women from rural China when riboflavin (1 mg) and vitamin A were administered in addition to iron and folate supplementation, compared to iron and folate alone(Reference Ma, Schouten and Zhang47). Decker et al. (1977) documented a higher erythrocyte count among pregnant women after supplementation with riboflavin (9 mg) and iron, compared with iron alone. Furthermore, the fall in Hb concentrations and haematocrit over the course of pregnancy was smaller (though insignificant) when riboflavin was administered in combination with iron(Reference Decker, Doris and Glatzle50). A study of over 800 Cambodian women found that daily iron supplementation for 12 weeks increased Hb concentrations; however, the addition of multiple micronutrient supplementation containing riboflavin (1·4 mg) did not confer additional significant benefit(Reference Karakochuk, Barker and Whitfield45). Inconsistencies in results may be attributed to differences in baseline riboflavin status which were not measured in all studies, differences in the additional micronutrients included in trials, the riboflavin dose and the duration of interventions.
Table 5. Randomised controlled trials investigating riboflavin intervention (alone or combined supplementation) in relation to haematological parameters and anaemia among pregnant, lactating and reproductive-aged women

Abbreviations: EGRac, erythrocyte glutathione reductase activation coefficient; FA, folic acid; Hb, haemoglobin; MMN, multiple micronutrient; RBC, red blood cell; vit, vitamin.
The majority of studies to date investigating the relationship between riboflavin and anaemia risk have been limited to low- and middle-income populations where clinical riboflavin deficiency is endemic owing to low dietary intakes of dairy products. Less is known about the role of riboflavin in cases where deficiency is less severe; however, one RCT has been conducted within a high-income population, albeit in young women, rather than pregnant women. Women aged 19–25 years who were riboflavin deficient, non-supplement users were randomised to either placebo or riboflavin (at doses of either 2 mg/d or 4 mg/d). There were no significant effects of treatment on haematological status when data for all participants were analysed together. However, when changes in Hb concentrations were examined relative to EGRac values at baseline, a clear pattern emerged, such that women with the worst riboflavin status at baseline (EGRac > 1·65) showed the greatest increase in Hb in response to riboflavin intervention(Reference Powers, Hill and Mushtaq46). This indicates that riboflavin supplementation may be effective in improving haematological status only under the condition of riboflavin deficiency.
Mechanisms linking riboflavin with iron metabolism and anaemia
Various mechanisms have been proposed to explain the relationship between riboflavin status and Hb. Evidence from animal studies shows that riboflavin deficiency can reduce iron absorption. In riboflavin-deficient rats, the total radiolabelled iron absorbed from a test meal was significantly lower than that which was absorbed from control rats(Reference Adelekan and Thurnham51,Reference Powers52) . When similar experiments were conducted in humans, however, these findings could not be confirmed, possibly due to the large variability in iron absorption among participants, thus requiring further investigation(Reference Powers, Hill and Mushtaq46,Reference Fairweather-Tait, Powers and Minski53) .
Animal studies have suggested that riboflavin deficiency may increase gastrointestinal iron losses via increased shedding of mucosal cells from the gastrointestinal tract(Reference Miyaji and Hara54,Reference Powers, Weaver and Austin55) . Moreover, young rats fed a riboflavin-deficient diet upon weaning exhibited morphological changes within the intestinal villi compared with controls(Reference Williams and Powers36). It is thought that the gastrointestinal development which occurs during critical periods in utero and during weaning may be irreversible and that riboflavin deficiency during these periods can precede lifelong changes such as a reduction in the number of villi, leading to a reduction in absorptive surface area(Reference Yates, Evans and Powers56). Thus, impaired gastrointestinal development is another route by which riboflavin deficiency may contribute to reduced iron absorption, but this requires further research.
Of all the mechanisms which have been proposed, the evidence linking riboflavin to iron utilisation or mobilisation is the most convincing. This involvement is almost certainly due to a flavin-dependent release of iron from ferritin(Reference Zaman and Verwilghen57,Reference Sirivech, Frieden and Osaki58) . Iron is retained in the protein shell of ferritin and its release is dependent upon the reduction of ferritin from Fe+3 to Fe+2 by a reducing agent(Reference Anderson and Frazer59). The reduction and release of ferritin-bound iron is crucial for the production of red blood cells. Numerous reducing agents have been found to be capable of mobilising iron from ferritin, however, only reduced riboflavin and riboflavin co-factors; FMN and FAD, have been shown to be effective in doing so at a physiologically relevant rate in vitro(Reference Sirivech, Frieden and Osaki58,Reference Ulvik60) . For this reason, riboflavin deficiency may impair iron handling, resulting in lower Hb concentrations.
Finally, riboflavin may be implicated indirectly in Hb synthesis through its metabolic relationship with vitamin B6 and the requirement for riboflavin to generate the active form of B6 in tissues. Specifically, pyridoxine 5´-phosphate oxidase requires riboflavin in its co-factor form FMN for the conversion of pyridoxine 5´-phosphate and pyridoxamine 5´-phosphate to pyridoxal 5´-phosphate (PLP), the active vitamin B6 form(Reference McCormick61). PLP is a co-factor for δ-aminolevulinate synthase in the erythrocytes, which is a crucial enzyme that catalyses the first step of haem biosynthesis(62). A prospective study of Japanese pregnant women who were both anaemic and B6 deficient demonstrated improvements in Hb concentrations following intervention with vitamin B6(Reference Hisano, Suzuki and Sago63). Moreover, within a recent, non-randomised trial of anaemic, Egyptian pregnant women, vitamin B6 supplementation in combination with iron resulted in greater increases in Hb concentrations, compared to iron therapy alone over a short 3-week period of intervention(Reference Ghannam, Hussain and Osman64). This study, however, did not include measurements of vitamin B6 status. The metabolic dependency of PLP on riboflavin was demonstrated in recent studies from our centre, including a large-scale investigation of over 5600 Irish adults which showed that PLP concentrations decreased in a stepwise manner according to riboflavin status from optimal, to suboptimal to deficient, independently of B6 intake(Reference Jungert, McNulty and Hoey65,Reference Jarrett, McNulty and Hughes66) . It could be speculated, therefore, that riboflavin may be a rate-limiting nutrient for the maintenance of normal plasma PLP concentrations, with knock-on effects for haem biosynthesis. Further research is required to determine the metabolic interaction between riboflavin and vitamin B6 in pregnancy and to understand the extent of their roles both independently and together in the context of anaemia during pregnancy.
Conclusions
Riboflavin deficiency during pregnancy is a significant, but unrecognised, problem globally, with associated adverse maternal and foetal health outcomes, the full extent of which is not known primarily because riboflavin biomarkers are rarely measured in human studies. Although overall dietary riboflavin intakes are often reported to be sufficient in most populations, the lowest intakes are typically found in young women. Correspondingly, high rates of functional (subclinical) riboflavin deficiency have been reported in reproductive-aged women in convenience samples and in the very few nationally representative samples from high-income populations where riboflavin status has been assessed. Poor riboflavin status among women entering pregnancy, coupled with increased requirements during pregnancy, makes this an especially vulnerable time for the development and progression of riboflavin deficiency. The limited available data suggests that current dietary riboflavin recommendations during pregnancy may be insufficient to meet the increased demands. In low- and middle-income countries, where status is poorest, RCTs in pregnant women have demonstrated beneficial effects of riboflavin on haematological status. In high-income populations, limited observational evidence suggests that suboptimal riboflavin status contributes to an increased risk of anaemia, but further investigation, in the form of randomised trials in women before and during pregnancy, is warranted. If shown to be effective, interventions to improve riboflavin status - through improved diet, food fortification or supplementation – could offer a novel and effective means of reducing the global burden of anaemia in pregnancy.









