Introduction
Lear’s Macaw Anodorhynchus leari is endemic to the ‘Caatinga’ biome and considered as globally “Endangered” by IUCN (BirdLife International 2012). Its distribution is restricted to a small area in the north-east of Bahia state, Brazil and is concentrated in two protected areas, Raso da Catarina Ecological Station (RCES) and Canudos Biological Station (CBS), where the whole population nests and roosts communally on sandstone cliffs (Menezes et al. Reference Menezes, Araujo, Nascimento, Rego, Paiva, Serafim, Dellabella and Lima2006). However, the birds perform daily movements from these sites to forage in neighboring unprotected areas (Brandt and Machado Reference Brandt and Machado1990, Santos-Neto and Camandaroba Reference Santos-Neto and Camandaroba2008, Silva-Neto et al. Reference Silva-Neto, Souza and Santos-Neto2012). The organizations CEMAVE/ICMBio and Biodiversitas Foundation have assessed changes in the population size of Lear’s Macaw through the post-breeding monitoring of the two communal roosts since 1998, with standardised annual censuses conducted since 2004 (IBAMA 2006). In recent years, a population increase from 570 in 2004 (IBAMA 2006) to 1,125 individuals in 2010 has been observed (Lugarini et al. Reference Lugarini, Barbosa and Oliveira2012). Although the long-term population increase is partially explained by a higher monitoring effort, there is a consensus that the species has been recovering in numbers over the past few decades (BirdLife International 2012). Due to these increases in overall population size, BirdLife International (2012) downgraded the threat category of the species from “Critically Endangered” (CR) to “Endangered” (EN) in the 2009 IUCN Red List of Threatened Species, based on the estimate of more than 250 mature individuals capable of reproduction (excluding those that will not produce new recruits; IUCN 2013).
Estimating the number of mature breeding individuals is challenging for many species for which accurate population biology information is not available. Therefore, this number is often obtained by applying an assumed proportion of individuals that are mature to the estimated whole population size, an approach that often leads to gross overestimates of number of mature individuals (IUCN 2013). Especially in the case of long-lived species with deferred maturity, as in Lear’s Macaw (Young et al. Reference Young, Hobson, Lackey and Wright2012), both the number of mature individuals and the breeding fraction may be much smaller than the non-breeding part of the population (Kenward et al. Reference Kenward, Walls, Hodder, Pahkala, Freeman and Simpson2000, Negro Reference Negro2011). There is however a marked scarcity of information on breeding to non-breeding ratios in birds, which may undermine the design of proper conservation strategies, since these population fractions are often exposed to different threats related to their different use of space and resources (Penteriani et al. Reference Penteriani, Ferrer and Delgado2011).
The overall population size of Lear’s Macaw is reasonably well known. However, there is no information on the proportion of breeding birds, and the fact that sub-adults may form pairs and behave like nesting birds for a number of years before they actually breed makes this estimation difficult (BirdLife International 2012). In the same way, most aspects of the breeding biology of the species are virtually unknown in the wild (Juniper and Parr Reference Juniper and Parr2010). A study of the reproductive success of Lear’s Macaw is therefore essential to design effective conservation actions (BirdLife International 2012) and to understand the population ecology of the species (e.g. Carrete et al. Reference Carrete, Donázar and Margalida2006a). This will allow a better assessment of the threats the species is facing and allows prediction of population growth and extinction risk in the long-term through population viability analyses (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008).
Given the importance of knowing the proportion of the population that is breeding and its breeding success, the Management Plan for the Conservation of Lear’s Macaw considers the assessment of its breeding population size and breeding parameters as high priorities (IBAMA 2006, Lugarini et al. Reference Lugarini, Barbosa and Oliveira2012). Therefore, the aim of this study was to estimate the number of breeding pairs and main breeding parameters for a better knowledge of the population ecology, conservation and monitoring needs of the species.
Methods
Study area
Surveys were conducted at the two breeding sites known for the species: Raso da Catarina Ecological Station (RCES; 09°52’S, 38°38’W), and Canudos Biological Station (CBS; 09°57’ S, 38°59’W), known as Serra Branca and Toca Velha, respectively. Both are composed of sedimentary rock, characterised by the alternation of calcareous sandstone outcrops and delimited by intermittent streams (Oliveira and Chaves Reference Oliveira and Chaves2010). The areas are inserted in the Caatinga biome in the ecoregion of Raso da Catarina, where elevation reaches 800 m and temperature varies between 15 and 45°C. Climate is semi-arid, rainfall being torrential and irregular, with annual averages between 450-650 mm concentrated between December and July (Velloso et al. 2002).
Nest identification
Lear’s Macaw breeds exclusively in pre-existing cavities of calcareous sandstone cliffs. Breeding activities start in September–October with the exploration of cavities and last until April when the last chicks leave their nests (IBAMA 2006). Nest searches were conducted by walking along the intermittent rivers located at the base of the cliffs looking for potential nest cavities and their exploration by macaw pairs (Renton and Brightsmith Reference Renton and Brightsmith2009). In order to identify the cavities actually occupied by breeding pairs for nesting (i.e. active nests), direct observation was undertaken for an average of 12 hr/day at each site, during three consecutive days, twice a month, from early January to late June during two breeding seasons (2009 and 2010). Nests are most easily identified in January as this coincides with Lear’s Macaws remaining for longer periods inside their nests as they sit on eggs and brood very young chicks (Pacífico Reference Pacífico2011). Breeding sites at RCES were monitored by E.A.B. and K.O., while E.C.P. and T.F., together with field assistants, monitored the breeding sites at CBS. Similarly to Schneider et al. (Reference Schneider, Serbena and Guedes2006) and Renton and Brightsmith (Reference Renton and Brightsmith2009), active nests were identified based on continued observation of the following behaviour for three consecutive days: (1) the pair remained inside or in the entrance of the cavity; (2) in the absence of the mate, one of the individuals remained inside the cavity; and (3) mate-feeding was performed in the entrance of the cavity. The cliffs were photographed to aid location of both potential and active nest sites in each breeding season. Observations were conducted from distant points (> 100 m) to avoid disturbance (Schneider et al. Reference Schneider, Serbena and Guedes2006). This observation protocol allowed us to estimate the breeding population size and the breeding parameters of a subsample of nests (see below).
Breeding parameters
Breeding parameters were obtained from those nests (focal nests) where it was possible to see the number of fledglings observed in the nest entrance (Renton and Brightsmith Reference Renton and Brightsmith2009). The number and distribution of nests varied slightly between 2009 and 2010. Therefore, 34 focal nests (24 at RCES and 10 at CBS) were monitored to estimate breeding parameters in 2009, while 41 focal nests were monitored in 2010 (29 at RCES and 12 at CBS). At CBS we were also able to determine the breeding output by combining observations with direct nest inspections of all focal nests, using abseiling techniques in the sandstone cliffs, three to five times until chicks were close to fledge. These additional inspections confirmed that the breeding parameters obtained by observation were valid (Pacífico Reference Pacífico2011). The observation protocol used for nest identification was extended to assess breeding output, but with increased efforts between March and June (c.6 hr of observation/researcher/day) coinciding with the period in which nestlings are first sighted at the entrance of nest cavities (between the 12th and 15th weeks after hatching). This is a good metric to determine breeding success as nestlings they end to spend most of the daylight hours at the entrance until they are able to fly (Pacífico Reference Pacífico2011). During this period nestlings were easily identified, as they have smaller and paler lappets bordering the lower mandible than adults (Brandt and Machado Reference Brandt and Machado1990, Juniper and Parr Reference Juniper and Parr2010). We defined breeding success as the percentage of pairs producing at least one fledgling, brood size as the average number of fledglings per successful pair, and productivity as the average number of fledglings per pair that attempt to breed (i.e. that occupied a nest).
Breeding population size
The number of breeding pairs in the population was estimated as the total number of nests occupied in the 2010 breeding season, pooling focal (confirmed) and probable nests. Probable nests were defined as those nests where intense activity by macaw pairs was observed throughout the entire breeding season, but given the difficulty of monitoring them from suitable observation points, it was not possible to determine breeding parameters.
Statistical analyses
Differences in breeding parameters between breeding sites (CBS and RCES) and years (2009 and 2010) were assessed through Generalized Linear Models, fitting site, year and their interaction as fixed effects. The binomial distribution and logit link function were used to analyse breeding success using nesting attempts as sampling units (0: unsuccessful, 1: successful), while the Poisson distribution and log link function were used for productivity (number of fledglings: 0–3) and brood size (number of fledglings: 1–3). Estimated marginal means (i.e. the mean response for each factor level adjusted for any other variables included in the model) were provided for significant effects. All analyses were performed using SPSS 15.0.
Results
Breeding parameters
As expected, fully-grown Lear’s Macaw nestlings were first observed at the entrance of their nests from March to May, showing a peak in April (65.6% in 2009 and 63.9% in 2010, Figure 1), indicating that most nestlings fledged during this month. Overall breeding success was 80% of the breeding attempts (n = 75) recorded in focal nests. Productivity averaged 1.33 + 0.86 SD fledglings per breeding attempt (n = 75, Figure 2), while brood size averaged 1.67 + 0.60 SD fledglings per successful nest (n = 60). Successful nests fledged two chicks (53.3%) one chick (40%) or three chicks (6.7%).

Figure 1. Percentage of full-grown nestling Lear’s Macaws observed between March and May at the entrance of the nest cavities.
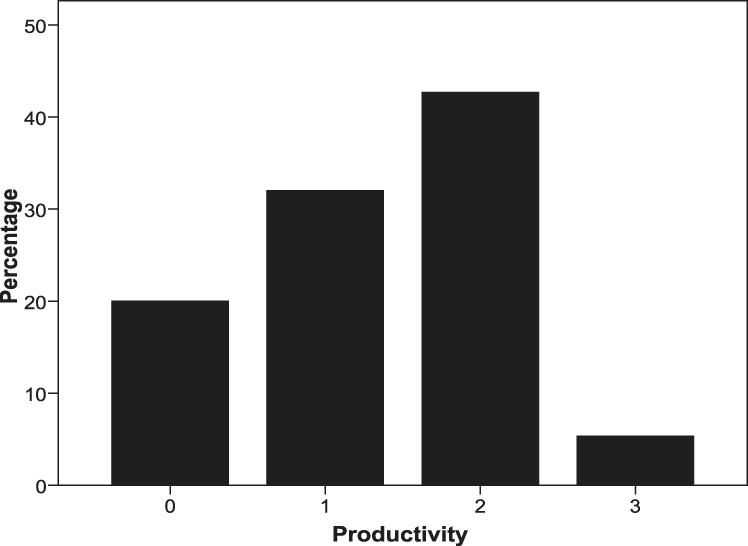
Figure 2. Productivity (number of fledglings per breeding attempt, n = 75) of Lear’s Macaws in 2009–2010. Raw data are depicted as the percentage of cases with 0–3 fledglings.
Breeding success did not differ between sites (Wald’s ᵡ2 1 = 0.069, P = 0.79) but was higher in 2010 (estimated marginal mean: 88% + 0.05 SE, Wald’s 95% CI: 0.78–0.98) than in 2009 (estimated marginal mean: 71% + 0.08 SE, Wald’ 95% CI: 0.55–0.88, Wald’s ᵡ2 1 = 3.27, P = 0.07; Figure 3). Similarly, productivity did not differ between sites (Wald’s ᵡ2 1 = 0.64, P = 0.42) but was higher in 2010 (estimated marginal mean: 1.55 + 0.14 SE) than in 2009 (estimated marginal mean: 1.15 + 0.14 SE) (Wald’s ᵡ2 1 = 3.79, P = 0.05). There was no significant interaction of site x year for breeding success (Wald’s ᵡ2 1 = 0.87, P = 0.35) nor productivity (Wald’s ᵡ2 1 = 1.13, P = 0.29). Brood size also did not vary between sites (Wald’s ᵡ2 1 = 1.14, P = 0.28) and between years (Wald’s ᵡ2 1 = 0.54, P = 0.46, interaction site x year Wald’s ᵡ2 1 = 0.21, P = 0.65; Figure 3).

Figure 3. Breeding success (percentage of successful -0- and unsuccessful -1- nests), productivity (percentage of nests raising 0-3 fledglings), and brood size (percentage of successful nests raising 1-3 fledglings) of Lear’s Macaws in relation to breeding site and year.
Breeding population size
In 2010, 20 probable nests were recorded at CBS and another 53 at RCES but could not be properly monitored because of the difficult visibility. These 73 probable nests together with the 41 monitored focal nests leads to the estimate of 114 breeding pairs (228 breeding individuals). Lugarini et al. (Reference Lugarini, Barbosa and Oliveira2012) censused a total of 1,125 Lear’s Macaws in 2010. Therefore, the 228 breeding individuals represented 20.3% of the population.
Discussion
Breeding parameters
The breeding success of Lear’s Macaw (80%) was much higher than in three species of the genus Ara (48%, Blue-and-yellow Ara ararauna, Green-winged A. chloropterus, and Scarlet Macaw A. macao) in lowland Amazonian forests (Renton and Brightsmith Reference Renton and Brightsmith2009) but only slightly higher than that of the Blue-and-yellow Macaw (72%) in Cerrado savannah (Bianchi Reference Bianchi1998). Estimates of reproductive success of the Hyacinth Macaw Anodorhynchus hyacinthinus in the northern (Antas et al. Reference Antas, Carrara, Yabe, Ubaid, Oliveira-Júnior, Vasques and Ferreira2010) and southern Pantanal (Guedes Reference Guedes2009) based on egg-laying records also yielded lower values (74% and 73%, respectively). Differences between species may be partially related to the sampling size or different methodologies applied (observations of nest occupation versus egg-laying recording though direct nest inspections). In Lear’s Macaw, however, breeding parameter estimates were consistent when obtained by nest inspections and observations at distance (Pacífico Reference Pacífico2011).
The average productivity (1.33) and brood size (1.67) of Lear’s Macaw indicate that each breeding pair normally produces 1–2 chicks, contrasting with its congener Hyacinth Macaw that usually rears only one chick (Guedes Reference Guedes1993, Reference Guedes2009). In other macaw species of genus Ara, however, successful broods of two or even three chicks are not rare, but average productivity (0.6–0.94) is also smaller than in Lear’s Macaw (Bianchi Reference Bianchi1998, Bravo and Brightsmith Reference Bravo and Brightsmith2006, Renton and Brightsmith Reference Renton and Brightsmith2009).
The species cited above, with the exception of Lear’s Macaw, nest mostly in tree holes. Given the higher breeding parameters of Lear’s Macaws, it is worth questioning whether nest substrate (tree holes vs. cliff cavities) may play a role in the breeding success of the species. Future studies of cliff nesting Ara macaws would be useful in addressing this issue. The availability of tree-holes is known to be a limiting factor in the density of parrot populations (Cockle et al. Reference Cockle, Martin and Drever2010). Forest removal, logging and natural or human-made fires diminish cavity availability, especially for large macaws (Bravo and Brightsmith Reference Bravo and Brightsmith2006), and this limited availability may increase competition. Nest losses due to interspecific competition compromise the reproductive success of Hyacinth Macaws (Guedes Reference Guedes2009, Antas et al. Reference Antas, Carrara, Yabe, Ubaid, Oliveira-Júnior, Vasques and Ferreira2010), while the main cause of breeding failure seems to be clutch predation in this large macaw species (Pizo et al. Reference Pizo, Donatti, Guedes and Galetti2008, Antas et al. Reference Antas, Carrara, Yabe, Ubaid, Oliveira-Júnior, Vasques and Ferreira2010). The colonial cliff-nesting behavior of Lear’s Macaws, however, could reduce predation risk as has been suggested for the cliff-nesting Burrowing Parrot Cyanoliseus patagonus (Masello and Quillfeldt Reference Masello and Quillfeldt2002). This hypothesis could be further tested by comparing breeding parameters of some macaw species which breed both in tree-holes and cliffs (Abramson et al. 1995, Rojas et al. Reference Rojas, Yucra, Vera, Requejo and Tella2013), and could add insight into the evolutionary transition in the use of nesting substrates by parrots (Brightsmith Reference Brightsmith2005).
Breeding parameters of Lear’s Macaw did not vary between the two breeding sites. Renton and Brightsmith (Reference Renton and Brightsmith2009) also did not find variations in productivity among breeding sites of three large macaw species. However, both breeding success and productivity were somewhat larger in 2010 than in 2009. These differences could be related to seasonal and inter-year variability in food resources for the species. Santos-Neto and Camandaroba (Reference Santos-Neto and Camandaroba2008) were able to map the 37 biggest patches of licuri palm tree Syagrus coronata, which provide the main food item of Lear’s Macaw (Brandt and Machado Reference Brandt and Machado1990) around breeding sites. The average distance from breeding sites to these licuri palm patches was 49.5 km for CBS and 45.9 km for RCES (Santos-Neto and Camandaroba Reference Santos-Neto and Camandaroba2008). Moreover, palm patches are small and highly degraded by humans and goats and show a marked fruit seasonality influenced by rainfall (Rocha Reference Rocha2009). Lear’s Macaws are not strictly dependent on licuri nuts as at least five other wild fruits are part of its diet during the breeding season, and macaws regularly consume maize perhaps as a response to the scarcity of wild fruits (Brandt and Machado Reference Brandt and Machado1990, Silva-Neto et al. Reference Silva-Neto, Souza and Santos-Neto2012). In fact, the Caatinga dry forest has been continuously devastated and its conservation status has received little attention by Brazilian governments (Leal et al. Reference Leal, Silva, Tabarelli and Lacher2005). Further studies of the spatial and temporal availability of food resources, related to rainfall regimes, are therefore needed for a better understanding of the variability in breeding parameters and the conservation problems faced by the species.
Breeding population size
Non-breeding population fractions are often cryptic and more difficult to estimate than their breeding counterparts since the later are attached to breeding sites and are easier to monitor (Penteriani et al. Reference Penteriani, Ferrer and Delgado2011). In the case of Lear’s Macaw, however, both breeding and non-breeding groups use the same cliffs for roosting, thus making both parts of the population equally easy to monitor but increases the possibility of inflating breeding estimates based on total counts of individuals. Moreover, individuals close to maturity could mate and prospect nest cavities before reproducing, which could introduce an important error in the breeding population estimate (BirdLife International 2012). Renton and Brightsmith (Reference Renton and Brightsmith2009) observed that 25% of nest cavities inspected by Ara macaws during the breeding season did not result in active nests. However, the combination of observations at distance with nest inspections of focal nests indicated that the survey methodology used to identify breeding pairs of Lear’s Macaws was appropriate. Using our estimate of 114 pairs breeding in 2010, the proportion of breeding individuals was about 20% of the whole population in 2010. This is similar to the proportion estimated for healthy populations of several Ara species (10–20%; Munn Reference Munn, Beissinger and Snyder1992) and for the whole population of the globally Endangered Red-fronted Macaw Ara rubrogenys (16–33%, Tella et al. in press.). However, it is lower than in other long-lived species with deferred sexual maturity like the Common Buzzard Buteo buteo (40%; Kenward et al. Reference Kenward, Walls, Hodder, Pahkala, Freeman and Simpson2000), Red-billed Chough Pyrrhocorax pyrrhocorax (40–72%; Blanco et al. Reference Blanco, Pais, Fargallo, Potti, Lemus and Dávila2009), Bearded Vulture Gypaetus barbatus (56%; Gómez de Segura et al. 2012), Egyptian Vulture Neophron percnopterus (c.45%; J.A. Donázar pers. comm.) and 18 seabird species (30–73%; Warham Reference Warham1996).
Conservation and monitoring implications
A recent increase in breeding numbers of Lear’s Macaws may be logically inferred from the positive population trend of the species recorded in recent decades (BirdLife International 2012). However, there are several reasons for not blindly assuming past or future linear relationships between breeding and overall population size. An overall population increase could result from conservation actions (BirdLife International 2012) that could significantly increase breeding output and adult survival without increasing the number of breeding pairs. Breeding numbers of hole-nesting parrots can be limited by the quantity and quality of nesting sites (Cockle et al. Reference Cockle, Martin and Drever2010), thus breaking the assumed direct relationship between number of individuals and number of breeding pairs. This could explain the lower breeding to non-breeding population ratios in macaws compared with other long-lived species (see above).
Currently, nearly all Lear’s Macaws are concentrated at two breeding/roosting sites separated by just 38 km and there is a strong suggestion that individuals moving between these sites belong to a single population (Menezes et al. 2006). A small group located in 1995 in an unprotected area between the Campo Formoso and Sento Sé municipalities in Bahia, 230 km to the west (BirdLife International 2012), seems to have been nearly extirpated with only two individuals located in 2012, probably due to trapping for illegal trade (ICMBio unpubl. data). The reduction to a single population not only makes the species more vulnerable to stochastic processes but also to crowding effects when facing nesting habitat limitations. If the Lear’s Macaw population does not expand to distant, potential nesting sites, it is likely that the breeding population size will not increase after exceeding the carrying capacity in terms of nest-site availability despite further increases in overall population size. Other social factors may also limit the number breeding as, for example, the percentage of breeding Puerto Rican parrots Amazona vittata decreased with an increase in the total population size in absence of nest-site limitation or skewed sex ratios (Beissinger et al. Reference Beissinger, Wunderle, Meyers, Saether and Engen2008). On a more positive note, the proximity of communal roosts of non-breeding individuals to nesting sites may contribute to supply mate losses (Blanco and Tella Reference Blanco and Tella1999) and buffer local extinction processes (Carrete et al. Reference Carrete, Grande, Sánchez-Zapata, Donázar, Tella, Díaz-Delgado and Romo2007). However, the spatial overlap of breeders and non-breeders in isolated populations of birds may also reduce their population growth through density-dependent processes. Negative effects can arise when non-breeders compete for resources with breeders or interfere with their breeding activities (Carrete et al. Reference Carrete, Donázar and Margalida2006a, Reference Carrete, Donázar, Margalida and Bertran2006b, Blanco et al. Reference Blanco, Pais, Fargallo, Potti, Lemus and Dávila2009). In the case of Lear’s Macaw, the large non-breeding population competes with breeders in foraging areas and this could compromise their breeding condition and success, especially in years of food scarcity. Moreover, interference of non-breeders with breeding activities could also increase in an overcrowding situation, thus further reducing breeding performance (Renton Reference Renton2004, Carrete et al. Reference Carrete, Donázar and Margalida2006a).
The above uncertainties on future population projections for Lear’s Macaw call for the necessity of new monitoring and conservation efforts. Further monitoring must focus on the breeding fraction of the population and its breeding parameters, rather than solely on overall population size, as done so far, to fully assess changes in population dynamics, threats related to life-stage and the conservation status of the species in the long term. There is also the need to investigate the annual rates of juvenile and adult survival which, together with population and breeding parameter estimates, will allow the creation of population viability models (PVA) that ultimately would determine the conservation status and conservation action priorities for the species. This would require capture-mark-resighting work and, ideally, tagging birds for remote tracking, which would add valuable information on the causes and rates of mortality that can vary between the different population fractions (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008, Grande et al. Reference Grande, Serrano, Tavecchia, Carrete, Ceballos, Díaz-delgado, Tella and Donázar2009). Remote tracking would also provide data essential to determine the range movements by breeding and non-breeding individuals in relation to the spatial and seasonal changes in food resources (Tanferna et al. Reference Tanferna, López-Jiménez, Blas, Hiraldo and Sergio2013), as well as to investigate whether non-breeders could prospect distant, potential but still unknown nesting areas for the species. This would help to delineate protected areas, covering the most important foraging areas, and planning the geographical expansion of the species. If the species is not able to disperse naturally, it could result in an overcrowded population suffering from negative density-dependent effects in a relatively short time frame.
Acknowledgements
This work is part of joint efforts by partner institutions that for 20 years have developed strategies to conserve Lear’s Macaw. We are grateful to Fundação Biodiversitas, ICMBio – CEMAVE, Fundação Grupo O Boticário de Proteção a Natureza, CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, and World Parrots Trust (James D. Gilardi and André Becker Saidenberg). LFS thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). JLT thanks C. Keller for a short stay funded by INPA-CBIO (Brazil), which allowed the initiation of his participation in this project. We thank all collaborators and volunteers who participated in this work, especially Dan, Tito, João Carlos Nogueira, Dorivaldo Alves and Máximus Cardoso. We also thank the biologist Paloma Fernandes João, for aiding in observations, and Francisco Denes, Vagner Cavarzere, Neiva Guedes, Cristina Miyaki, Pedro F. Develey, and Donald J. Brightsmith for participating in the revision and discussion of this article. We further recognize the valiant actions realized by Kilma Manso Rocha and Simone Fraga Tenório in relation to local communities, including efforts in awareness so that communities may alter their relationship with macaws and the degraded Caatinga biome.





