Fatigue is extreme tiredness resulted from physical and mental exertion(Reference Finsterer and Mahjoub1). Fatigue is a common problem in different parts of the world. During the past years, reports on the prevalence of fatigue has been varied from 33 % in Singapore taxi drivers(Reference Lim and Chia2) to 58 % in the US manufacturing workers(Reference Lu, Megahed and Sesek3) and to 81 % in internal medical students of Japan(Reference Kato, Burger and Emoto4). Fatigue is also very frequent in educational environments. A study in Poland found that fatigue symptoms in school and university students were as frequent as that in hard-working adults(Reference Oginska and Pokorski5).
Physical and mental fatigue have different descriptions, causes and consequences; however, they often occur together, decrease functional performance and efficiency, and are improved by rest and sleep(Reference Finsterer and Mahjoub1). Physical fatigue is caused by intense or prolonged physical activity and characterised by tiredness, exhaustion, lethargy, languidness, languor, lassitude, listlessness, weakness, low vitality and anergia(Reference Zengarini, Ruggiero and Pérez-Zepeda6). In contrast, mental fatigue is a psychophysiological state that results from sustained cognitive activity(Reference Morris and Christie7). Mental fatigue compromises executive control over daily cognitive activities which leads to declined attention and cognitive performance. Reaction, response time and accuracy, decision-making, planning, motivation, and even psychomotor and physical performance are affected by metal fatigue(Reference Verschueren, Tassignon and Proost8,Reference Hachard, Noé and Ceyte9) . Cross-sectional studies have shown that in students, fatigue is associated with decline in school performance and working memory, negative health outcomes and refusal to attend the school(Reference Fukuda, Yamano and Joudoi10,Reference Mizuno, Tanaka and Fukuda11) . Hence, alleviation of fatigue may improve educational performance and achievement in students.
Fatigue may also be caused by psychological disorders such as depression or chronic inflammatory diseases such as cancer, what is called disease-related fatigue(Reference Finsterer and Mahjoub1). Inflammatory reactions are suggested to contribute, at least partly, to the pathophysiology of disease-related fatigue(Reference Yang, Yang and Wang12). Thus, pharmacologic treatments to target inflammation may alleviate symptoms of fatigue(Reference Theoharides, Asadi and Weng13). Likewise, anti-inflammatory dietary components can be of help(Reference Haß, Herpich and Norman14). Long-chain n-3 fatty acids are largely recognised as anti-inflammatory agents(Reference Calder15). Patients with chronic fatigue syndrome have generally low serum concentrations of n-3 fatty acids(Reference Castro-Marrero, Zaragozá and Domingo16). Accordingly, consumption of n-3 fatty acids may help in alleviation of fatigue.
A number of clinical trials have reported beneficial effects of n-3 fatty acids on fatigue(Reference Yehuda, Rabinovitz and Mostofsky17–Reference Black, Witard and Baker20), but none of these trials has measured fatigue as the primary outcome. In addition, they except one(Reference Yehuda, Rabinovitz and Mostofsky17) examined disease-related fatigue and recruited participants with inflammatory diseases. We hypothesised that conditions with low-grade inflammation such as overweight and obesity(Reference Zatterale, Longo and Naderi21) may also benefit from fatigue-lowering effect of n-3 fatty acids. On the other hand, most of the relevant trials have investigated the effect of long-chain n-3 fatty acids, mainly EPA and DHA, on fatigue, while the literature on the effect of their precursor, α-linolenic acid, is lacking. Compared with EPA and DHA, α-linolenic acid has more contribution to the diet total n-3 fatty acids. In fact, only 18 % of dietary n-3 fatty acids is ingested as EPA and DHA(Reference Welch, Shakya-Shrestha and Lentjes22). That is because of limited dietary sources of EPA and DHA (marine foods) compared with α-linolenic acid, which is found in plant sources such as flaxseed, walnuts, soyabean, hemp seeds, their oil, rapeseed oil and green-leafy vegetables(Reference Rodriguez-Leyva, Dupasquier and McCullough23). Hence, it would be worthwhile to find out if α-linolenic acid has also the potential to attenuate fatigue. Flaxseed is one of the best plant sources of this fatty acid with 23 g α-linolenic acid per 100 g seeds(Reference Rodriguez-Leyva, Dupasquier and McCullough23). In addition to anti-inflammatory effect, flaxseed may have advantages, such as appetite suppression(Reference Ibrügger, Kristensen and Mikkelsen24,Reference Kristensen, Savorani and Christensen25) which benefits overweight individuals by reducing their energy intake. In this regard, previous studies have shown that flaxseed fibre suppresses appetite and energy intake(Reference Ibrügger, Kristensen and Mikkelsen24).
On the other hand, fatigue and depression are interrelated as both are associated with increased inflammation(Reference Lee and Giuliani26). Fatigue and depression are common in patients with autoimmune disorders, and treatment of inflammation may improve their depression and fatigue condition(Reference Lee and Giuliani26). In this regard, higher levels of inflammation in depressive patients may cause less effectiveness of anti-depressive treatments, while anti-cytokine therapy may improve their depression symptoms(Reference Kappelmann, Lewis and Dantzer27). Accordingly, we additionally assessed mood feelings including depression, anxiety and stress, some of which may be important in our target society (children and adolescents). Hence, in the present study, we examined the effect of flaxseed on fatigue, mood, appetite, energy intake, weight and other anthropometric measures in children and adolescents with overweight and obesity.
Methods
Study design and participants
The study was a randomised parallel-group controlled clinical trial conducted in winter 2019 in Shiraz, Iran. Due to the lack of reports for the effect of flaxseed on fatigue, the sample size was estimated thirty-three in each group for observation of a 25 % change in fatigue of the treatment group compared with the control, considering a 5 % error and 80 % power. Participants were recruited through convenience sampling from referees to Public Health Centres. Inclusion criteria were as follows: healthy children and adolescents aged 7–18 years, BMI > 25 kg/m2, and willingness to participate in the study. Children and adolescents with physical and mental illness and those on special diets, such as isoenergetic regimens, nutritional supplements, appetite suppressants and anti-psychotic medications, were not included.
In order to participate in the study, the consent of both children and their parents was necessary. Written informed consent was obtained from parents after explaining the study aim and procedure. The study was performed according to the guidelines of 1964 Declaration of Helsinki and its later amendments. The trial was approved by Ethics Committee of Shiraz University of Medical Sciences (approval code IR.SUMS.REC.1396.172) and registered in the Iranian Registry of Clinical Trials with code IRCT20190408043204N1.
Intervention
The trial had two groups: treatment that received 20 g/d flaxseed and control that received 25 g/d puffed wheat. Stratified simple randomisation was used to allocate participants to the flaxseed and puffed wheat groups. Stratification was performed based on age group, that is, separately for children (7–12 years) and adolescents (13–18 years). In each age group, random allocation was performed using a random number table.
Participants were not blinded to the treatments because the parents did not agree participation of their children unless they saw the foods that were given to the children. Therefore, flaxseed and puffed wheat were provided in intact forms. The treatments were isoenergetic. Puffed wheat was chosen as control because it is low in fibre, contains mainly carbohydrate and is unlikely to have ingredients that affect the outcomes of the present study. The amount of α-linolenic acid in the puffed wheat is about 0·08 g/100 g compared with 22·8 g/100 g in flaxseed.
Participants in the flaxseed and wheat groups were recommended to consume the corresponding product daily for a period of 4 weeks. The energy content of the treatments was equal (418·4 kJ (100 kcal) in 20 g flaxseed or 25 g puffed wheat). Flaxseed, puffed wheat and measuring cups suitable for each group were provided. Participants in the flaxseed group were asked to grind the flaxseed and consume it in uncooked form with meals (lunch and dinner), for instance, with yogurt, salad or food dishes. Participants in the wheat group were asked to consume puffed wheat as a snack between main meals. In order to prevent oxidation of unsaturated fatty acids in flaxseed, participants were instructed to grind flaxseed in small amounts, for 2–3 d use, and store it in a tight container in a refrigerator. A registered dietitian gave a diet plan to every participant according to his/her individual energy needs and considering the energy received from the treatments. Diet recommendations were given to all participants according to the guidelines of healthy eating with special emphasis on main food groups(28). However, diet energy was kept the same as that before trial. Reminders were given during the intervention for consumption of the treatments and following the dietary recommendations. In order to evaluate the compliance, diary sheets were given to the subjects to mark them each time that they consumed flaxseed or puffed wheat.
Fatigue and mood assessment
Fatigue, including physical and mental fatigue, and mood states were considered as primary, and appetite, energy intake, weight, BMI and waist circumference were measured as secondary outcomes. All outcomes were measured at baseline and the end of the 4-week intervention. Fatigue was evaluated by Multidimensional Fatigue Inventory which is a twenty-item self-report instrument consisting of the five dimensions including general, physical, and mental fatigue, reduced motivation and reduced activity(Reference Smets, Garssen and Bonke29). Each dimension rated on a five-point Likert scale and scored from 4 to 20, resulting in a total score of 20–100. Higher scores indicated higher degree of fatigue. Mood was assessed by the Short Mood and Feeling Questionnaire which measures child and adolescent depression(Reference Angold, Costello and Messer30). Depression, anxiety and stress were assessed by Depression Anxiety Stress Scales which is a twenty-one-item with every seven items on one of the subscales(Reference Le, Tran and Holton31). Appetite was estimated by the eight-item Council on Nutrition Appetite Questionnaire(Reference Wilson, Thomas and Rubenstein32). Sleep was considered as a confounder for mood and fatigue and measured by Pittsburgh Sleep Quality Index which assesses sleep quality during the past month(Reference Buysse, Reynolds and Monk33).
Anthropometric assessment
Weight was measured with minimal clothing to the nearest 0·1 kg using a digital scale (Glamor BS-801, Hitachi). Height was estimated with the precision of 0·5 cm using a non-stretchable tape fixed on a wall. BMI was computed by weight in kilograms divided by height squared in metres. Z scores of height, weight and BMI were obtained by Epi Info software version 3.5.4 based on the standards of Centers for Diseases Control 2000. Waist circumference was measured without pressure to the abdomen at the middle of the distance between the lowest rib and the iliac crest using a non-stretchable tape.
Biochemical assessment
Blood samples were collected after 12-h overnight fast. Serum was separated immediately and stored in –70°C for later analysis. Glucose, blood lipids, Fe and total iron-binding capacity were determined by commercially available kits (Pars Azmun) and an auto-analyser (BT 1500, Biotecnica Instruments). Transferrin saturation was calculated by serum Fe concentration divided by total Fe-binding capacity multiplied by 100. Anti-coagulated blood was used for determination of Hb with the cyanmethaemoglobin method(Reference Amatuzio, Grande and Wada34).
Dietary intakes and physical activity
Diet was questioned by diet record technique for 3 d (two random weekdays and one weekend day). Food records were checked with the participants and incomplete records were completed through onsite or phone interview. Nutrient composition was determined with Nutritionist IV version 3.5.2 (Hearst Corp.). Physical activity was assessed by a validated International Physical Activity Questionnaire and expressed as metabolic equivalent task in minutes per week (MET-min/week)(Reference Craig, Marshall and Sjöström35).
Statistical analysis
Data were analysed with SPSS software version 21 (SPSS Inc.). Analysis was performed based on per-protocol and intention-to-treat (ITT) approaches. Per-protocol analysis was performed for those who consumed at least 50 % of the administered materials (n 30 in flaxseed and n 22 in puffed wheat groups) and ITT was performed for all subjects who entered the trial (n 38 in flaxseed and n 34 in puffed wheat groups). For ITT analysis, imputed missing values as well as data of low adherent participants were used in the analysis. Missing values were replaced using multiple imputation model based on the available data(Reference Liao and Stack36). Missing-at-random assumption was used to generate ten sets of imputed data, which then were analysed to produce a set of ten results, the pooled of which was considered as the ITT outcome.
Normality of data was checked with the Shapiro–Wilk test, and abnormally distributed data were log-transformed before analysis. Baseline values between the flaxseed and puffed wheat groups were compared with the independent t test. For comparison of baseline and post-intervention values in each group, the paired sample t test was used. For anthropometric and biochemical variables, between-group comparisons were performed with ANCOVA with age, sex and corresponding baseline values as confounders. Because mood and fatigue are affected by Fe deficiency, for mood and fatigue variables (Table 2), between-group comparisons were performed with ANCOVA with age, sex, sleep, total iron-binding capacity and corresponding baseline values as confounders. The average of baseline and endpoint dietary intakes and physical activity were compared with the independent t test. Statistical analysis was set at P < 0·05.
Results
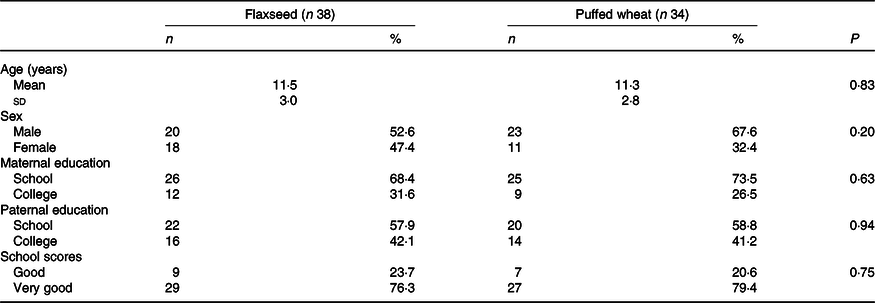
Seventy-two eligible children and adolescents were randomised to the flaxseed (n 38) and puffed wheat (n 34) groups. During the 4-week trial, five individuals in the flaxseed and nine in the puffed wheat group were excluded due to physical illness (n 1), possible side effects (heart burn) (n 1), withdrawal of consent (n 10) and personal reasons such as busy schedule (n 2). Thus, fifty-eight individuals (n 33 and 25 in the flaxseed and puffed wheat groups, respectively) completed the trial. The flow chart of the trial is depicted in Fig. 1. Demographic characteristics showed that participants of the two groups did not differ in age, sex, parental education and school scores (Table 1).
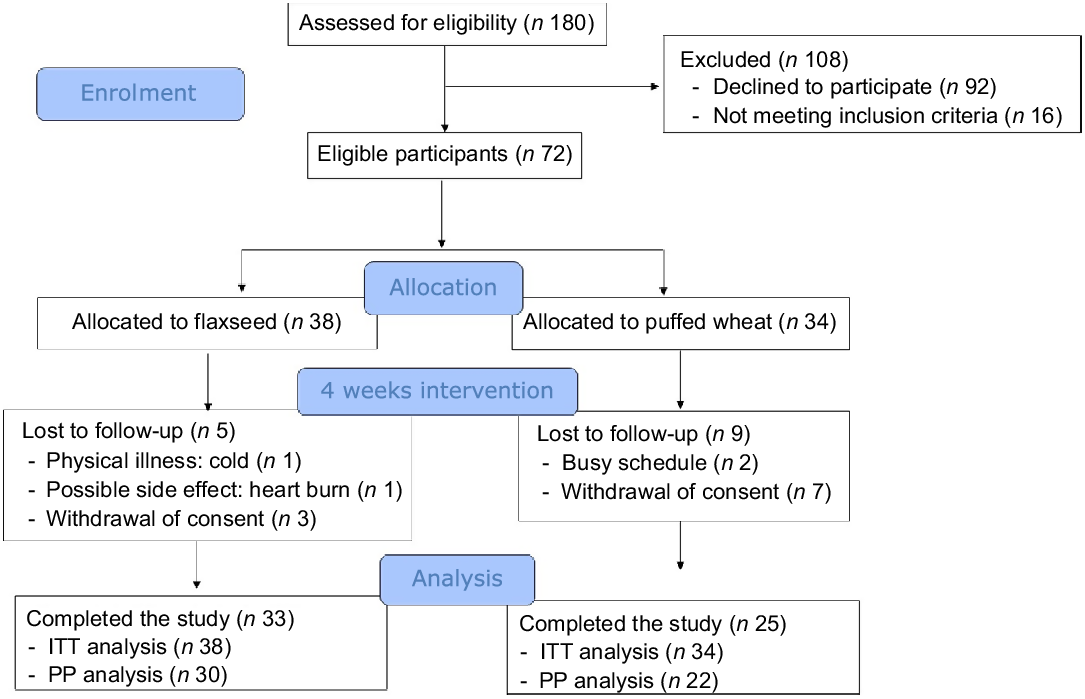
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow chart of the trial. Intention-to-treat (ITT) analysis was performed for all participants who started the intervention. Per-protocol (PP) analysis was performed on those who completed the study and consumed at least 50 % of the test products.
Table 1. Demographic characteristics of the participants
(Numbers and percentages; mean values and standard deviations)
Based on per-protocol analysis which included those with at least 50 % adherence to the administered products, fatigue decreased in both groups; although the alteration was not significant within groups, a significant difference was observed between groups as a result of greater fatigue reduction in the flaxseed group (–3·13 (sd 11·3) v. –0·62 (sd 8·22), P = 0·03) (Table 2). Physical (–1·10 (sd 4·11)) and mental (–1·93 (sd 3·67)) fatigue decreased in the flaxseed group, although only the decrease in mental fatigue was statistically significant (P = 0·007). Both physical (P = 0·04) and mental fatigue (P < 0·001) demonstrated significant difference between the groups. Mood which was measured as an indicator of depression decreased in both groups, but the reduction was only significant in the puffed wheat group (–3·33 (sd 6·93), P = 0·04). However, this reduction did not cause a significant between-group difference (P = 0·78). Likewise, depression which was measured by Depression Anxiety Stress Scales questionnaire showed reductions in both groups, but the reductions were not significant within or between groups. Anxiety and stress also did not show significant alterations within groups. Appetite decreased to the same extent in both groups (P = 0·001) and thus no difference was observed between the groups.
Table 2. Fatigue, appetite and mood characteristics pre- and post-intervention based on the per-protocol approach
(Mean values and standard deviations)
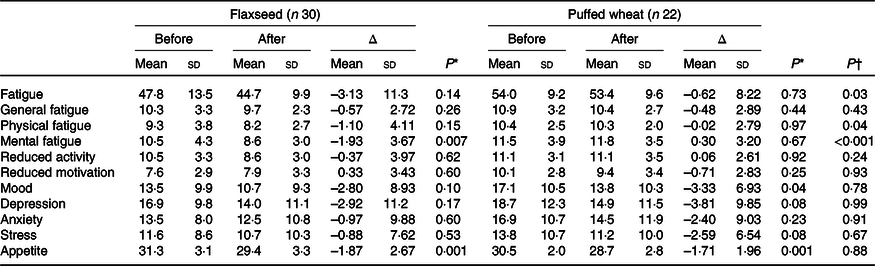
* Within-group comparisons were performed with the paired t test.
† Between-group assessments were performed with ANCOVA with age, sex, sleep, total Fe-binding capacity and corresponding baseline values as confounders.
Based on per-protocol analysis, height and height Z score increased significantly in both groups, but the increase was greater in the wheat group (for height 1·09 (sd 0·87) v. 0·53 (sd 0·89) cm), resulting in a significant difference between the groups (P = 0·03) (Table 3). Weight and weight Z score decreased in both groups, but the decrease was only significant in the wheat group (–0·73 (sd 1·16) kg, P = 0·008). BMI and BMI Z score significantly decreased in both groups and a greater reduction was observed in the wheat group (for BMI –0·67 (sd 0·56) v. –0·25 (sd 0·63) kg/m2), causing a significant between-group difference (P = 0·01). Waist circumference also significantly decreased in both groups with an average of –1·23 (sd 2·48) cm, but no significant difference was observed between groups (P = 0·79). Fasting blood glucose, lipid fractions and Fe status indicators did not show significant alterations within groups. The average of dietary intakes and physical activity during the study did not differ between groups (Table 4).
Table 3. Anthropometric and biochemical variables pre- and post-intervention based on the per-protocol approach
(Mean values and standard deviations)
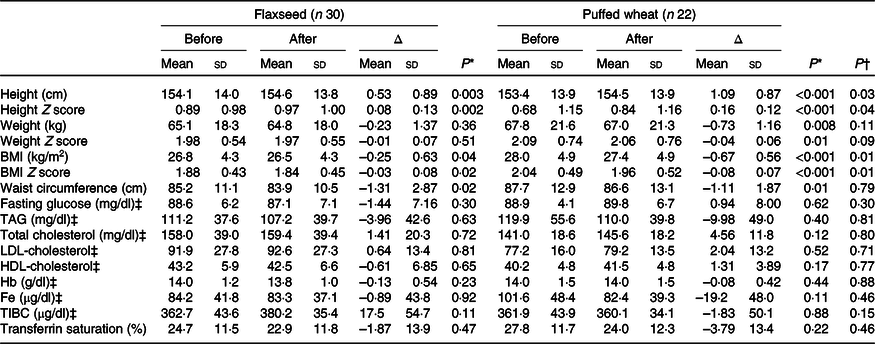
TIBC, total Fe-binding capacity.
* Within-group values were compared with paired t test.
† Between-group assessments were performed with ANCOVA with age, sex and corresponding baseline values as confounders.
‡ To convert glucose in mg/dl to mmol/l, multiply by 0·0555. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert Hb in g/dl to g/l, multiply by 10. To convert Fe in μg/dl to μmol/l, multiply by 0·179. To convert TIBC in μg/dl to μmol/l, multiply by 0·179.
Table 4. Daily dietary intakes and physical activity of the participants during the study in the flaxseed and puffed wheat groups
(Mean values and standard deviations)
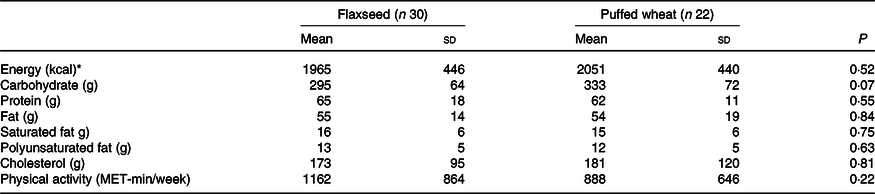
MET, metabolic equivalent task.
* To convert energy values from kcal to kJ, multiply by 4·184.
In ITT analysis, mental fatigue decreased significantly in the flaxseed group which caused a significant difference between groups (P = 0·028) (online Supplementary Table S1). There was also a trend for decreased appetite in the flaxseed group (P = 0·055), but no significant difference was observed between groups. None of anthropometric and biochemical variables changed significantly in the ITT analysis (online Supplementary Table S2).
Discussion
During the 4-week trial, consumption of 20 g/d flaxseed improved mental fatigue without affecting general fatigue, motivation and activity as well as psychological feelings such as depression and anxiety. Although no significant change in physical fatigue was observed in either group, a significant difference was observed between groups in favour of flaxseed. Nevertheless, flaxseed showed less benefit than puffed wheat for anthropometric measures as it caused smaller increase in height and reduction in BMI than puffed wheat. Appetite decreased to the same extent in both groups.
Fatigue
n-3 Fatty acids are likely the compounds responsible for decreased fatigue in the flaxseed group. n-3 Fatty acid-rich foods from both plant and fish sources are one of the components of fatigue reduction diet(Reference Zick, Colacino and Cornellier37). In a randomised clinical trial, the reduction in fatigue following consumption of this diet was associated with increased serum n-3 fatty acids(Reference Zick, Colacino and Cornellier37). Evidence from cross-sectional studies have also indicated the association between long-chain n-3 fatty acids and fatigue. For instance, in breast cancer survivors, higher intake of long-chain n-3 fatty acids (EPA and DHA) relative to n-6 fatty acids was associated with lower sensory and total fatigue(Reference Alfano, Imayama and Neuhouser38). Also, patients with chronic fatigue syndrome had low concentrations of EPA and DHA in their erythrocytes(Reference Castro-Marrero, Zaragozá and Domingo16). Clinical trials have also shown the benefit of a mixture of α-linolenic acid and linoleic acid on fatigue of healthy individuals(Reference Yehuda, Rabinovitz and Mostofsky17) and that of long-chain n-3 fatty acids on patients with disease-related fatigue, such as patients with rheumatoid arthritis(Reference Berbert, Kondo and Almendra18), psychotic disorders(Reference Robinson, Gallego and John19) and also in sport-induced fatigue(Reference Black, Witard and Baker20).
The association between n-3 fatty acids and fatigue may rely on the anti-inflammatory potential of long-chain n-3 fatty acids(Reference Calder15). Growing evidence suggests a link between inflammatory conditions and chronic disease-related fatigue(Reference Haß, Herpich and Norman14). Stimulators of inflammatory conditions have shown to induce fatigue and sleepiness concomitant to the cytokine arousal(Reference Lasselin, Karshikoff and Axelsson39). Although participants of the present study were not implicated in chronic diseases, overweight and obesity may have caused chronic low-grade inflammation(Reference Saltiel and Olefsky40), which may have affected mental fatigue in our participants, providing a chance for α-linolenic acid to exert its benefits for alleviation of mental fatigue.
The majority of the reports on the link between n-3 fatty acids and fatigue point at physical aspects of fatigue, especially chronic disease-related fatigue. To the best of our knowledge, only one study has examined the effect of long-chain n-3 fatty acids on mental fatigue of healthy individuals, presenting evidence for a beneficial effect from EPA, but not DHA(Reference Jackson, Deary and Reay41). For the first time, we tested the effect of α-linolenic acid on different aspects of fatigue and found advantages on mental fatigue. However, to exert anti-inflammatory effects, α-linolenic acid needs to be converted to longer chain fatty acids, EPA and DHA, through desaturation and chain-elongation processes. Using isotopically labelled fatty acids or analysis of serum fatty acid profile after taking α-linolenic acid supplements, it has been found that about 5 % of α-linolenic acid is converted to EPA and less than 0·5 % of which is converted to DHA(Reference Plourde and Cunnane42). Nevertheless, the results of the present study suggest that despite this low conversion rate, it is still likely that we see benefits of n-3 fatty acids if we consume α-linolenic acid-rich foods. The amount of α-linolenic acid in 20 g of flaxseed consumed in this trial was about 4·6 g, which could have produced approximately 230 mg of EPA and 23 mg of DHA in the body. The produced EPA is comparable to the EPA present in 100 g of baked sea trout fish, but this amount of fish contains almost ten times more DHA than 20 g flaxseed(43). It is noteworthy that, in addition to α-linolenic acid, flaxseed contains other biocompounds such as polyphenols(Reference Garros, Drouet and Corbin44) which are also known to protect against fatigue and fatigue-associated inflammation(Reference Liu, Wu and Zhang45). It is likely that these compounds have contributed to the observed effects. Randomised controlled trials with supplements of α-linolenic acid are needed to confirm these results and determine the extent of the effects by this fatty acid.
Appetite
Both treatments suppressed appetite; although the suppression was significant in both groups, no difference was observed between groups. Protein and fibre are two major diet components that may contribute to appetite suppression(Reference Korczak, Timm and Ahnen46). Based on data of nutritional values, flaxseed and puffed wheat have almost equal amounts of protein (3·7 v. 3·8 g in the consumed flaxseed and puffed wheat, respectively), but flaxseed has more fibre than puffed wheat (5·5 v. 2·8 g). Thus, flaxseed would be expected to confer stronger effect for appetite suppression than puffed wheat. However, appetite was suppressed to the same extent in the flaxseed and puffed wheat groups. Suppression of appetite in the wheat group could be due to the time of puffed wheat consumption which was between meals. Investigations have acknowledged the effect of mid-day snacks on appetite control and induction of satiety(Reference Guo, Totosy de Zepetnek and Chang47,Reference Njike, Smith and Shuval48) . In this regard, we observed higher reduction in energy intake in the puffed wheat group compared with the flaxseed group (–1920 (sd 1807) v. –1360 (sd 2611) kJ/d (–459 (sd 432) v. –325 (sd 624) kcal/d); P = 0·43).
Anthropometric measures
Although weight reduction is not appropriate for children, but it could be beneficial in children with overweight and obesity provided that growth rate is not affected. The puffed wheat group demonstrated a greater decrease in weight and BMI compared with the flaxseed group which could be partly due to a more substantial height increase in this group. The greater weight reduction of the puffed wheat group could also be due to the time of puffed wheat consumption which was between meals and may have caused a satiety effect as mentioned above. In fact, consumption of energy-containing snacks may help in appetite control and reducing food intake at following meals(Reference Guo, Totosy de Zepetnek and Chang47). Hence, intake of flaxseed as a snack may cause higher satiety and induce more reduction in overall energy intake, something that needs to be examined in future investigations.
As stated, height and height Z score were also improved in puffed wheat consumers to a greater extent than in the flaxseed group. Findings of this trial are not sufficient to justify the larger increase in height of the puffed wheat group, but α-linolenic acid of flaxseed may be involved in a smaller height increase in flaxseed consumers. In this regard, a follow-up study indicated that higher intake of PUFA, from either n-3 or n-6 type, during mid-pregnancy was associated with lower height in offspring during peripuberty(Reference Al-Hinai, Baylin and Tellez-Rojo49). Also, mice pups who consumed long-chain n-3 fatty acid-rich milk had slower pre-weaning growth rates compared with pups reared on regular milk(Reference Bongiovanni, Depeters and Van Eenennaam50). Thus, α-linolenic acid in flaxseed may have affected height growth of children and adolescents in the flaxseed group. However, a systematic review on randomised controlled clinical trials did not show any effect from consumption of long-chain n-3 fatty acids supplements during pregnancy on the growth of children(Reference Campoy, Escolano-Margarit and Anjos51).
Mood disorders
Flaxseed did not affect mood disorders, including depression, anxiety and stress. Long-chain n-3 fatty acids have been proposed as a strategy for preventing or treating mood disorders such as depression and anxiety in certain conditions(Reference Zick, Colacino and Cornellier37,Reference Berger, Smesny and Kim52,Reference Larrieu and Layé53) , although no such document is available for α-linolenic acid. However, the lack of effect in the present study is likely due to healthy mental status of our participants, as in previous studies benefits of long-chain n-3 fatty acids were observed in individuals at-risk of mental states(Reference Berger, Smesny and Kim52) or recent-onset psychosis(Reference Robinson, Gallego and John19).
Limitations
This was the first study that examined the effect of a plant source of n-3 fatty acids on various aspects of fatigue, including mental fatigue. However, we experienced limitations. A limitation was that we could not blind the participants to the treatments because the parents did not agree participation of their children unless they knew the content of the treatments. Due to financial limitations, we were not able to measure the levels of serum cytokines to see if fatigue improvement was associated with suppression of inflammatory markers. Also, lack of budget did not allow us to measure circulating fatty acids which could be of benefit for assessing the compliance of participants with test products and dietary recommendations. Moreover, the results could be interpreted better, if puffed wheat had consumed at the same time as flaxseed. Future investigations need to take these points into consideration.
Concluding remarks
In conclusion, consumption of 20 g/d flaxseed attenuated mental fatigue of children and adolescents with overweight/obesity. However, the effect size was small and the improvement was not observed in other aspects of fatigue. According to the baseline values, the participants had mostly very low to low fatigue. Therefore, it is likely that a floor effect has occurred in which the magnitude of the effects has been small due to low baseline fatigue, while better results can be achieved if participants with more severe fatigue are recruited. The lack of effect on mood feelings including depression, anxiety and stress should be considered with caution as the participants were not patients with mood disorders. For the same reason, the lack of effect on cardiometabolic risk factors cannot be substantiated. Smaller increase in height of flaxseed consumers suggest that before getting confidence on its safety, it is better to avoid administration of flaxseed during periods of growth. Considering the low conversion rate of α-linolenic acid to EPA and DHA, results of the present study seem promising. Nevertheless, as the observed effects may have been caused by other components in flaxseed, randomised controlled trials with supplements of α-linolenic acid are needed to clarify if these effects have been exhibited by α-linolenic acid.
Acknowledgements
We thank the patients who dedicated their time to participate in this study.
The results presented herein were extracted from the thesis written by Ms Zohreh Gholami. The project was financially supported by Shiraz University of Medical Sciences, grant number: 96-01-84-15712.
Both authors contributed equally to the study design, data analysis, and manuscript preparation. The trial was carried out by Z. G. with the assistance of research staff.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520003888








