Introduction
Bleaching is a crucial step in the oil-refining industry, where clays are used to remove impurities such as beta-carotene, chlorophyll, trace metals, peroxides, and phosphatides from edible vegetable oils and fats (Richardson, Reference Richardson1978; Sabah and Çelik, Reference Sabah and Çelik2005). Commonly used industrial clays for bleaching include bentonite, sepiolite, palygorskite, and fuller’s earth, a natural mixture of Al-smectite or Ca-montmorillonite-rich clay, and palygorskite (Manning, Reference Manning and Manning1995; Keegan, Reference Keegan2000). Bentonite clays are typically acid activated to enhance their bleaching efficiency, although this increases cost and can affect oil taste (Richardson, Reference Richardson1978; Merkl, Reference Merkl1989; Christidis and Kosiari, Reference Christidis and Kosiari2003; Murray, Reference Murray and Murray2007).
Palygorskite and sepiolite clays, in contrast, are often used in their natural or thermally activated forms, by-passing the need for chemical treatment. These clays inherently possess a large surface area (up to 300–400 m2 g–1) due to their rod-like crystal structure and fine particle size (Suárez and García-Romero, Reference Suárez and García-Romero2012; Suárez et al., Reference Suárez, García-Rivas, Morales, Lorenzo, García-Vicente and García-Romero2022a). Thermal activation at temperatures above 200°C increases their surface area further, and enhances bleaching performance by removing physiosorbed water and increasing surface oxidation and porosity (Jones and Galán, Reference Jones, Galán and Bailey1988; Galán, Reference Galán1996; Frini-Srasra and Srasra, Reference Frini-Srasra and Srasra2008; Xavier et al., Reference Xavier, Santos, Osajima, Luz, Fonseca and Filho2016). The effectiveness of bleaching clays hinges on their surface area, pore-size distribution, and the presence of active sites for adsorption of pigments and Fe oxides in oils (Sabah and Çelik, Reference Sabah and Çelik2005; Huang et al., Reference Huang, Liu, Liu and Wang2007). Despite similar chemical compositions, clays can exhibit varying bleaching performance, emphasizing the need for thorough laboratory and industrial testing to assess their suitability (Stout et al., Reference Stout, Chamberlain and Mcklvey1949; Oboh et al., Reference Oboh, Aworh and Agagu1987; Usman et al., Reference Usman, Ekwueme, Alaje and Mohammed2012).
The Ventzia Basin clays of Greece consist of Mg-Fe rich palygorskite and mixed palygorskite-smectite formed by the alteration of ultramafic rocks (Kastritis et al., Reference Kastritis, Kacandes, Mposkos and Eliopoulos2003). Several studies have revealed the complex structure and mineralogy of the Ventzia Basin clays (Gionis et al., Reference Gionis, Kacandes, Kastritis and Chryssikos2006; Gionis et al., Reference Gionis, Kacandes, Kastritis and Chryssikos2007; Chryssikos et al., Reference Chryssikos, Gionis, Kacandes, Stathopoulou, Suarez, Garcia-Romero and Sanchez Del Rio2009; Stathopoulou et al., Reference Stathopoulou, Suárez, García-Romero, Del Río, Kacandes, Gionis and Chryssikos2011; Kypritidou et al., Reference Kypritidou, Argyraki, Chryssikos and Stamatakis2016; Kaufhold et al., Reference Kaufhold, Chryssikos, Kacandes, Gionis, Ufer and Dohrmann2019). Palygorskite has a mixed dioctahedral-trioctahedral character and is Fe rich. The smectite of Ventzia is a mixture of clay minerals with dioctahedral (e.g. Fe-montmorillonite and nontronite) and trioctahedral (e.g. saponite) character.
These types of clays require a wide suite of evaluation methods to determine possible industrial end uses including oil bleaching. The mineralogical compositions of clays and the clay content are essential parameters to determine market value and industrial end uses, but it is equally important to know the physical properties determined in a manner to deem them comparable to reference materials, e.g. thermally activated palygorskite-smectite from the USA (Oil-Dri Pure-Flo B80). A number of studies performed in the past have investigated the rheological properties of Ventzia Basin clays that are directly related to their industrial uses (Christidis et al., Reference Christidis, Katsiki, Pratikakis and Kacandes2010) and their effectiveness for environmental applications such as the removal of potentially toxic elements from aqueous solutions (Kypritidou and Argyraki, Reference Kypritidou and Argyraki2018; Kypritidou et al., Reference Kypritidou, Gatou, Argyraki and Zotiadis2022) or the stabilization of Pb in contaminated soil (Zotiadis et al., Reference Zotiadis, Argyraki and Theologou2012; Kypritidou and Argyraki, Reference Kypritidou and Argyraki2021). However, systematic research linking their geological occurrence to specific clay properties that might affect the clay’s adsorption capacity and particularly its oil bleaching performance is still lacking.
This study aimed to evaluate the bleaching performance of thermally activated and raw (unheated) clays mainly composed of palygorskite-smectite mixtures from the Ventzia Basin, west Macedonia, Greece. The specific objectives of the study were to investigate how: (1) the mineralogical compositions, (2) the chemical compositions, and (3) selected physicochemical properties of samples originating from four deposits being exploited from the Ventzia Basin influence their efficiency in bleaching degummed rapeseed oil.
Geological setting
The clays studied were deposited in the Pliocene-Pleistocene sedimentary succession of Ventzia Basin in west Macedonia, Greece (Fig. 1). They have a large palygorskite content (60–95%), which was formed diagenetically by transformation of smectite eroded from the saprolite bodies of the ultramafic bedrock, and the smectite-bearing sands of the Tsotyli Formation (Kastritis et al., Reference Kastritis, Kacandes, Mposkos and Eliopoulos2003). The substrate of the basin is composed of Mesozoic limestones that are members of the Pelagonian Unit, the Mesozoic Vourinos ophiolite complex, and Miocene conglomerates, marls, and sandstones that belong to the Tsotyli Formation (Liati et al., Reference Liati, Gebauer and Fanning2004; Kilias et al., Reference Kilias, Vamvaka, Falalakis, Sfeikos, Papadimitriou, Gkarlaouni and Karakostas2015; Rassios et al., Reference Rassios, Tzamos, Dilek, Bussolesi, Grieco, Batsi and Gamaletsos2020). Within the Ventzia Basin there are several occurrences of palygorskite and natural mixtures of palygorskite and Mg-Fe smectite; however, deposits of commercial interest are limited to four major currently exploited clay deposits, namely: Knidi, Pilori, Harami, and Velanida (Kaufhold et al., Reference Kaufhold, Chryssikos, Kacandes, Gionis, Ufer and Dohrmann2019), which have been sampled and analyzed.

Figure 1. Geological map of the Ventzia Basin in west Macedonia, Greece, showing major geological formations and the locations of the four deposits studied (modified after Mavridis and Kelepertzis, Reference Mavridis and Kelepertzis1993; Mavridis et al., Reference Mavridis, Kelepertzis, Galanakis and Photiades2015).
Materials and methods
Sampling and sample preparation
The samples used for the bleaching tests were collected from exploratory boreholes installed in the privately owned mines of Geohellas S.A. (Fig. 1). An initial set of 159 samples was collected from seven boreholes that were installed at the depocenter (thickest clay beds), off-center, and the periphery or margin of each of the four deposits. Based on sample mineralogy, 70 samples were subsequently selected due to their high clay mineral content for further analysis. The sampling protocol included all clay beds of every deposit (Fig. 2). Clay-rich samples were collected, air-dried, and quartered from cores that were initially 10 cm wide by ~30 cm long. Samples were subsequently dried to 150°C to remove moisture, gently crushed, and sieved to retain the 0.25–3.35 mm portion.
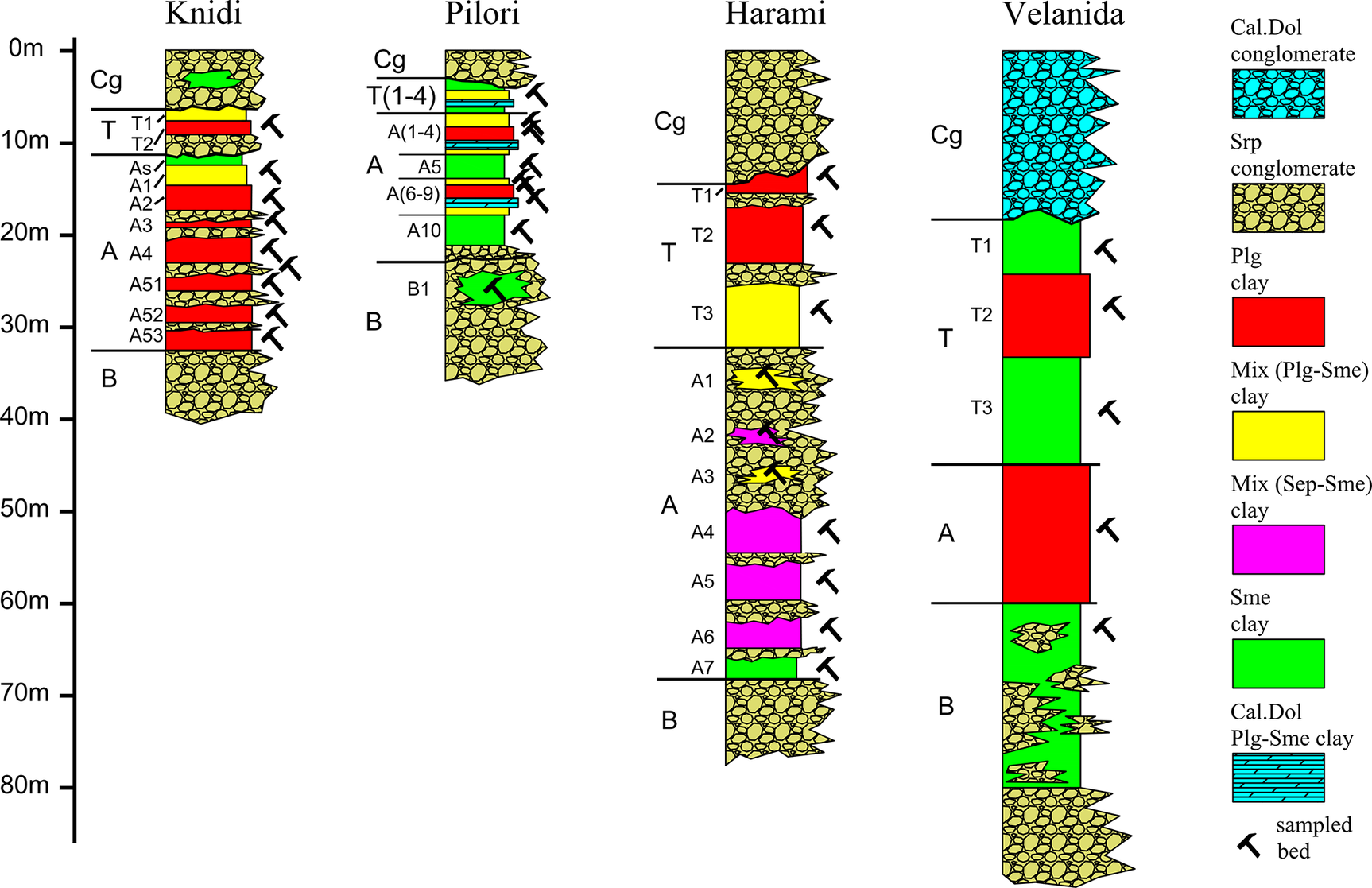
Figure 2. Simplified stratigraphic columns of the clay deposits studied (Cg = top conglomerate unit, T = transitional clay succession (T1, T2, etc. = clay beds), A = main clay succession (A1, A2, etc. = clay beds), B = basal conglomerate unit, Cal = calcite, Dol = dolomite, Plg = palygorskite, Sme = smectite, Sep = sepiolite, Srp = serpentine). Mineral abbreviations adapted from Warr (Reference Warr2021).
Mineralogical, chemical, and textural analysis
The bulk mineralogy of the 70 samples was determined by powder XRD using a Thermo ARL diffractometer (Model X’TRA 17) instrument with Cu radiation (Kα = 1.54056) operating at 45 kV and 40 mA, angle range from 2 to 70°2θ, step size of 0.04°2θ, and step time of 2 s at ambient conditions. Mineralogical evaluation was conducted using the DIFFRAC PLUS v2.2 software developed by Bruker. The characterization of the clay minerals was carried out on a subset of 12 samples that included one palygorskite, one palygorskite-smectite, and one smectite from each of the four deposits based on the large amounts of these minerals. The <2 μm fraction (clay fraction) was separated from this subset of 12 samples by sedimentation using the method described by Poppe et al. (Reference Poppe, Paskevich, Hathaway and Blackwood2001). Oriented mounts were also prepared and examined by XRD on air-dried, ethylene glycol-treated and heat-treated samples at 550°C. Semi-quantitative analysis for palygorskite was performed using a procedure devised by Gionis et al. (Reference Gionis, Kacandes, Kastritis and Chryssikos2007). The semi-quantitative analysis for the other major mineral phases (Sme, Srp, Qz, Cal, and Dol) and the corresponding clay fraction minerals was performed by mineral intensity factors calibrated against internal standards (Schultz, Reference Schultz1964; van der Marei, Reference van der Marei1966).
The same 70 clay samples were also analyzed chemically for ten major oxides. The analytical procedure included fusion with lithium metaborate (LiBO2/LiB4O7), dissolution in HCl, and analysis by ICP-OES. The loss on ignition (LOI) was measured after incineration of oven-dried (at 105°C) samples to 1050°C. Sample preparation was carried out at the Geohellas S.A. laboratories, and they were shipped subsequently to Bureau Veritas Commodities laboratories in Vancouver, British Columbia, Canada for analysis.
Taking into consideration the results of the mineralogical analysis, the fresh broken surfaces of 25 samples were examined under the scanning electron microscope (SEM), using a JEOL JSM 5600 instrument equipped with an energy dispersion spectrometer (EDS) Oxford Link ISIS 300. The 25 samples examined were selected randomly to represent all types of clays from the four deposits studied. Samples were coated with thin films (~350 Å) of carbon using an Agar SEM carbon coater. The SEM studies were performed at the Laboratory of Economic Geology and Geochemistry of the National and Kapodistrian University of Athens.
Near-infrared spectroscopy (NIR) analysis was conducted on 57 clay samples to confirm the clay mineralogy but also to explore the octahedral composition of palygorskite. The Fe content at the dioctahedral position was expressed as x while the trioctahedral Mg-component was expressed as y (Gionis et al., Reference Gionis, Kacandes, Kastritis and Chryssikos2006; Gionis et al., Reference Gionis, Kacandes, Kastritis and Chryssikos2007). The x and y values were estimated based on the normalized intensities of the AlAlOH, AlFe3+OH, Fe3+Fe3+OH, and Mg3OH overtone bands, respectively (Chryssikos et al., Reference Chryssikos, Gionis, Kacandes, Stathopoulou, Suarez, Garcia-Romero and Sanchez Del Rio2009). A Vector 22N Fourier-transform spectrometer by Bruker Optics was used to obtain an NIR spectrum over the 4000–8000 cm–1 range. The NIR analysis was conducted at the National Hellenic Research Foundation. The NIR analytical procedure and interpretation of the results have been described in detail by Gionis et al. (Reference Gionis, Kacandes, Kastritis and Chryssikos2006, Reference Gionis, Kacandes, Kastritis and Chryssikos2007).
Physicochemical properties
The oil (WO%) and water (WW%) adsorption capacity of the 70 clay samples were measured using an empirical method devised by Westinghouse S.A. The Westinghouse method (Pabis-Mazgaj et al., Reference Pabis-Mazgaj, Pichniarczyk, Stempkowska and Gawenda2022) has no standard, but it has been accepted worldwide as a common industry practice. Methylene blue adsorption (MBA) capacity was also measured as an estimate of the smectite proportion of clays (Miyoshi et al., Reference Miyoshi, Tsukimura, Morimoto, Suzuki and Takagi2018). The MBA capacity was determined using the spot method described by Yukselen and Kaya (Reference Yukselen and Kaya2008) and recorded as the amount of methylene blue adsorbed per milliliter. The MBA procedure was calibrated against a Wyoming bentonite standard that contains 68% Na-montmorillonite. Apparent bulk density (ABD) measurements were based on the ASTM D1895 2017 standard method. Each sample was allowed to free fall from a funnel set at a certain height, into a 100 mL volumetric cylinder filled to the top and the net mass of the clay (g) was determined. ABD was accordingly expressed as g cm–3. The measurements of the physicochemical properties were performed at Geohellas S.A. laboratories.
The N2 isotherms (adsorption-desorption) were utilized to evaluate the surface area and porosity of the subset of the 12 selected powdered clay samples dried at 105°C by a TriStar II Plus surface area and porosity analyzer developed by Micromeritics at the analytical laboratories of the Centre for Research & Technology Hellas (CERTH). Pore-size distribution, mean pore diameter (PSav), micropore volume (PVμp), total pore volume (TPV), specific surface area (SSABET), external surface area (SAext), and micropore surface area (SAμp) were subsequently calculated for the subset of the 12 samples. The t-plot method was used in the calculation of PVμp, SAμp, pore size distribution, and SAext. The Brunauer–Emmett–Teller (BET) equation (Brunauer et al., Reference Brunauer, Emmett and Teller1938) was utilized to calculate the surface area of the samples whereas the Barrett–Joyner–Halenda (BJH) model (Barrett et al., Reference Barrett, Joyner and Halenda1951) was employed for analysis of the pore-size distribution. The range of relative pressures used for measuring textural properties in the microporous range was between 0.05 and 0.10.
Bleaching test method
Bleaching tests were performed on crude, but degummed, rapeseed oil obtained from the Bunge oil refinery plant in Romania. Rapeseed oil has a dark green color, strong fruity odor, and is usually difficult to bleach. The sample preparation of the clays is the result of meticulous in-house experiments (Geohellas, unpublished data, 2023) that provided optimal results for bleaching performance and included extrusion, drying at 105°C, heating to 300°C, hammer milling for 2 min to <250 μm, and high-speed air rotating techniques to separate the finer grain sizes through centrifugation processes. The bleaching contact time was set at 30 min at 105°C while the clay dosage was 1.5%. The clay particle size ranged between 5 and 100 μm with d 50 (the particle size of at least 50% of the material) <27 μm and d 90 (the particle size of at least 90% of the material) <70 μm. Special attention was given to maintaining the particle size at between 5 and 70 μm to ensure consistency and repeatability of the results among all samples. Particle-size analysis was conducted on a MasterSizer 2000 particle size analyzer (Malvern Panalytical Company). The visible light absorption of the bleached oil, expressed as Vis (violet at 420 nm), Red and Yellow hue, and Chl (chlorophyll content), were measured using a tintometer (Lovibond Tintometer Company) and with a UV-Vis spectrophotometer (Shimadzu Corporation). The bleaching performance of raw (unheated) clays yielded low Vis values of between 3 and 17 (Geohellas, unpublished data, 2024).
Results and Discussion
Mineralogical assemblages
The mean, standard deviation, and range (min–max values) of the bulk sample compositions are summarized in Table 1. Semi-quantitative analysis of the mineralogical phases contained in the 70 bulk samples and the clay fractions of the subset of 12 samples from all four deposits are presented in Table S1 of the Supplementary material. The XRD patterns of the 12 samples are presented in Figs S1–S12 in the Supplementary material. Dolomite and to a lesser extent calcite occur as secondary minerals in fissure fillings in the upper clay beds of Pilori and Velanida. Dolomite is scarce in the Knidi deposit and identified only locally in fissure fillings of the upper clay beds of the Harami deposit. Magnesite is associated with smectite clays in the deeper parts of the Harami deposit. The samples contain Fe/Mg-rich clay minerals consisting of palygorskite, smectite, and a mixture of palygorskite and smectite. Certain samples from the Knidi and Pilori deposit have elevated serpentine and quartz contents, respectively. Similar results obtained for some of the deposits were described previously by Kypritidou et al. (Reference Kypritidou, Argyraki, Chryssikos and Stamatakis2016), Kypritidou and Argyraki (Reference Kypritidou and Argyraki2018), and Kaufhold et al. (Reference Kaufhold, Chryssikos, Kacandes, Gionis, Ufer and Dohrmann2019). Common mineral phases associated with palygorskite and smectite are serpentine, dolomite, and quartz. Minor calcite, feldspars, magnesite (Harami deposit), and sepiolite were also identified, while enstatite, talc, tremolite, kaolinite, illite, and chlorite occur in trace amounts (Table 1; Table S1; Figs S1–S12).
Knidi deposit
Palygorskite-rich clays (on average >50% Plg), hereafter referred to as ‘palygorskite’, from the Knidi deposit, contain, on average, 25% serpentine but no dolomite. In this deposit, palygorskite is assembled in seven distinct clay beds grouped in successions T and A that are intercalated with serpentine gravel and sand (Fig. 2). Their color ranges from gray to brown and turn light gray upon drying. Palygorskite-smectite clays (24–55% Plg), referred to hereafter as ‘palygorskite-smectite’, are located near the top and the periphery of the clay deposit. Smectite-rich clays (on average >34% Sme), referred to hereafter as ‘smectite’, occur in lenses within the overburden serpentine gravel and sands. Mineral abbreviations have been adapted from Warr (Reference Warr2021).
Pilori deposit
Palygorskite (on average >63% Plg) from the Pilori deposit occurs in two clay beds grouped in succession A (Fig. 2). These clays are always associated with quartz (average 20%), while dolomite is prominent with smectite (>39% Sme), mixed palygorskite-smectite (19–47% Plg), and occasionally with palygorskite (Table 1). Dolomitic smectite occurs principally at the top and close to the periphery of the deposit. The presence of serpentine is limited compared with palygorskite from the Knidi deposit. Palygorskite beds are purple to gray, sometimes green, and turn light gray upon drying. The presence of smectite is more prominent compared with clays from the Knidi deposit and has a green to brown color that changes only slightly to lighter hues upon drying. Smectite is usually concentrated at the top, bottom, and periphery of the deposit.
Table 1. XRD semi-quantitative (%) analysis and selected physical properties of palygorskite (Plg), palygorskite-smectite (Mix), and smectite (Sme) clay samples from the four studied deposits

Mean values are shown in bold, standard deviation values follow the ± sign, and the min–max value ranges are shown in italic.
Harami deposit
Clays from the Harami deposit are slightly different, as they are devoid of dolomite or calcite and have a small quartz content (Table 1). The presence of disseminated opal intergrowths and micro-nodules from the center of the deposit are evident in Harami palygorskite (on average >75% Plg) that appear in irregular shapes ~1 cm wide. The XRD analysis on hand-picked opal inclusions from palygorskite revealed the presence of opal-A′ with traces of quartz, talc, chlorite, and baryte in close association with palygorskite (Fig. S13 in the Supplementary material). Two main clay successions occur, namely T and A, separated by thick gravelly sands and gravel that narrow toward the center of the deposit. The serpentine content is small compared with clays from the Knidi deposit and is typically encountered at the bottom and periphery of the deposit. Palygorskite accumulates at the depocenter of the deposit in thick beds (10–12 m) and typically associates with amorphous silica (opal-A′) intergrowths that make it difficult to excavate the material. It has a gray color that turns to lighter shades of gray upon drying. The palygorskite beds at the center of the deposit grade abruptly to mixed palygorskite-smectite (22–50% Plg) beds. Smectite (on average >75% Sme) has a brown to reddish color and, like Pilori smectite, changes color only slightly upon drying. Sepiolite becomes apparent toward the bottom of the deposit where mixed smectite-sepiolite beds exhibit a pinkish color (Fig. 2).
Velanida deposit
The main characteristic of Velanida palygorskite (on average >68% Plg) is the presence of fine-grained euhedral dolomite aggregates and, to a lesser extent, calcite, as the main non-clay mineral phases (Table 1). Quartz and serpentine in the form of clastic grains occur in lesser amounts compared with palygorskite from the Pilori and Knidi deposits, respectively. Usually gray but locally purple, palygorskite beds occur in thin layers that turn to lighter shades upon drying. The palygorskite beds are also concentrated in two thick successions T and A separated by smectite (on average >61% Sme) beds, unconsolidated serpentine gravel, and sandy claystone (Fig. 2). Smectite is ubiquitous and usually appears brown to green, changing color only slightly upon drying. It occurs in thick beds at the bottom and the periphery of the deposit. Thinner smectite inserts intercalate palygorskite beds throughout the deposit. The smectite along with the serpentine content increases within the palygorskite beds toward the periphery and the bottom of the deposit.
Textural features of clay and non-clay minerals
Investigation by SEM of the clay samples revealed that palygorskite fibers form bundles that differ in size among the studied deposits. Palygorskite fibers from the Knidi and Pilori deposits are embryonic, smaller, and barely visible compared with palygorskite fibers from Harami and Velanida deposits (Fig. 3a,b). A characteristic intermediate composition of palygorskite and smectite is shown in the Knidi deposit (Fig. 3a). Palygorskite fibers from Knidi and Pilori deposits are tightly interwoven and as a result, form a massive bundle. Smectite aggregates are not easily detectable due to their small size, but they form randomly oriented flakes (Fig. 3c) usually in close association with palygorskite. Palygorskite fibers from the Harami deposit create an open-space texture with larger and smaller pores that vary in size (Fig. 3d). The present SEM studies of clays provide an overall picture of the microtexture; however, more systematic work is warranted to explore a possible relationship between microporosity data and the observed microtexture.

Figure 3. Representative SEM-SEI images of Ventzia Basin clays: (a) intermediate composition sample from the Knidi deposit, showing palygorskite and smectite aggregates in close association; (b) barely visible, short palygorskite fibers in a characteristic sample from the Pilori deposit; (c) smectite aggregates from the Velanida deposit forming randomly oriented flakes; (d) open space texture of palygorskite from the Harami deposit; (e) rhombohedral dolomite (Dol) aggregates rest on pore spaces within palygorskite (Plg) mats from the Velanida deposit; (f) amorphous silica (opal-A′) intimately interwoven with palygorskite (Plg) fibers forming a highly porous spongy structure and lightweight nature of the mixture in characteristic samples from Harami deposit.
Rhombohedral dolomite crystal aggregates of authigenic origin are abundant and inherited serpentine grains are intertwined with palygorskite fibers especially visible in samples from the Velanida deposit (Fig. 3e). Opal-A′ is intimately associated in the form of intergrowths with high-purity palygorskite from the center of Harami deposit. An SEM image of a palygorskite from the center of the Harami deposit reveals amorphous silica that coats and, in certain areas, glues palygorskite fibers together (Fig. 3f). Although there is an open space texture, i.e. high porosity, created by the randomly oriented palygorskite fibers, certain areas display a tighter microtexture, i.e. reduced porosity.
Chemical compositions of clays
All clay samples had large MgO and Fe2O3 and small Al2O3 contents compared with typical palygorskite from the Attapulgus-Meigs-Quincy district, Georgia and Florida of the United States (Merkl, Reference Merkl1989) (Table S2). The mean values of Fe2O3 and MgO were ~12 and 14%, respectively, while the mean value of Al2O3 was only 5% (Table 2). Harami clays had the smallest Al2O3 content at 3%, while Pilori clays had the largest Al2O3 content at 9%, compared with the other three clay deposits of the Ventzia Basin (Table 2). Larger MgO contents have been measured in both the Harami and Knidi clay deposits, compared with those of Velanida and Pilori. The Fe2O3 content was comparable in all palygorskite samples of the four deposits, i.e. less than in the corresponding smectite samples at each deposit. The CaO levels were greater in the Velanida deposit, which is the only deposit in contact with the carbonate basement rock. The largest SiO2 content was measured in two instances, in the Pilori smectite and the Harami palygorskite. The SiO2 enrichment in Pilori clays can be attributed to the larger amount of quartz and feldspars found in the smectite clayey material, while in Harami it is due exclusively to amorphous silica (opal) identified in the palygorskite clayey material (Fig. S13). The elevated alkali content in Pilori samples, associated with large amounts of Al2O3, is indicative of the presence of clastic aluminosilicate minerals from the feldspar group (K-feldspar, plagioclase) in Pilori clay beds.
Table 2. The chemical composition of palygorskite (Plg), palygorskite-smectite (Mix) and smectite (Sme) clay samples from the four studied deposits
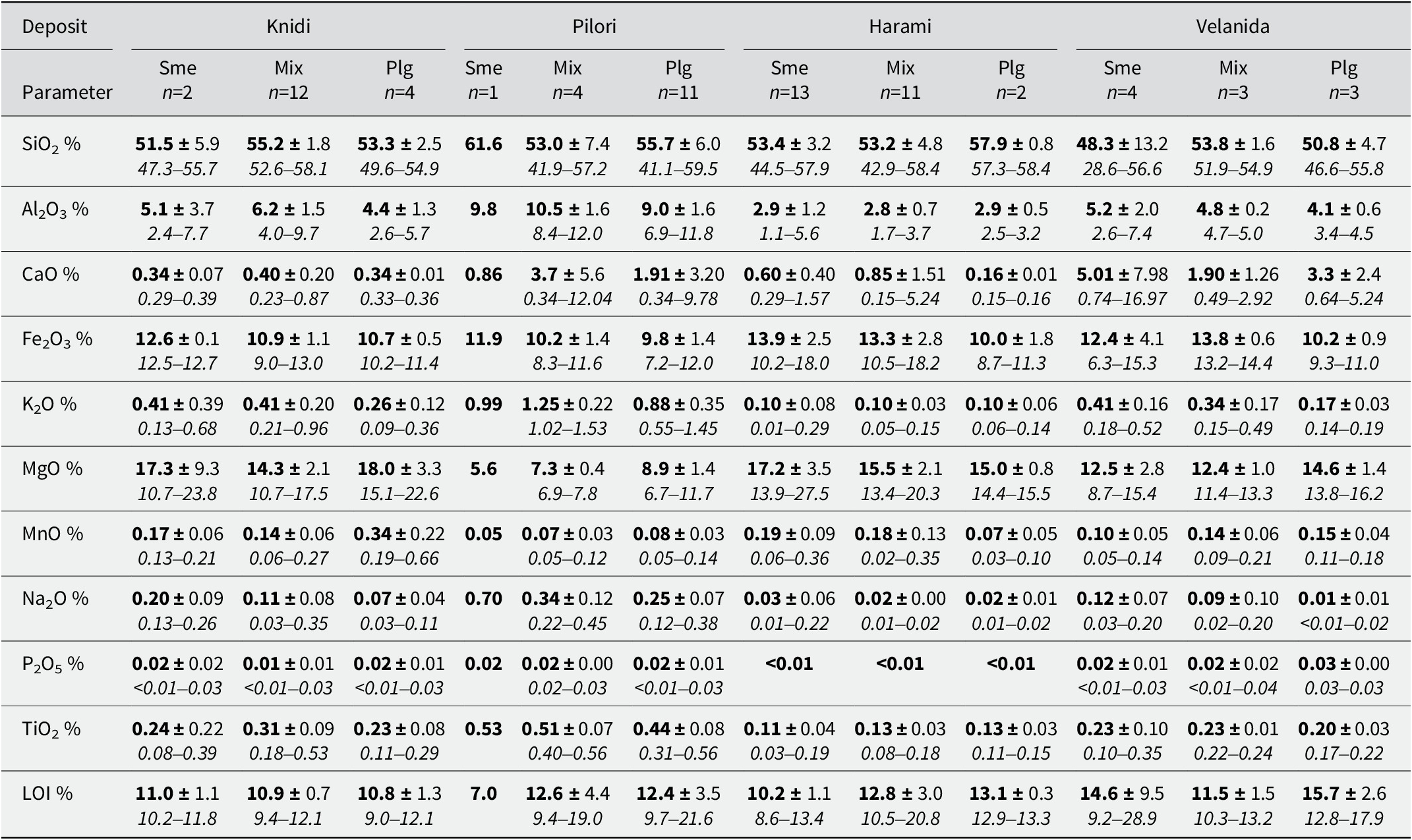
Mean values are shown in bold, standard deviation follows the ± sign and the min–max value ranges are shown in italics.
Dioctahedral and trioctahedral character of palygorskite
Based on the NIR spectroscopy of the samples studied, x (Fe in dioctahedral positions) and y (trioctahedral Mg component) values were large while the Al content at the dioctahedral position (1–x) was small (Table S2), in agreement with the bulk chemical analytical results. The absorption and details of the second derivative OH overtone spectra for three characteristic samples under study are shown in Fig. S14a and S14b, respectively. The y value ranged between 0.06 and 0.49 with a mean value of 0.33, while the x value ranged between 0.34 and 0.69 with a larger mean value of 0.55 (Table S2). A value of 1 for x and y corresponds to a fully ferric dioctahedral and fully trioctahedral Mg component for palygorskite, respectively (Chryssikos et al., Reference Chryssikos, Gionis, Kacandes, Stathopoulou, Suarez, Garcia-Romero and Sanchez Del Rio2009). The y value was smaller in palygorskite from the Pilori deposit and greater in palygorskite from the Harami deposit that is directly influenced by the depositional environment, closely related to the different geochemical backgrounds of the bedrock underlying each deposit. The amount of octahedral Mg in palygorskite-rich samples is positively correlated (R 2=0.71) to the BET surface area (Fig. S15). Crystallochemical work performed on bentonites by Suárez et al. (Reference Suárez, Lorenzo, García-Vicente, Morales, García-Rivas and García-Romero2022b) has shown an effect of trioctahedral Mg content on the lattice dimensions that correspondingly influence BET surface area and microporosity. However, due to the limited number of samples (n=4) in the present study, further work is needed to verify whether this relationship is significant (Fig. S15).
Physicochemical characteristics of the clay materials
The measured apparent bulk density (ABD), light absorption parameters of bleached oil (Vis, Yellow, Red, Chl), water (WW%), oil (WO%) adsorption, and methylene blue adsorption (MBA) of all samples (n=70) are shown in Tables S3 and S4. Palygorskite had larger WO% and smaller ABD values while smectite had larger WW% and MBA values. Mixed palygorskite-smectite from the Knidi and Harami deposits had greater Vis and lower Yellow, Red, and Chl values than palygorskite. Small values of Red (<3), Yellow (<69), and Chl (<0.400) and high Vis values (>18) correspond to high bleaching ability and are considered to be competitive bleaching earths in the market.
Bleaching behavior
The Vis values were selected in this study to evaluate the bleaching performance of the clays because the main impurities in vegetable oils absorb light in the blue-violet range (Stout et al., Reference Stout, Chamberlain and Mcklvey1949). Interestingly, mixed palygorskite-smectite yielded, on average, larger Vis values than palygorskite and especially smectite (Fig. 4a). Based on this finding and the results of surface properties of the materials studied (Table 3), a synergistic effect of smectite presence on bleaching performance is proposed. The small Vis values of Plg recorded from samples 23 and 24 (Fig. 4a) belong to the Pilori deposit and are due to the elevated amount of dolomite. The small Vis value of Plg sample 35 (Fig. 4a) originating from the Harami deposit is due to opal-A′ intergrowths. Plg sample 37 is characterized by an increased smectite content (~30%) but not large enough to be considered a mixed (palygorskite-smectite) clay.

Figure 4. A box-and-whisker plot showing the variation of Vis values (bleaching ability) between smectite (Sme), palygorskite-smectite (Mix), and palygorskite (Plg) clays from all four deposits: (a) comprehensive n=70 samples and (b) grouped according to the Knidi (K), Pilori (P), Harami (M), and Velanida (L) deposits.
Table 3. Values of specific surface area (SSABET, SAμp, and SAext) and porosity (PVμp, TPV, and PSav) from all four deposits for the subset of 12 samples
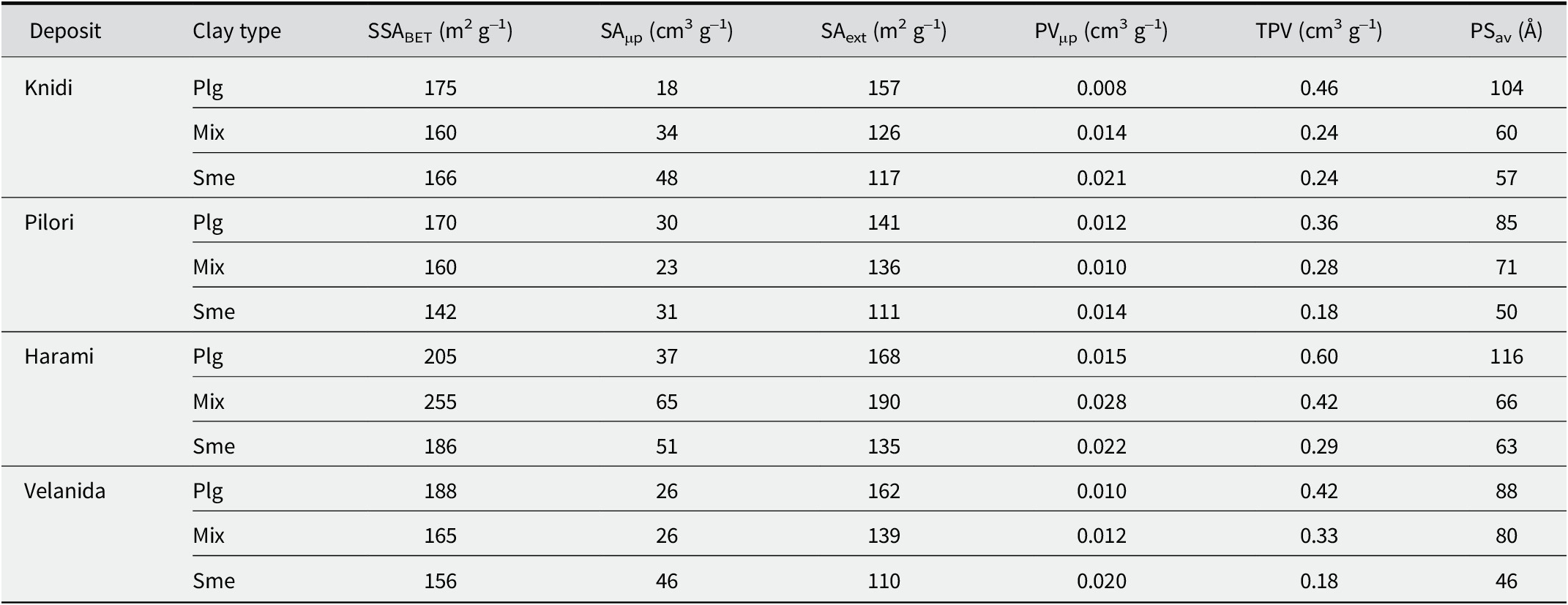
Plg = palygorskite, Mix = palygorskite-smectite, Sme = smectite.
Slightly different bleaching abilities were observed among the various deposits (Fig. 4b). Palygorskite-smectite (Mix) samples from the Knidi and Harami deposits had larger Vis values than palygorskite from the same deposits, i.e. better bleaching performance. This is due to the elevated serpentine content in Knidi palygorskites (Table 1) and amorphous silica (opal-A′) in Harami palygorskites (Fig. S13), both of which impair bleaching performance. On the other hand, palygorskite-smectite samples from the Pilori and especially the Velanida deposit had smaller Vis values than their respective palygorskite samples (Fig. 4b). Therefore, the larger Vis values recorded from the Knidi and Harami palygorskite-smectite are due to their larger palygorskite contents compared with palygorskite-smectite from the Pilori and Velanida deposits. Indeed, Knidi and Harami palygorskite-smectite contained, on average, 34–39% palygorskite as opposed to 28–29% palygorskite from the Pilori and Velanida deposits (Table 1). A slight 5–10% increase in the palygorskite content is sufficient to create a substantial difference in their bleaching performance. The larger non-clay minerals (quartz and carbonates) content in Pilori and Velanida deposits appears to impair their bleaching performance; however, the elevated serpentine content found in Knidi clays seems to not have a significant effect on bleaching performance. It is suggested that fine-grained serpentine in Knidi clays may have a positive control on the bleaching process and this requires further investigation.
Porosity and specific surface area
The measured SSABET and calculated SAμp, SAext, PVμp, TPV, and PSav values of the subset of 12 samples from all four deposits are listed in Table 3. According to Table 3, all three types of clays studied (palygorskite, mixed palygorskite-smectite, and smectite) had large SSABET values, ranging from 142 to 255 m2 g–1. These values are high compared with data gathered from an extensive review on the surface properties of palygorskite by Suárez et al. (Reference Suárez, García-Rivas, Morales, Lorenzo, García-Vicente and García-Romero2022a). The large SSA values of the present samples is in accord with their large TPV values recorded at between 0.18 and 0.60 cm3 g–1. Palygorskite had consistently larger SSA, TPV, and PSav values than palygorskite-smectite and, moreover, smectite, which implies that diagenetic processes had an important impact on the physicochemical properties as they increased the specific surface area and porosity of the clay. On the other hand, smectite samples had larger SAμp and PVμp values than palygorskite. An exception is the Harami palygorskite-smectite that had the largest SSA and SAμp/PVμp values of all the clays. This corresponds to the greatest bleaching performance among all Ventzia clays (Table 1; Table S4). Palygorskite crystallite sizes from Harami palygorskite-smectite were about half the size, measured at 6 nm, compared with sizes of 11–13 nm from all other studied palygorskites.
The N2 isotherms of all 12 samples indicated a reversible section at low pressures and a hysteresis loop at higher pressures (Fig. 5). However, isotherms of smectite differed as they have a larger hysteresis loop. Palygorskite and palygorskite-smectite to a lesser degree, presented narrow hysteresis loops (type A), that point to the presence of tubular pores while smectites look more like type B that indicates the presence of slit-shaped pores (de Boer and Lippens, Reference de Boer and Lippens1964). Palygorskite-smectite from Pilori were similar to a type E character indicative of wider pores (de Boer and Lippens, Reference de Boer and Lippens1964). Palygorskite samples revealed a type H3 hysteresis curve (meso–macroporous materials) while smectite samples revealed a type H4 hysteresis curve characteristic of micro–mesoporous materials (Thommes et al., Reference Thommes, Kaneko, Neimark, Olivier, Rodriguez-Reinoso, Rouquerol and Sing2015). Interestingly, Harami palygorskite-smectite (highest bleaching performance) yielded greater adsorption at lower relative pressures that is characteristic of microporous solids (Suárez et al., Reference Suárez, García-Rivas, Morales, Lorenzo, García-Vicente and García-Romero2022a).

Figure 5. N2 adsorption-desorption isotherms of smectite (Sme: in orange), mixed palygorskite-smectite (Mix: in blue), and palygorskite (Plg: in gray) from: (a) the Knidi (K) deposit; (b) the Pilori (P) deposit; (c) the Harami (M) deposit; and (d) the Velanida (L) deposit.
Effect of examined factors on bleaching performance
The correlation matrix of Spearman correlation coefficients, calculated with IBM’s SPSS software for the set of 70 samples (Table 4), indicated a statistically significant (p<0.01) negative correlation between bleaching ability expressed as Vis and ABD. Correlation coefficients between ABD and bleaching performance were also calculated for the three types of clay, i.e. palygorskite-smectite (n=25), palygorskite (n=21), and smectite (n=24) (Tables S5–S7). A significant, negative correlation (p<0.01) was observed between ABD and Vis only for palygorskite-smectite. This is explained as, although palygorskite had, on average, a smaller ABD value than palygorskite-smectite (Table 1), Vis for certain palygorskite samples from the Harami deposit yielded a poor bleaching performance due to amorphous silica (Fig. 3f; Table S13). Similarly, significant Spearman’s correlation coefficients (p<0.01) were obtained between ABD and Red, Yellow, Chl, and WO% for palygorskite-smectite only (Table S5). The binary plots of ABD–Vis for palygorskite-smectite and palygorskite, presenting the best bleaching performance as indicated by Vis≥18 for a considerable number of samples, were plotted with samples identified by deposit (Fig. 6). The detrimental effect on bleaching performance due to the presence of opal in the Harami deposit, as well as of carbonates or quartz in the Pilori and Velanida deposits, can be seen clearly in these diagrams. The bleaching performance of smectite is negligible, although a poor but significant (p<0.05) negative Spearman’s correlation coefficient was obtained for this clay (Table S7).
Table 4. Correlation matrix (Spearman’s correlation) for palygorskite, palygorskite-smectite and smectite from all four studied deposits (n=70)
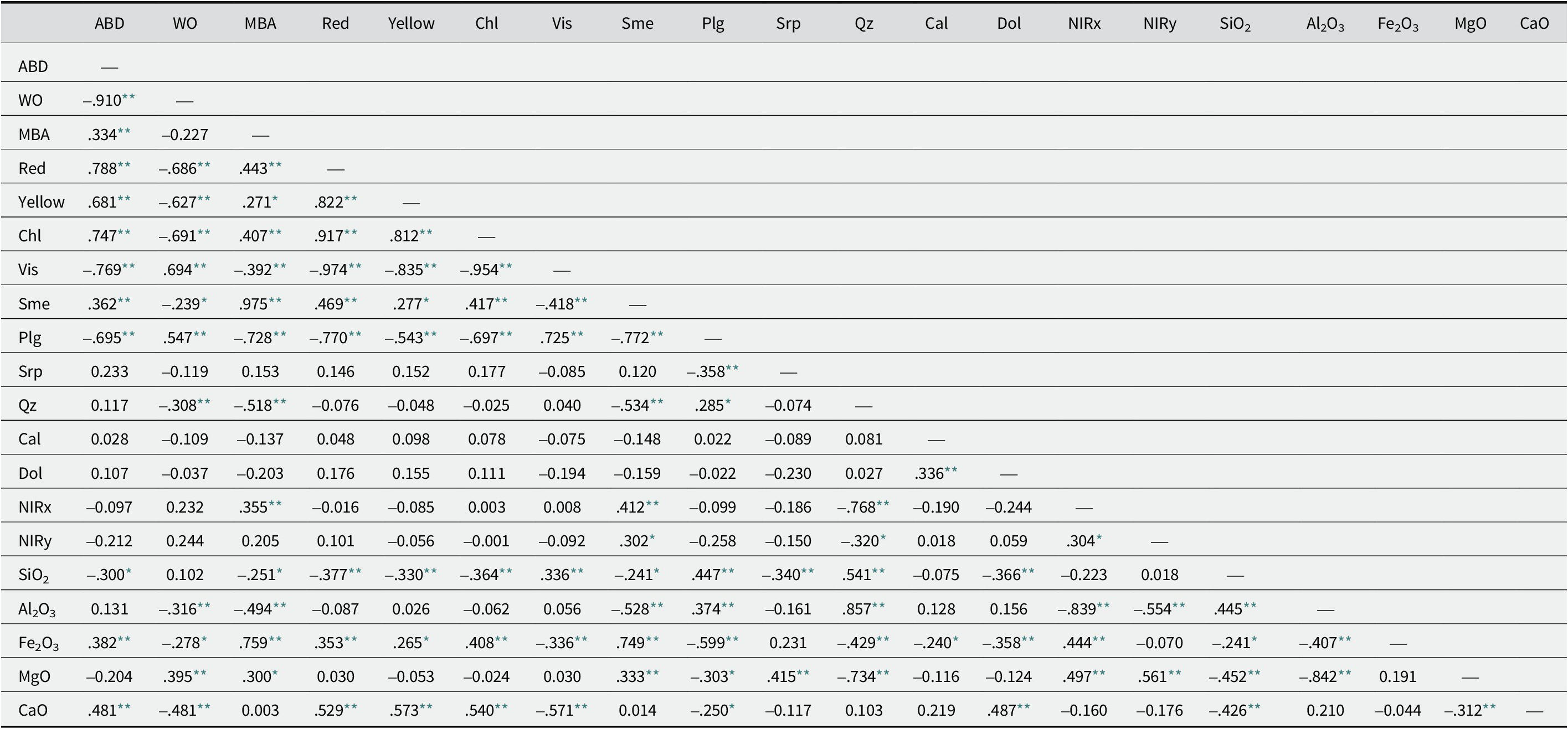
** Correlation is significant at the 0.01 level (2-tailed)
* correlation is significant at the 0.05 level (2-tailed). n=57 for NIR data.

Figure 6. Binary plots of ABD vs Vis: (a) palygorskite showing a poor correlation between ABD and Vis, and (b) palygorskite-smectite showing a strong correlation between ABD and Vis.
All 70 samples were plotted on a MgO–Fe2O3–Al2O3 ternary diagram grouped in clays with high bleaching performance (Vis≥18) and in clays with poor bleaching performance (Vis<18). No association was found between Vis and the concentrations of the main oxides MgO, Fe2O3, and Al2O3 as both groups plot in the same area (Fig. S16a). The bleaching performance is clearly independent of the Fe, Al, and Mg variance of the palygorskite-rich clays studied. The presence of CaO in large amounts has an indirect impact on the bleaching performance as it reflects the presence of carbonate minerals which are detrimental to bleaching.
The Mg5, Mg2Fe2, and Mg2Al2 values of palygorskite that correspond to the octahedral occupancy of Mg, Fe, and Al, respectively, were also plotted on a ternary diagram (Fig. S16b). These values were drawn from NIR analysis based on the method proposed by Gionis et al. (Reference Gionis, Kacandes, Kastritis and Chryssikos2006, Reference Gionis, Kacandes, Kastritis and Chryssikos2007). As with chemical composition, no correlation was observed between the dioctahedral composition or the trioctahedral fraction of palygorskites and their bleaching ability. In Fig. S16b the plotted values showed no distinction between samples that have a good bleaching ability (Vis≥18) as opposed to samples with poor bleaching performance (Vis<18) as they plot in the same region.
Based on the total sample dataset, all bleaching earth parameters were closely related to ABD and WO% but less relevant to WW% and MBA of clays (Tables 1 and 4). The WW% values along with MBA were directly correlated and influenced by the smectite contents of the clays that demonstrate minimal effect on bleaching performance.
Furthermore, based on the results from the 12 samples that were studied by means of N2 adsorption (Table 3), a statistically significant, linear, negative correlation was observed between ABD and TPV (p<0.05, R 2=0.79) and between ABD and SSAext (p<0.05, R 2=0.77) which indicates the close relationship between porosity and ABD. A less pronounced but nevertheless significant negative correlation was also observed between ABD and PSav (p<0.05, R 2=0.62), and between ABD and SSABET (p<0.05, R 2=0.50). Additionally, and more importantly, a significant, negative correlation was observed between Vis and ABD (p<0.05, R 2=0.56) and between Vis and SSAext (p<0.05, R 2=0.51) which indicates that lighter clays (lower ABD and more porous) have larger Vis values or better bleaching ability.
Based on these findings, an experimental formula was computed from the x-y correlation diagram between ABD and Vis values:
where y = Vis and x = ABD. To establish this relationship, the opal-laden palygorskite sample from the Harami deposit was not taken into account. The correlation between measured and predicted values of Vis based on Eqn (1) was calculated for the 70 samples and yielded a high value (R 2=0.82). Therefore, ABD can be considered a sufficient proxy to safely correlate the porosity and the external surface area (SSAext) of a clay, which are the controlling factors for bleaching performance. This is in accord with Sabah and Çelik (Reference Sabah and Çelik2005) who reported that the surface area and porosity of an acid-activated sepiolite were the main factors that controlled the adsorption of undesirable coloring substances from rapeseed oil. ABD is primarily related to specific surface area and porosity (Chaudhari et al., Reference Chaudhari, Ahire, Vidya, Chkravarty and Maity2013; Kakaire et al., Reference Kakaire, Makokha, Mwanjalolo, Mensah and Menya2015) but also indirectly to the mineralogical composition. Accessory minerals such as quartz or dolomite (less porous minerals) increase the ABD of a sample and impair bleaching performance. In this study, Vis values plotted on ternary diagrams revealed that the bleaching efficiency of the studied clays is not affected by the chemical composition of the clay or the octahedral composition of the palygorskite (Fig. S16) but from ABD, i.e. porosity.
The poor correlation between bleaching performance and ABD in certain palygorskite samples has been attributed to the presence of amorphous silica, especially evident in palygorskite from the Harami deposit. As opal-A′-laden palygorskite from the center of the Harami deposit has poor bleaching performance (Fig. 6a; Table S4) it is suggested that amorphous silica intergrowths in palygorskite interfere and obstruct active sites responsible for the bleaching process. On the other hand, no amorphous silica was observed in palygorskite-smectite samples. Palygorskite crystals free of opal A′ intergrowths are expected to exhibit even better bleaching performance. Indeed, this has been demonstrated by high-purity palygorskite samples with exceptionally good bleaching performance (Geohellas, unpublished data, 2012).
Overall, the findings of the present study indicated that the bleaching efficiency is significantly affected by the clay’s mineralogical composition, porosity, and specific surface area, while the chemical composition does not directly affect bleaching performance. Palygorskite-smectite, particularly those from the Knidi and Harami deposits, exhibited superior bleaching performance due to their larger palygorskite contents and despite the presence of serpentine. The present study has emphasized that smaller ABD values correlated with better bleaching performance is attributed to greater porosity and larger specific surface area. Accessory minerals such as quartz and dolomite also impaired bleaching performance by increasing the ABD. Table 5 summarizes the main points of these findings and provides a comparison with clay materials from other regions. According to Table 5, palygorskite-smectite yielded superior bleaching performance results than a thermally activated palygorskite-smectite from the United States (Pure-Flo B80) and was only slightly inferior to acid-activated sepiolite and bentonite from Turkey and Greece, respectively. Chlorophyll removal was greatest in palygorskite-smectite while the BET surface area achieved was close to that for the acid-activated sepiolite and bentonite (Table 5).
Table 5. Comparative data for three representative clay samples from the present study and four clays with similar compositions from other regions

n.a. = data not available.
a In-house measurements on a natural mixture of palygorskite and smectite from the United States (not acid-activated)
b bleaching performance of a Turkish acid-treated sepiolite on degummed rapeseed oil for a contact time of 25 min (table 3, Sabah and Çelik, Reference Sabah and Çelik2005)
c bleaching performance of an acid-treated Greek bentonite from S & B Industrial Minerals in Milos Island on sunflower oil (Taxiarchou and Douni, Reference Taxiarchou and Douni2014)
d bleaching performance of an acid-activated bentonite with 1% dosage at 90°C on degummed rapeseed oil (Sabah and Çelik, Reference Sabah and Çelik2005).
Conclusions
The bleaching performance of thermally activated (300°C) palygorskite and palygorskite-smectite has been assessed and shown to be controlled primarily by the number of active sites associated with the external surface area, the porosity, and the pore volume of the clay. All three parameters were inversely proportional to the ABD of clays which can be measured by an easy and cost-effective industrial test. ABD can be used safely to evaluate the bleaching performance of a clay at industrial scale instead of gas porosimetry tests which are more time-consuming and require expensive instrumentation. Tests performed on thermally treated palygorskite, palygorskite-smectite, and smectite from the Ventzia Basin, Greece, showed that enhanced bleaching of rapeseed oil was principally related to both the ABD and the palygorskite content of mixed palygorskite-smectite, i.e. clays with an ABD of <0.65 g cm–3, a palygorskite content of >50% and an average smectite content of 20%. Clays that are free of opal, with an external BET surface area of >160 m2 g–1, a total pore volume of >0.40 cm3 g–1 and an elevated mean pore diameter of 85 Å demonstrated the best bleaching performance (Vis>24). The chemical composition and the octahedral speciation of palygorskite do not play a significant role in the bleaching earth ability of the clays. An exception is the enrichment in calcium, associated with the presence of carbonates, that negatively affects bleaching performance. Other accessory minerals such as quartz (>10%) or opal intergrowths also proved detrimental to the bleaching performance. However, fine-grained serpentine (Knidi deposit) does not seem to impair the bleaching performance of clays significantly, and warrants further investigation. On average, palygorskite-smectite from the Knidi and the Harami deposit demonstrated better purification (bleaching) of rapeseed oil than palygorskite.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/cmn.2024.36.
Author contribution
Konstantinos Vythoulkas: Writing – original draft, Funding acquisition, Conceptualization, Methodology, Investigation, Visualization, Software, Data curation; Michael Stamatakis: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources; Manuel Pozo: Writing – review & editing, Methodology, Investigation, Software, Formal analysis, Data curation; Ariadne Argyraki: Writing – review & editing, Methodology, Investigation.
Acknowledgements
Special thanks to Mr Vassilis Skounakis for the technical support during the SEM studies, Mrs Eleftheria Typa and Mr Paschalis Kalimeris for guidance on the bleaching tests, and Dr Georgios Chryssikos for the NIR analysis. The input of four anonymous reviewers and editors is greatly acknowledged for improving the initial manuscript. The publication of the article in OA mode was financially supported by HEAL-Link.
Financial support
The authors thank Geohellas S.A. for financial support of the fieldwork and laboratory analysis related to this study.
Competing interest
The authors declare that they have no competing interests related to this work.
Data availability statement
All data generated or analyzed during this study are included in this published article and its Supplementary material.














