INTRODUCTION
Production of extended-spectrum beta-lactamases (ESBL) in Enterobacteriaceae has been associated with a delay in effective treatment and increased mortality [Reference Schwaber and Carmeli1]. Nosocomial transmission and hospital outbreaks caused by these bacteria have been reported [Reference Laurent2, Reference Quale3], but there is also evidence that ESBL-producing Enterobacteriaceae, especially ESBL-producing Escherichia coli, are emerging in the community [Reference Rodriguez-Bano and Navarro4, Reference Pitout5]. ESBL-producing Enterobacteriaceae have been isolated from different environments such as food, animal farms and sewage [Reference Mesa6] as well as from different human populations such as healthy carriers, household contacts of infected patients, nursing-home residents and international travellers [Reference Valverde7–Reference Laupland10].
The prevalence of ESBL-producing Enterobacteriaceae has been increasing worldwide and has been recognized as an emerging public health concern [Reference Coque, Baquero and Canton11–Reference Pitout and Laupland14]. Third-generation cephalosporin resistance in E. coli and Klebsiella pneumoniae as a marker for ESBL production has also shown a substantial increase in German intensive care units (ICUs) during recent years [Reference Meyer, Gastmeier and Schwab15, Reference Meyer16]; however, the mode and extent of the hospital-wide spread of these ESBL-producing bacteria remains unclear.
A hospital-wide surveillance system for ESBL-producing E. coli, K. pneumoniae and K. oxytoca was implemented in 39 German hospitals starting in 2007. The aims of this study were to use the data gathered in 2008 in order to determine the extent of spread and the incidence densities (IDs) of ESBL-producing bacteria in hospitals and to analyse influencing factors by means of a linear regression analysis.
METHODS
Surveillance of ESBL-producing Enterobacteriaceae
Surveillance of all cases of ESBL-producing E. coli, K. pneumoniae and K. oxytoca was performed by trained infection control personnel in 39 hospitals of a hospital group located in 39 cities throughout Germany. Thirty-one hospitals were acute care hospitals and eight hospitals were rehabilitation hospitals. Data from each hospital were submitted to a central database. Data were collected from all hospitalized patients.
Definitions
An ESBL-E/K case was defined as a patient with any type of culture positive for ESBL-producing E. coli (ESBL-E) or ESBL-producing Klebsiella spp. (ESBL-K) recovered during the hospital stay or as a patient known to be a carrier of these bacteria on admission. Every patient was counted only once during his hospital stay regardless of the number of positive cultures.
Each case was differentiated between community-onset and hospital-onset based on a temporal definition. A community-onset case was defined as a case with known carriage of ESBL-E/K on admission or with the first culture positive for these bacteria within 48 h of admission. Patients were considered carriers on admission if they had a history of previous colonization or infection with ESBL-E/K and had written evidence of a positive culture result.
A hospital-onset case was defined by the first culture positive more than 48 h after admission. All cases occurring between 1 January and 31 December 2008 were included in the analysis. Denominator data on the number of patient-days in 2008 were extracted from hospital administration systems. The patient-days of ESBL-E/K cases were not excluded from the denominator.
Statistical analysis
The numbers of cases were determined for all ESBL-E/K cases and the subcategories community-onset cases, hospital-onset cases, ESBL-E cases and ESBL-K cases. Incidence densities/1000 patient-days (pd) for these case categories were calculated for each hospital and mean and median IDs with 25% and 75% quartiles were calculated from the pooled data of all hospitals. In addition, the proportions of community-onset and hospital-onset ESBL-E and ESBL-K cases were determined and tested for significant differences with Fisher's exact test.
To analyse the association between the outcome ‘ID of hospital-onset ESBL-E/K cases’ and the ID of community-onset ESBL-E/K cases (categorized into four groups by the quartiles ⩽25%/>25%, 50%/>50%, 75%/>75%) and structural variables such as type of hospital (acute care hospital or rehabilitation hospital), hospital size (<400 or ⩾400 beds) and presence of an ICU in the hospital, a multiple linear regression analysis was performed. The structural variables were intended to provide a proxy measure for the intensity of care provided in the hospitals. To achieve normal distribution, the IDs of hospital-onset ESBL-E/K cases were log-transformed. The regression coefficients were converted to measures of effect using an exponential transformation and referred to as the multiplicative effect (ME) of the categories of ID of community-onset ESBL-E/K cases and structural variables. All parameters were included in the full model. From this model, parameters with the smallest χ2 value and P⩾0·05 were excluded stepwise until the P values of all parameters included in the model were <0·05. Similar linear regression analyses were performed for the outcome ‘ID of hospital-onset ESBL-K cases’ and the outcome ‘ID of hospital-onset ESBL-E cases’ including the same structural variables as described above and the variables ID of community-onset ESBL-K cases or ID of community-onset ESBL-E cases in the same categorization, respectively. For all tests a P value of <0·05 was considered significant. All analyses were performed with SPSS version 18.0 (IBM Corporation, USA).
Control measures
Hand disinfection according to the indications of the WHO ‘Clean care is safer care’ campaign and the German national hand hygiene campaign was promoted in all hospitals during the period of analysis [Reference Sax17]. For ESBL-E/K carriers, contact precautions, i.e. wearing single-use gloves and gowns during patient care were implemented in all participating hospitals; however, patients carrying ESBL-producing Enterobacteriaceae were not isolated in single rooms. No systematic admission screening for ESBL-producing Enterobacteriaceae was performed in any of the hospitals and no restrictions were placed on antibiotic use.
RESULTS
Thirty-nine hospitals with 3836449 pd performed surveillance of ESBL-E/K cases and submitted complete data for analysis to the central database. The hospital size in this group ranged from 25 to 1401 hospital beds and 24/31 acute care hospitals had an ICU. A total of 2081 ESBL-E/K cases occurred in these hospitals in 2008, of these 1930 (92·7%) cases were in the 31 acute care hospitals and 151 (7·3%) cases in the eight rehabilitation hospitals. ESBL-E (1330 cases, 63·9%) was more common than ESBL-K (751 cases, 36·1%), including 666 cases of ESBL-producing K. pneumoniae and 85 cases of ESBL-producing K. oxytoca. In total, 1113 (53·5%) ESBL-E/K cases were community-onset cases, while 968 (46·5%) cases were hospital-onset cases according to the definitions. The mean and median numbers of all ESBL-E/K cases and of the different subcategories with 25% and 75% quartiles and the respective IDs with 25% and 75% quartiles are shown in Tables 1 and 2. The numbers of ESBL-E/K cases of individual hospitals reported for 2008 varied substantially with a range from a minimum of 0 cases to a maximum of 224 reported cases. Of the seven hospitals that did not observe any cases, five were rehabilitation hospitals and two were acute care hospitals, but none of these hospitals had more than 250 beds. The IDs ranged from 0 to 2·53 cases/1000 pd for all ESBL-E/K cases and from 0 to 1·82 cases/1000 pd for hospital-onset ESBL-E/K cases (Table 2, Fig. 1).

Fig. 1. Incidence densities (ID) per 1000 patient-days of community-onset (▪) and hospital-onset (□) cases of ESBL-producing E. coli and Klebsiella spp. (ESBL-E/K) in 39 German hospitals in 2008.
Table 1. Numbers of cases of ESBL-producing E. coli (ESBL-E) and ESBL-producing Klebsiella spp. (ESBL-K) and of the subcategories community-onset and hospital-onset cases in 39 German hospitals, 2008
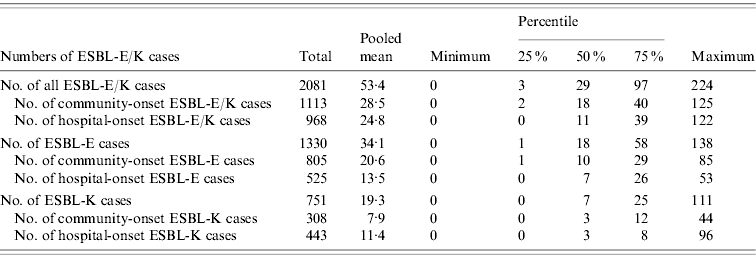
ESBL, Extended-spectrum beta-lactamase; ESBL-E, ESBL-producing E. coli; ESBL-K, ESBL-producing Klebsiella spp.
Table 2. Incidence densities of ESBL-producing E. coli (ESBL-E) and ESBL-producing Klebsiella spp. (ESBL-K) and of the subcategories community-onset and hospital-onset cases in 39 German hospitals, 2008
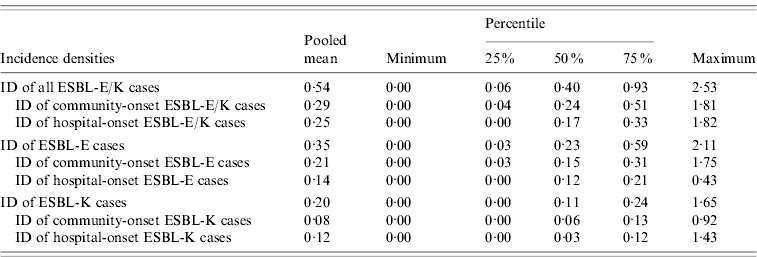
ESBL, Extended-spectrum beta-lactamase; ESBL-E, ESBL-producing E. coli; ESBL-K, ESBL-producing Klebsiella spp.; ID, incidence density.
Multiple linear regression analysis showed that the IDs of community-onset cases were independently associated with the IDs of hospital-onset cases in all three models (Table 3). In addition, in the model including ESBL-K cases as well as in the model including ESBL-E cases the variable ‘hospital with >400 beds’ was an independent protective factor for the outcome ID of hospital-onset cases (Table 3).
Table 3. Results of the linear regression analyses with the outcome incidence density (ID) of hospital-onset ESBL-E/K-cases, ID of hospital-onset ESBL-E cases and ID of hospital-onset ESBL-K cases in 39 German hospitals, 2008
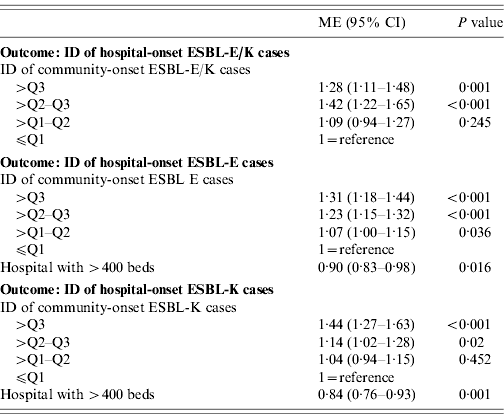
ESBL, Extended-spectrum beta-lactamase; ESBL-E, ESBL-producing E. coli; ESBL-K, ESBL-producing Klebsiella spp.; ME, multiplicative effect; CI, confidence interval; Q1, 25% percentile; Q2, 50% percentile; Q3, 75% percentile.
There were differences between ESBL-E and ESBL-K in the distribution of community-onset and hospital-onset cases. While a higher proportion of ESBL-E cases were community-onset cases, a higher proportion of ESBL-K cases (59·0%) than of ESBL-E cases (39·5%) were hospital-onset cases (P<0·001).
DISCUSSION
Surveillance of multidrug-resistant pathogens such as ESBL-producing Enterobacteriaceae in hospitals is the basis for analysis of risk factors and the implementation and evaluation of control measures [Reference Kaier18, Reference Meyer19]. The described hospital-wide surveillance system for ESBL-E/K documented 2081 ESBL-E/K cases in 39 hospitals in 2008. The analysis of these cases showed a higher proportion of ESBL-E cases compared to ESBL-K cases being of community-onset and a significant association between the IDs of community-onset and hospital-onset cases.
Surveillance for ESBL-producing E. coli and K. pneumoniae in ICUs as high-risk areas for antimicrobial resistance has already been established within the German Nosocomial Infection Surveillance System [Reference Kohlenberg20], but a recent hospital-wide study showed that only 34·7% of patients were admitted to an ICU at the time of their first culture testing positive for ESBL-producing organisms [Reference Kola21]. Restriction of surveillance to ICUs might therefore miss a substantial proportion of cases. In addition, the increasing number of reports about community-acquisition of ESBL-producing bacteria and their import into hospitals stress the need for hospital-wide surveillance [Reference Rodriguez-Bano and Navarro4, Reference Pitout5, Reference Ben-Ami22]. For other pathogens with a known substantial community reservoir and import into hospitals such as methicillin-resistant Staphylococcus aureus (MRSA) and Clostridium difficile systems for hospital-wide surveillance have already been established [Reference Chaberny23, Reference Gastmeier24].
The pooled mean hospital-wide ID of 0·54 ESBL-E/K cases/1000 pd confirms that ESBL-producing Enterobacteriaceae are already a serious problem in hospitals. In comparison, the surveillance systems named above reported pooled mean hospital-wide IDs of 1·03 and 0·68 cases/1000 pd for MRSA and Clostridium difficile-associated diarrhoea, respectively, for 2008. However, our study shows substantial heterogeneity of IDs of ESBL-E/K cases between hospitals (Fig. 1). Many factors might contribute to this heterogeneity including differences in the patient population, regional variation and differences in the frequency and quality of microbiological tests. Although our sample of hospitals might not be representative for all German hospitals, this variation of the IDs of ESBL-E/K cases between hospitals underscores the importance of endemic baseline values determined from a number of hospitals to enable the identification of outliers and focus further interventions on especially affected hospitals.
Even without admission screening more than half of the ESBL-E/K cases in this study were classified as community-onset cases. In addition, the regression analysis showed a linear association between community-onset and hospital-onset ESBL-E/K cases. The ID of community-onset cases might have affected the ID of hospital-onset cases in our study in two ways – by increasing the likelihood of hospital transmission or, more directly, by hidden import. The optimal methods and procedures for ESBL screening are still under investigation [Reference Meyer25, Reference Reddy26]. The current evidence does not support hospital-wide admission screening or justify the costs that would have been incurred by screening all admissions in 39 hospitals. However, it is a limitation of this study that the lack of admission screening might have resulted in a misclassification of a number of community-onset cases as hospital-onset cases. Further limitations are related to the definitions and metrics used in this study, such as the exclusively temporal definition of cases. Moreover, we did not investigate the ability of the local laboratories to detect ESBL production in Enterobacteriaceae. Despite these limitations, our data show that community-onset cases are an important part of the current problem of ESBL-producing Enterobacteriaceae in hospitals. The regression analysis also showed that the variable ‘hospital with >400 beds’ was a protective factor for hospital-onset ESBL-E and ESBL-K cases. Possible explanations for this finding are that the larger hospital perform microbiological diagnostics earlier and are therefore more likely to identify carriers within the 48-h interval of admission or that they are more experienced with the control of multidrug-resistant bacteria.
In addition, the analysis indicates that there are differences in the epidemiology of ESBL-E and ESBL-K. Because ESBL-E cases were more common, exceeding ESBL-K cases by 2:1, this difference is best seen in the proportions of community-onset and hospital-onset cases. While a higher proportion of ESBL-E cases were community-onset cases, a significantly higher proportion of ESBL-K cases were hospital-onset cases. This is in accordance with reports of ESBL-K in association with nosocomial transmission and outbreaks [Reference Laurent2, Reference Quale3], while ESBL-E have often been described as community-acquired [Reference Rodriguez-Bano and Navarro4, Reference Pitout5]. In addition, a recent study has shown a low transmission rate of ESBL-E in the hospital setting [Reference Harris27]. For this reason, it is advisable that surveillance systems differentiate between ESBL-E and ESBL-K and adjust infection control interventions accordingly, for example by screening risk groups for ESBL-E on admission or by implementing contact precautions to reduce hospital transmission of ESBL-K.
In summary, our analysis shows a linear association between the IDs of community-onset and the IDs of hospital-onset ESBL E/K cases and differences in the distribution of community-onset and hospital-onset cases between different species of ESBL-producing bacteria. The large number of ESBL-E/K cases and their wide dissemination in hospitals emphasize the need for continuous hospital-wide surveillance and increased efforts to prevent further spread guided by the analysis of surveillance data.
ACKNOWLEDGEMENTS
The authors thank the infection control personnel in the participating hospitals for their efforts in collecting the data on ESBL-E/K cases.
DECLARATION OF INTEREST
None.






