Lower post-prandial blood glucose and insulin responses (post-prandial glycaemia (PPG) and post-prandial insulinemia (PPI), respectively) are widely associated with a reduced risk of development and progression of cardiometabolic diseases(Reference Thomas and Elliott1–Reference Livesey, Taylor and Livesey4). More recently, it has been proposed that diets generating lower PPG and PPI responses might also be beneficial for weight control(Reference Ludwig and Ebbeling5). One potential approach to reduce the PPG and PPI responses to commercial foods is the use of plant extracts that may slow the rate of carbohydrate digestion and glucose uptake(Reference El-Abhar and Schaalan6).
Extracts derived from mulberry products (mainly mulberry leaf) have been widely proposed and tested for their potential PPG-lowering effect(Reference Chan, Phui-Yan and Siu-Kuin7–Reference Jeong, Jang and Kim10). Mulberry products are a source of 1-deoxynojirimycin (DNJ), which can inhibit the action of α-glucosidase in the human small intestine(Reference Thakur, Zhang and Mocan11). This mechanism of action, which is also the basis for a class of drugs used in managing diabetes, slows the final step in the release of glucose from dietary carbohydrates and thus also reduces the rate of uptake and appearance of glucose in blood. While this mechanism of action is widely understood and exploited, it carries a potential risk of causing significant carbohydrate malabsorption(Reference Khwaja and Arunagirinathan12), which would typically be marked by rises in breath hydrogen and gastrointestinal discomfort.
We have recently reported a series of studies which confirmed and replicated the efficacy and tolerability of a specific, well-characterised mulberry fruit extract (MFE) for reducing the PPG and PPI responses to rice meals(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) . In that research, we showed that a dose as low as 0·37 g MFE containing about 2 mg DNJ significantly reduced the PPG and PPI response to about 50 g available carbohydrate in rice meals, with no indications of carbohydrate malabsorption or intolerance. Subsequent research confirmed that MFE reduces the rate but not amount of glucose absorption, with no significant effects on glucose disposal or endogenous production(Reference Boers, van Dijk and Duchateau15).
MFE has a number of attractive features as a candidate food ingredient: mulberry fruit has a long history of safe consumption, the extract has acceptable sensory attributes, and the proposed active component DNJ shows good chemical and thermal stability(Reference Yoshihashi, Do and Tungtrakul16,Reference Gao, Zheng and Wang17) . Almost all other prior related research on mulberry for PPG-lowering effects has used mulberry leaf extracts, typically at doses containing at least 2–10 times more DNJ than the lowest effective dose in our previous studies with MFE(Reference Phimarn, Wichaiyo and Silpsavikul8,Reference Jeong, Jang and Kim10) . In some of the trials using mulberry leaf extracts, rises in breath hydrogen were reported, indicative of carbohydrate malabsorption(Reference Zhong, Furne and Levitt18–Reference Mudra, Ercan-Fang and Zhong20).
Most research using mulberry extracts has tested the glycaemic response to purified (oligo)saccharides or a range of foods with no clear characterisation of the carbohydrate component. Our previous research demonstrated the efficacy of MFE using both a rice porridge and boiled Sona Masoori (SM) (Sona Masuri, Sona Mahsuri) rice(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) . That rice type was initially selected because it is widely available, has grain characteristics in the middle range of common Indian varieties and has a medium/high glycaemic index of 72(Reference Shobana, Kokila and Lakshmipriya21). However, there is considerable inherent variation in the glycaemic and insulinemic responses to different rice types, mainly depending on starch characteristics and (post-harvest and consumer) processing(Reference Boers, Seijen ten Hoorn and Mela22,Reference Kaur, Ranawana and Henry23) . The present trial was therefore intended to assess the generalisability of the efficacy of a single dose of MFE for reducing acute PPG and PPI responses, by testing this in healthy Indian participants with four rice types selected to broadly reflect the naturally occurring range of starch-related characteristics in rices commonly consumed globally.
Materials and methods
General
The primary objective was to assess whether the addition of MFE to four different rice varieties has a statistically significant effect (as percent change relative to the corresponding non-MFE control) on venous post-prandial blood glucose positive incremental AUC during the 2 h after study product intake (PPG; +iAUC2 h). The secondary objectives were to test the corresponding percent change in post-prandial insulin responses (PPI; tAUC2 h) and tolerance as reflected in self-reported gastrointestinal symptoms. The effects on PPG and PPI responses over 3 h (percent changes in +iAUC3 h and tAUC3 h, respectively) were included as exploratory measures. The clinical phase was executed between 5 December 2013 and 3 January 2014 at Lambda Therapeutics Research Ltd (LTRL), India. The sponsoring company provided study materials but had no part in the study execution, participant contact or outcome measurement and recording.
Ethical approval
The trial was conducted in compliance with the Declaration of Helsinki, and the protocol and informed consent forms were approved by the Independent Ethics Committee – Aditya (Ahmedabad, India; Project 578-13, Protocol FDS-NAA-1373) on 2 November 2013.
Participants and allocation to treatments
Potential participants aged 20–49 years were recruited from a database of healthy volunteers at LTRL. To reduce the likelihood of including individuals with (undiagnosed) impaired glucose tolerance or diabetes, participants had to be in a normal range of BMI (≥ 18·5 and < 25 kg/m2) and fasting blood glucose (> 3·4 and < 6·1 mmol/l) (24,25) . All participants were native to the area of the test location, but there was no specification or collection of data on ethnicity. Potential participants were screened in two sessions prior to the start of the intervention. The full inclusion and exclusion criteria were identical to those used in our previous research(Reference Mela, Cao and Govindaiah14) and listed in online Supplementary Table S1. All participants signed written informed consent followed by a verbal and written explanation of the study before initiating any protocol-specific procedures. Each participant was given opportunity to inquire about details of the study and was informed of their right to withdraw from the study at any time. Instructions and self-report interviews and data collection were all undertaken in the native language of participants.
One hundred twenty male and female participants were planned for and randomised into the study. Using a balanced incomplete block design and computer-generated sequences and allocations, participants were randomly allocated to a treatment order in which they each received four test products over 4 weeks (two out of four rice types, with and without MFE). Individuals who dropped out before the first treatment were replaced, while those who dropped out after participating in any of the treatments were not replaced. When a participant decided to withdraw, or failed to attend a session, efforts were made to perform all planned assessments and record the reasons.
Characterisation, selection and preparation of test products
The main factors responsible for variation in PPG responses to different rice types are the cultivar, amylose content, and post-harvest processing and consumer preparation(Reference Boers, Seijen ten Hoorn and Mela22). In order to identify a limited set of rice types to reflect the range of potential variation in rices, measurements were made of relevant physical and chemical properties of a wide selection of rices commercially available in India and the Netherlands (online Supplementary Table S2 and text). Statistical analyses based on these data showed that SM, Bora Saul (BS), Gobindobogh (Gb) and Banskati (Bn) segregated into different clusters (online Supplementary Fig. S1) and were also well separated along the two axes of a multidimensional space describing most of the variability. Therefore, the BS, Gb and Bn rice types were selected for use in clinical testing, in addition to the SM variety previously used. The main characteristics of these are shown in Table 1.
Table 1. Selected characteristics of rice types used in this study: description (cultivar, post-harvest processing), grain size and shape(35), carbohydrate content and percent amylose, and percent rapidly digestible starch (RDS), slowly digestible starch (SDS), and resistant starch (RS) analysis from an in vitro digestion model(Reference Boers, MacAulay and Murray28)
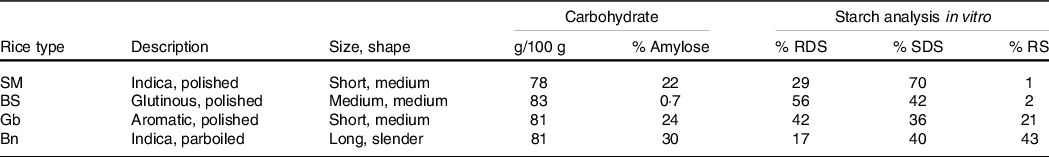
Each test meal consisted of a serving of boiled rice with 0 or 0·37 g of MFE added. Each single serving of rice was prepared using 64, 60, 62 or 62 g of the raw SM, BS, Gb or Bn rice, respectively, the amounts containing about 50 g of available carbohydrate (defined as total carbohydrate excluding fibre). The complete macronutrient composition per serving of each rice type is given in online Supplementary Table S3. To achieve identical total volumes, carbohydrate contents and hydration of the final cooked rice as served, slightly different rice weights and water additions were needed, depending on carbohydrate content, the physical characteristics and cooking methods for each rice type. The SM, BS, Gb or Bn rices were prepared by the addition of 140, 130 (100 from pre-soaking), 140 or 180 ml water, respectively, and then cooked in an automatic rice cooker, yielding portions of about 190 gm cooked rice of each type. The rice was removed after 30 min and allowed to cool for 5 min before stirring in the contents of pre-weighed, individually coded alumnum sachets containing either the control (1·0 g mannitol) or MFE (0·37 g MFE and 0·63 g mannitol). Participants and staff serving the rice were blinded to the presence or absence of MFE, which at this dose has negligible effects on sensory attributes.
To ensure similar final weights (about 550 g in total) and total water contents (about 490 ml) of the different meals, participants consumed the cooked SM, BS, Gb and Bn rice with 350, 360, 350 or 310 ml water, respectively. The rice with water meals were consumed within a timed 15-min period. If any individual was not able to finish within 15 min, the time (t) = 15 min blood sample was taken and they finished consuming the test product immediately after blood sampling. If any individual was unable to consume the full quantity of the test product within 30 min, they would be excluded from the study. Participants were not allowed to consume any food during the 3 h after test meals when blood samples were being collected and were provided with lunch afterwards. They were not allowed to drink water 1 h prior to test meals but thereafter were allowed to consume a maximum additional 500 ml of water until blood sampling was completed.
Data and sample collection
The night before each test day, participants stayed at the test facility and were fed a standard meal about 12 h before the baseline (fasting) blood samples. Data and sample collection procedures were identical to our previous research(Reference Mela, Cao and Govindaiah14) and are detailed in the online Supplementary Material. In brief, blood samples were collected via an intravenous indwelling cannula. Two baseline blood samples were collected in a period of 15 min before the start of test product ingestion (time = 0 min), and subsequent samples were collected 15, 30, 45, 60, 90, 120 and 180 min after eating commenced.
A paper-based questionnaire for gastrointestinal discomfort was completed by participants before each blood sampling and at 240 ± 5 min after eating commenced. Intensity of nausea, flatulence, bloating and pain were each rated as ‘none’ (=0), ‘mild’ (=1), ‘moderate‘ (=2) or ‘severe’ (=3).
Source and characterisation of MFE
The MFE (batch No. MF-DC-KQ-111207 Draco Natural Products Inc., San Jose CA, USA) contained 0·5 % (w/w) of DNJ (about 1·85 mg DNJ per 0·37 g MFE used per serving). We previously tested and confirmed the DNJ content and the in vitro bioactivity of this specific source and batch of MFE for α-glucosidase inhibition, as well as the efficacy of this dose for reducing PPG and PPI(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) .
Analytical procedures
All analyses were carried out by LTRL at the clinic test facility, using an enzymatic-photometric method for plasma glucose and immunoassay for serum insulin, following procedures used in our previous studies(Reference Mela, Cao and Govindaiah14), and detailed in the online Supplementary Material.
Statistical analyses
The study was powered for 80 % of power with an overall error rate of 0·20 for the primary outcome, percent change in PPG +iAUC2 h. The sample size was based on a 20 % reduction, using mean and variance estimates taken from our previous research with a similar population(Reference Mela, Cao and Govindaiah14). This resulted in a target sample size of 120 participants.
Statistical analyses were carried out according to a pre-specified plan. No interim analyses were planned or performed, and treatment assignments were revealed only after blind review of the data following the clinical data collection phase. The blind review was undertaken by the principle investigator, statistician, and principle sponsor contact, and the treatment code was broken only after a hard lock of the data was agreed following this blind review.
Due to hemolysis, a number of samples could not be used for the insulin analysis. For these samples, values were updated in the database by the statistician using the value from other treatment periods for that individual (for baseline values) or for single missing value a linear interpolation of neighbouring time points (t = 15 to t = 60 min), log-linear interpolation (for t = 90 and t = 120 min) or log-linear extrapolation (t = 180 min) using three insulin values in window t = 60 to t = 180 min. When there were two or more hemolytic samples per treatment, the AUC was excluded from the analysis.
The primary end point was tested using a linear mixed model of the form Log(+iAUC2 h) = sex + baseline + subject_baseline + weight + visit + treatment + error, where baseline is the mean baseline value for that visit for that individual and subject_baseline is the mean baseline score over all visits for the individual. This latter term is included to avoid possible bias in the estimates of the product effect due to the use of a mixed model and the inclusion of a different baseline value at each visit. Visit is a categorical variable for the visit number (1 to 4). Treatment is a categorical variable representing control rice or rice with added MFE. Sex and weight were covariates as stipulated in the protocol. There was no a priori hypothesis for nor intent to specifically test for the influence of those factors. The error terms were assumed to be normally distributed. An analogous approach was used for the secondary and exploratory glucose and insulin outcomes. For the comparison of the individual or combined rice types to their respective controls, P-values were determined using Dunnett’s method, which is identical to a paired two-sample t test for this study design. The criterion for statistical significance was a two-sided P ≤ 0·05.
Frequencies of individuals who had a gastrointestinal discomfort score of two or three after study product intake were tabulated. The proportion of participants giving a score of 2 or 3 were compared between active and reference treatments using a logistic regression model allowing for basal level as a covariate. This was done for each symptom separately.
Availability of data and materials
All materials were commercially available at the time the research was conducted. Data from this study may be available for research purposes from author HMB on reasonable request, noting that some caveats may apply.
Results
Analysis population
Of 120 individuals entering the trial, 111 completed all allocated treatments and 9 discontinued prematurely, missing one or more test sessions (see Consolidated Standards of Reporting Trials (CONSORT) subject flow diagram, online Supplementary Fig. S2). The participants had a mean (sd, range) age of 37 (8·50, 20–49) year and BMI of 22·3 (2·19, 18·5–24·9) kg/m2. There were only trivial differences in the characteristics of the subgroups of individuals providing data for each treatment (online Supplementary Table S4). The intention-to-treat and per-protocol populations for the primary outcome were the same, and usable data from all 120 participants were included in the analyses.
Primary and secondary outcomes
The response data for the primary outcome measure of percent change in PPG +iAUC2 h are presented in Table 2.
Table 2. Baseline-adjusted values for plasma glucose response over 2 h to the addition of mulberry fruit extract (MFE) to Sona Masoori (SM), Bora Saul (BS), Gobindobogh (Gb) and Banskati (Bn) rice types, and all types combined
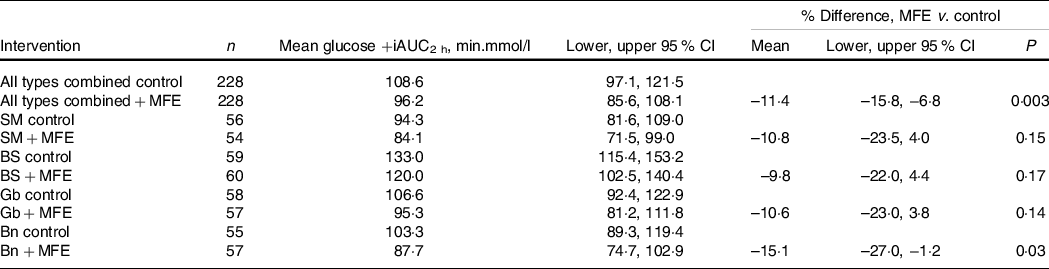
Addition of MFE produced similar mean reductions in PPG in all rice types. This effect was statistically significant for all rice types together and for rice type Bn when these were considered separately. The mean glucose response profiles for each rice type, with and without addition of MFE, are illustrated in online Supplementary Fig. S3.
The response data for the secondary outcome measure of percent change in PPI tAUC2 h are presented in Table 3.
Table 3. Baseline-adjusted values for serum insulin response over 2 h to the addition of mulberry fruit extract (MFE) to Sona Masoori (SM), Bora Saul (BS), Gobindobogh (Gb) and Banskati (Bn) rice types, and all types combined
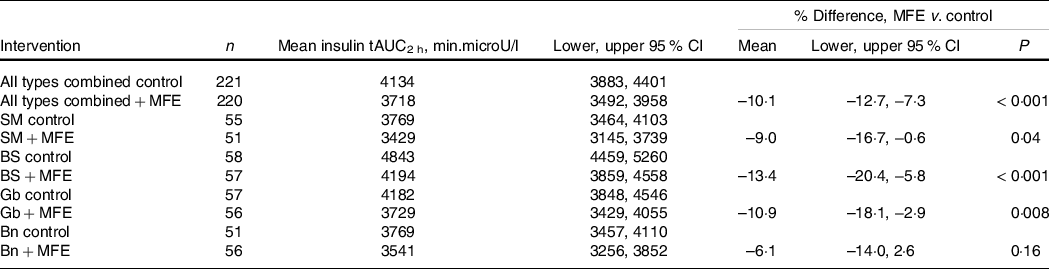
Addition of MFE produced absolute reductions in PPI in all rice types, with variable effect sizes. This effect was statistically significant for all rice types together, and for all rice types except Bn when these were considered separately. The mean insulin response profiles for each rice type, with and without addition of MFE, are illustrated in online Supplementary Fig. S4.
There were no symptoms of bloating, flatulence, nausea or pain reported in the gastrointestinal discomfort questionnaire following consumption of any of the test products.
Exploratory outcomes
The response data for PPG +iAUC3 h are presented in online Supplementary Table S5. The addition of MFE produced similar mean reductions in PPG over 3 h in all rice types. This effect was statistically significant for all rice types together, but not for any specific rice type when considered separately.
The response data for the PPI tAUC3 h are presented in online Supplementary Table S6. The addition of MFE produced reductions in PPI over 3 h that were statistically significant for all rice types together and for rice types BS and Gb when considered separately.
Discussion
These results support the efficacy and tolerability of MFE for reducing the PPG and PPI responses to rices in general, although there was variation in the absolute effect sizes and statistical significance of these when considered for individual specific rice types. The study was not designed or powered for comparisons of each rice type to each other; however, the substantial overlap of the 95 % CI between each rice type for percent change in 2-h PPG (79–99 %) and PPI (52–88 %) indicates that such analyses would not reject the null hypotheses of equality of the means (online Supplementary Tables S7 and S8) (Reference Cumming and Finch26,Reference Austin and Hux27) . Consistent with our previous research, in which we also tested higher doses of MFE, there were no indications of gastrointestinal intolerance.
No previous study has assessed the efficacy of mulberry extracts in relation to the natural range of variation in the characteristics of widely consumed food starch sources such as rice. In our previous trials using 0·37 g of MFE added to SM rice, with similar procedures and test populations, we observed higher mean percentage reductions in the 2 h PPG and PPI than those observed here(Reference Mela, Cao and Govindaiah14). In the present trial, there was also a smaller mean absolute PPG and PPI responses to SM rice itself (i.e. response to the control without MFE). This study was powered for a 20 % reduction in PPG for the individual rice types based on our previous research, and the smaller MFE effect sizes for PPG in SM (and BS and Gb) rice seen here did not meet the criterion for statistical significance. Nevertheless, there was substantial overlap in the 95 % CI for the effect of 0·37 g MFE in SM rice observed here (–23·5 to 4·0) and in previous trials (–37·1 to −5·6 and −29·0 to −10·8) (Reference Mela, Cao and Govindaiah14).
The dose of MFE used here (0·37 g MFE, containing 1·85 mg DNJ) is probably close to the lower limit of efficacy for this extract. We previously reported that a dose one natural log-step lower (0·12 mg MFE containing about 0·6 mg DNJ) gave a PPG response in SM rice similar (–12·1 %) to that observed here(Reference Mela, Cao and Govindaiah14). In the present study, the assessment of all rice types combined provides the most robust estimate of the effect of this dose MFE on glycaemic responses to rices in general. More recently, we have reported a mean decrease in PPG of 11·4 % (95 % CI –7·3, –26·3 %) when 0·75 g MFE (about 3·7 mg DNJ) was added to a meal containing about 50 g of starch as wheat porridge(Reference Boers, van Dijk and Duchateau15). Given these results and the possible effects of natural variation in food starch characteristics, that higher MFE (and DNJ) dose may be advised to ensure greater and more reliably consistent efficacy.
In our previous studies, MFE-induced reductions in PPG were generally also paralleled by reductions in PPI(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) . In contrast, the largest reduction in PPG here was observed for the rice type (Bn) with the smallest reduction in PPI. The mean PPG response was also somewhat lower for the SM rice control than Gb and Bn, although the latter types have a higher percent amylose content and a higher resistant starch content as determined by the in vitro glucose release assays. Although the in vitro starch measures were used to characterise and differentiate the rices used here, the control PPG values strongly suggest that the fraction designated ‘resistant starch’ was not resistant to proximal intestine digestion in vivo and contributed to the acute glycaemic responses. We previously also found that the individual parameters derived from in vitro digestion had inconsistent relationships with PPG responses, which were better predicted by models based on measures of the rate of digestion(Reference Boers, MacAulay and Murray28). In general, the amylose and resistant starch content of rices were both positively and negatively associated with the effect size of MFE for PPG- and PPI-lowering effects, respectively. Overall, however, the present results do not provide a basis for drawing firm conclusions on these relationships, which could be of interest to explore further in an experiment specifically designed for that purpose. It is also possible that starch characteristics influence the rates of passage and uptake of DNJ, and measurement of circulating DNJ levels could therefore also provide further mechanistic insight.
This research gave primary consideration to PPG and PPI responses in the 2-h post-prandial time frame. The duration and timings of blood sampling here have been widely used and recommended as the primary measurement period in clinical testing of glycaemic control and nutrition interventions in healthy individuals, as well as for the glycaemic index of foods(Reference Brouns, Bjorck and Frayn29,Reference Martini, Biasini and Rossi30) . Our previous research had shown that, even with addition of higher doses of MFE, this time frame captured the largest and most physiologically relevant part of the glycaemic excursion, including the peak, with mean values already approaching baseline by 120 min(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) . Subsequent research furthermore confirmed that the delay in rate of absorption attributable to MFE is modest (about 10 %), even at a dose two times greater than used here(Reference Boers, van Dijk and Duchateau15). This may reflect the fact that DNJ itself is highly bioavailable and fairly rapidly absorbed, so its presence and effects on carbohydrate digestion in the gut are of relatively short duration(Reference Parida, Takasu and Nakagawa31). However, for novel functional ingredients or doses with greater effects on gastrointestinal transit times, or a more sustained presence in the gut, it may be appropriate to focus on the 3-h or even longer post-prandial periods and to include more blood collection time points to capture the response profiles with sufficient resolution.
Excessive inhibition of carbohydrate digestion would carry risks of malabsorption and poor gastrointestinal tolerance. We previously found no indications of malabsorption or intolerance using doses of MFE up to 1·5 g with boiled SM rice(Reference Mela, Cao and Dobriyal13,Reference Mela, Cao and Govindaiah14) , and subsequent research confirmed that MFE reduces the rate but not amount of glucose uptake(Reference Boers, van Dijk and Duchateau15). Given unknown potential interactions between MFE and rice type, self-reported gastrointestinal symptoms were also included in this trial, although no indications of any adverse effects were observed.
There are a number of limitations to this research. We matched rice quantities based on available carbohydrate determined from the reported proximate composition. However, given differences in measured amylose and resistant starch contents, it is likely that the actual amount of available carbohydrate may have differed amongst the rices. The trial was carried out in a single test site with a healthy weight, normoglycaemic population. Other independent or multi-site verification of the effects of MFE, also in other populations, may be of interest. We sampled venous blood, to assure we could get the necessary quantities of blood whilst minimising discomfort and risk to participants. While this is unlikely to generate a directional bias favouring any specific treatment, it is likely to have resulted in lower and more variable glucose values than, for example, capillary blood(Reference Brouns, Bjorck and Frayn29), and thus perhaps also lower sensitivity in general to the effects of MFE. The study was also not designed or powered to consider the relative effects on insulin release and action, so further research could place more focus on this, including related measures such as C-peptide. Future research may also consider effects beyond this acute time frame and the potential impact of sustained use of MFE on glycaemic responses or other markers of glycaemic control.
Overall, although there were some differences in mean effect sizes from our previous results using MFE, the study reinforces evidence for the general efficacy of mulberry extracts for reducing PPG responses. This was shown in a product format relevant to many consumers globally, accompanied by desirable outcomes for PPI and tolerance. In addition to evidence from our previously published trials with MFE(Reference Mela, Cao and Dobriyal13–Reference Boers, van Dijk and Duchateau15), data from this study are in line with other published data showing reductions of PPG in the range of 10–30 % following co-ingestion of various carbohydrate loads, including rice, with mulberry leaf extracts containing between 6 and 12 mg DNJ(Reference Lown, Fuller and Lightowler9,Reference Nakamura, Nakamura and Oku32–Reference Asai, Nakagawa and Higuchi34) . While direct comparisons are made with caution due to differences in the study designs, extracts and populations, the literature overall indicates that mulberry extracts with substantiated PPG-lowering effects are likely to be efficacious when consumed with a wide range of difference starch sources.
Conclusions
Addition of 0·37 g MFE reduced the PPG and PPI response to rice in general, with qualitatively modest variation in the mean effect sizes for specific rice types. A higher dose may be required to assure a consistent efficacy across the natural variation in dietary starch characteristics.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523001319
Acknowledgements
The authors acknowledge the important support and contributions to this research from a number of current and former Unilever colleagues and the staff of Lambda Therapeutics Research Ltd.
Funding for this research came entirely from Unilever, a food and beverage manufacturer, and Unilever employees were primarily responsible for the study design, material selection and characterisation, statistical analyses and preparation of the manuscript. Unilever employees had no part in the intervention study execution or participant contact or data collection and were blind to treatment codes until these were revealed after the data were unlocked. Participant recruitment and contact, trial execution, clinical chemical analyses, and all raw data collection and recording were carried out by Lambda Therapeutics Research Ltd acting as a contracted research organisation, independent of any direct Unilever involvement other than financial support and the sourcing and provision of test products.
All authors contributed to the conceptualisation, data interpretation and writing, and have approved the manuscript. T. K. was additionally responsible for study management and liaison with the clinical test site, R. K. and J. W. M. S. ten H. developed and carried out analytical procedures on the test materials, and H. H. was responsible for the statistical design and analyses.
H. M. B., T. K. and R. K. are employees of Unilever, the sponsor of the study and a manufacturer of carbohydrate-containing foods. D. J. M., H. H. and J. W. M. S. ten H. were employees of Unilever at the time the research was carried out but have no current affiliation with the company. The authors declare no other conflicts of interest related to the topic of this research.






