Adolescence constitutes a period of nutritional vulnerability due to increased dietary requirements for growth and development and their special dietary habits. An adequate Zn intake throughout this period is necessary due to particular physiological requirements during the pubertal growth spurt. Physiological processes that accompany puberty, including sexual maturation, onset of menarche and increased erythropoiesis, have a major impact on adolescents’ requirements for Zn( 1 ). Even when the growth spurt has ceased, additional Zn may be required to fill the tissue Zn pools depleted as a result of these increased demands( Reference King 2 ). It has been established that Zn is an essential trace element that plays an important role in homeostasis in man, and over 200 enzymes have been confirmed to be Zn-dependent( Reference Gibson, Hess and Hotz 3 ). It is an essential component of the brain, and participates in central nervous system development and functions. It is related to the synthesis of proteins and, therefore, to growth, differentiation and cellular metabolism. Moreover, Zn is involved in the synthesis of growth hormone( Reference Salgueiro, Zubillaga and Lysionek 4 ) and is an important factor in maturation and skeletal growth( Reference King 2 ) being intimately linked to bone metabolism, which is of special importance during adolescence( Reference Favier 5 ).
Zn deficiency is a serious nutritional problem that negatively affects growth and intellectual and sexual development( Reference Salgueiro, Zubillaga and Lysionek 4 ), and has been related with attention or behaviour alterations, hypogonadism, impotence and other reproductive disorders( Reference Favier 5 – Reference Brown, Wuehler and Peerson 7 ) as well as a broad range of neurobehavioural abnormalities( Reference Brown, Wuehler and Peerson 7 ). This deficiency is mainly due to an insufficient mineral intake or to the consumption of diets with low Zn bioavailability. According to Brown et al.( Reference Brown, Wuehler and Peerson 7 ), approximately 50 % of the world's population is at risk of inadequate Zn intake. Individuals at risk of low Zn status include those with low absorption, increased losses or increased needs due to growth, heightened by low Zn intake or by a diet with low Zn bioavailability. The level of adequate Zn utilization is greatly influenced by the composition of the diet, regarding both the concentration of Zn and the presence of enhancers and inhibitors of Zn absorption( Reference Brown, Wuehler and Peerson 7 ). Due to the above-mentioned needs for growth, adolescent diets should contribute sufficient and suitable nutrients to provide adequate mineral bioavailability and cover Zn requirements at this time of life. Unfortunately, however, many adolescents fail to meet Zn needs during puberty, mainly due to the poor quality of their diets( Reference Gibson, Heath and Ferguson 8 ).
The Mediterranean diet has been proposed as one of the healthiest dietary models available, mainly due to its potential protective role against the most common chronic diseases, and its health benefits have been demonstrated in many studies( Reference Serra-Majem, Ribas and Ngo 9 ). It is generally agreed that the main characteristics of the Mediterranean diet include a high intake of plant foods (vegetables, fruits, cereals, legumes, nuts and seeds, and olive oil); a low to moderate intake of dairy products (mainly in the form of cheese or yoghurt); low to moderate consumption of poultry and eggs; a moderately high intake of fish and shellfish; and a low intake of red meat and processed meat products( Reference Trichopoulou and Vasilopoulou 10 ). Cereals, legumes and vegetables are foods containing large amounts of phytate; this is known to form an insoluble phytate–mineral complex that is not absorbed by the human gastrointestinal tract, and thus the bioavailability of minerals is reduced( Reference Lönnerdal 11 ). Moreover, Gibson et al.( Reference Gibson, Heath and Limbaga 12 ) reported that decreased consumption of red meat may compromise the intake and bioavailability of Zn, which could also be provoked by an increased consumption of cereals. In this sense, there is insufficient information on the effects of the Mediterranean diet on Zn absorption, especially among adolescents. Thus, the aim of the present study was to evaluate dietary Zn bioavailability and status in a group of adolescents consuming a varied diet based on Mediterranean patterns.
Experimental methods
Participants and diet design
Participant selection and diet design have been described previously( Reference Mesías, Seiquer and Muñoz-Hoyos 13 ). Briefly, twenty male adolescents (mean age 12·9 (se 1·1) years) were recruited among adolescents aged 11–14 years, after evaluating their health status by medical questionnaire and physical examination and performing food habit and lifestyle nutritional surveys. Exclusion criteria included diseases or disorders that might modify the results of the parameters under study, medication, eating disorders and alcohol or tobacco use. The use of mineral or vitamin supplements and fortified foods was not allowed during the study. An experimental diet was designed on the basis of the main Mediterranean eating patterns( Reference Trichopoulou and Vasilopoulou 10 ), in compliance with Recommended Intakes for Spanish Adolescents( Reference Moreiras, Carbajal and Cabrera 14 ). Moreover, the dietary habits of the adolescents, previously evaluated by nutritional survey, were taken into account as far as possible. A 7 d menu was drawn up, in which several modifications were made with respect to the participants’ habitual diet in order to fit the Mediterranean dietary pattern, namely a higher content of fish, legumes, cereals, fruits and vegetables, a lower content of meat and meat products, and a higher MUFA:SFA ratio as the main differences. Daily number of servings of the main food groups was as follows: meat 1·2, fish 0·4, dairy products 2·7, cereals 4·3, legumes 0·4, potatoes 0·8, fruits and vegetables 2·3. Olive oil was used as the main dietary fat. The food content of the diet was transformed into energy and nutrient values using the Spanish Food Composition Tables( Reference Mataix, Mañas and Llopis 15 ) and with AYS44 Diet Analysis software (ASDE SA, Valencia, Spain).
Lunch and dinner were prepared by a local catering firm( Reference Mesías, Seiquer and Muñoz-Hoyos 13 ) and were distributed daily to the homes of the participants and to the investigators to enable analysis of the Zn content in the experimental diet. The participants and their parents received specific instructions for preparing the breakfast and the afternoon snack at home; these were prepared in the same way in the laboratory for subsequent mineral analysis. The daily content of fat, carbohydrate and protein of the experimental diet was calculated to be 100 g (36 % of energy), 317 g (50 % of energy) and 88 g (14 % of energy), respectively, at an energy content of 10 565 kJ/d (2525 kcal/d; determined using AYS44 Diet Analysis).
Study design
The experimental study included a 28 d nutritional intervention period, in which the experimental diet was consumed in four cycles of the designed 7 d menu. During the 28 d, compliance with the diet was assessed by daily record sheets, on which participants noted the details of their food consumption. If the prepared meals were not consumed entirely, the participants were asked to weigh and record all the food that was left. They also weighed and recorded the food consumed at breakfast and for the afternoon snack. The participants were asked to totally avoid restaurant and takeaway food during the period of dietary treatment, and to comply strictly with the recommendations. Data on the food consumed were transformed into values of energy and nutrients using the above-mentioned computer program.
The study was approved by the Ethics Committee of the San Cecilio University Hospital of Granada and was in accordance with the Helsinki Declaration of 2002, as revised in 2004. Informed consent was obtained from the parents of all the children participating in the study.
Dietary Zn utilization
The balance technique was followed to assess Zn dietary utilization. The balance comprised the last 4 d of the nutritional intervention period, during which each participant collected urine and faeces daily. The initial 20 d were considered to be an equilibration period, which was longer than the minimum recommended to allow gastrointestinal clearance of unabsorbed minerals from the previous diet( Reference Zhi, Moore and Kanitra 16 ). Each 24 h urine pool collection began with the second voiding of the day and finished with the first voiding of the following day. The total urine volume collected each day was recorded. The participants were asked to report any problem with the collections, such as spillages or missed specimens. Individual faecal samples were weighed, diluted with 6 m-HCl and homogenized with a hand blender (Vital CM, Taurus, Lleida, Spain). Aliquots were frozen at −20°C until analysis.
On the day immediately after the excreta collection and following a 12 h overnight fast period, blood samples were obtained from each participant by venepuncture, left to clot for 30 min and centrifuged at 1700 g for 15 min to obtain serum and blood cells. Supernatant-containing cells were stored at −20°C until analysis. Erythrocytes were isolated by hypotonic haemolysis in accordance with the method of Hanahan and Ekholm( Reference Hanahan and Ekholm 17 ) and the cytosol fraction was preserved for superoxide dismutase (SOD) analysis. Zn was determined in both erythrocytes and serum. SOD was determined following Fridovich( Reference Fridovich 18 ), based on the inhibition by SOD of cytochrome C reduction and spectrophotometric measurement at 550 nm.
Hair samples from each participant were also obtained on the day of blood sampling, to determine Zn concentration. About 3 cm of hair was cut from the nape of the neck close to the scalp. The individual hair sample was washed with acetone, ethanol and Milli-Q water, and dried overnight.
Before the Zn analysis, samples of faeces, urine, erythrocytes, serum and hair were completely digested by the addition of concentrated HNO3–HClO4 (1:4 v/v) and by heating at high temperature (180−220°C) in a sand beaker. Zn analysis, for all samples, was performed by flame atomic absorption spectroscopy in a Perkin-Elmer Analyst 700 Spectrophotometer (Norwalk, CT, USA). Samples of bovine liver reference standard (certified reference material BCR No. 185; Community Bureau of Reference, Brussels, Belgium) were simultaneously used to validate the accuracy of the Zn assay. The inter-assay CV for Zn was 3·26 % in faeces and 4·12 % in urine. All glassware and the polyethylene sample bottles were washed with 10 m-HNO3, and demineralized water (Milli-Q) was used throughout.
The following indices were calculated from the data obtained for Zn intake and faecal and urinary excretion: absorption Zn (A = intake Zn – faecal Zn); retention Zn (R = absorption Zn – urinary Zn); fractional absorption or digestibility (%A/I = absorption/intake Zn × 100); utilization efficiency or bioavailability (%R/I = retention/intake Zn × 100).
Body weight and height were recorded at the beginning of the study and at the end of the nutritional intervention period, and the body mass index (BMI = kg/m2) was calculated.
Statistical analyses
The SPSS for Windows statistical software package version 13·0 (SPSS Inc., Chicago, IL, USA) was used for data entry and statistical analysis. Relationships between the variables were determined by computing the corresponding correlation coefficients (Pearson linear correlation) at the P < 0·05 confidence level.
Results and discussion
The weight, height and BMI of the participants before starting the nutritional intervention period were 57·6 (se 2·3) kg, 161·4 (se 2·2) cm and 22·1 (se 0·8) kg/m2, respectively; eight of them (38 %) were overweight. The overall increase in weight and height during the experimental period was 0·6 kg (final weight 58·2 (se 2·2) kg) and 0·7 cm (final height 162·1 (se 2·2) cm), respectively. As a result, the BMI of the boys did not change.
Adherence to the Mediterranean diet patterns during the nutritional intervention period was tested by applying the Mediterranean Diet Quality Index for children and adolescents( Reference Serra-Majem, Ribas and Ngo 9 ) based on principles sustaining Mediterranean dietary patterns as well as those that undermine it. The index ranges from 0 to 12 and is based on a sixteen-question test. The results of this index are classified as poor (≤3), average (4–7) and good (≥8). In our study, the average degree of compliance with the experimental diet was 88·5 %, and the overall Mediterranean index value of the participants was 7.
The average daily intakes of energy and nutrients by the adolescents during consumption of the experimental diet are presented in Table 1. The average energy intake (9351 kJ/d, 2235 kcal/d) comprised about 91 % of the recommended intake for Spanish adolescents( Reference Moreiras, Carbajal and Cabrera 14 ). Contributions of protein, carbohydrate and lipids to total energy intake were 14·8 (se 0·2) %, 47·0 (se 0·3) % and 38·1 (se 0·3) %, respectively.
Table 1 Daily intakes of energy and nutrients during consumption of the experimental diet by Spanish male adolescents aged 11–14 years (n 20)

Zn intake
The contribution of the different food sources to the total Zn intake is illustrated in Fig. 1. Cereals, together with meat and dairy products, were the major contributors of dietary Zn, which is in agreement with previous studies conducted among Mediterranean adult populations in southern Spain( Reference Sánchez, López-Jurado and Planells 19 ). Moreover, similar data have been reported in other epidemiological studies with adolescents in the UK( Reference Thane, Bates and Prentice 20 ) and Germany( Reference Kersting, Alexy and Sichert-Hellert 21 ), with children in New Zealand( Reference Gibson, Bailey and Parnell 22 ) and in the total Italian diet( Reference Lombardi-Boccia, Aguzzi and Cappelloni 23 ). Fish and seafood, despite being important sources of Zn, made minor contributions to daily Zn intakes because these foods were partially rejected by some of the participants, which may account for their relatively low consumption (0·4 servings/d).
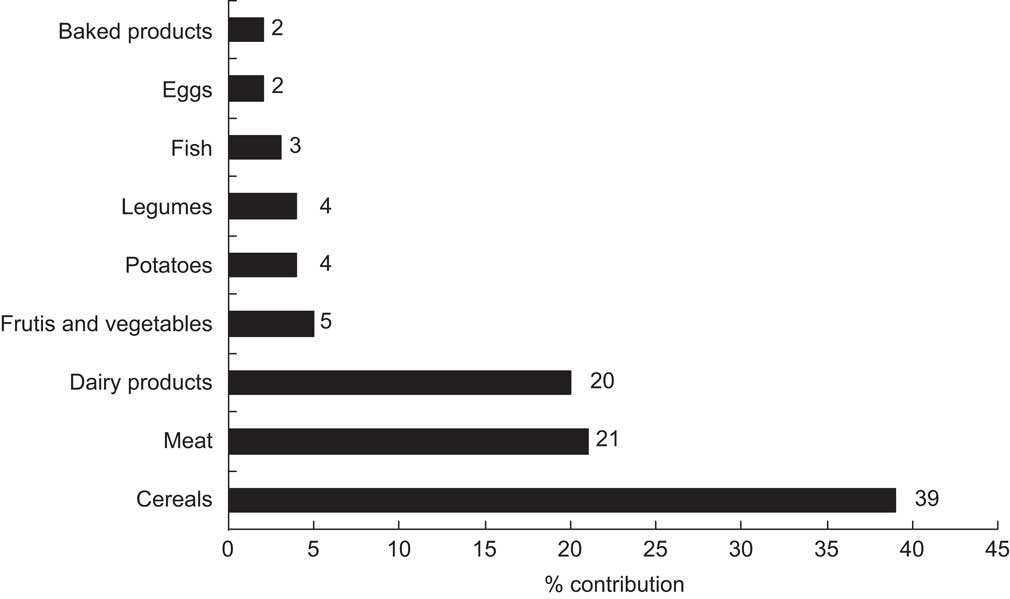
Fig. 1 Contribution (%) of main food sources to daily zinc intake among Spanish male adolescents aged 11–14 years (n 20) consuming an experimental diet
Zn intake ranged from 8·42 to 13·80 mg/d, with a mean value of 11·36 mg/d (Table 2), equivalent to 76 % of the recommended value for Spanish adolescents (15 mg/d)( Reference Moreiras, Carbajal and Cabrera 14 ). This value is similar to that reported in another study of Spanish adolescents (11·50 mg/d)( Reference Fernández-Morales, Aguilar Vilas and Mateos Veja 24 ) but lower than that for American boys aged 12–19 years (14·3 mg/d)( Reference Ervin, Wang and Wright 25 ). Regarding European studies, our average Zn intake was exceeded by that reported for male adolescents in Finland and Estonia (15·3 and 12·9 mg/d, respectively), whereas Austria, Netherlands and the UK reported lower values (9·6, 8·9 and 7·8 mg/d, respectively)( Reference Lambert, Agostoni and Elmadfa 26 ). Epidemiological studies have shown that adolescents usually have dietary Zn intakes that fail to meet their requirements( Reference Elmadfa and Weichselbaum 27 ), particularly among girls( Reference Gibson, Heath and Ferguson 8 ). The adolescents in the present intervention study did not meet Spanish Zn intake recommendations probably because they did not totally consume the experimental diet; in particular, some fish and seafood were rejected. However, the participants did meet the RDA for Zn of 8–11 mg/d for male adolescents aged 9–18 years( 1 ).
Table 2 Dietary zinc utilization during consumption of the experimental diet by Spanish male adolescents aged 11–14 years (n 20)

A correlation between Zn and energy intake was observed (r = 0·5737, P = 0·0065), in agreement with Gibson et al.( Reference Gibson, Hess and Hotz 3 ), who reported that in Western countries, the intake of dietary Zn is frequently associated with energy intake, which in the studied participants was also below recommended levels.
Zn absorption and bioavailability
Faecal excretion of Zn (78 % of the intake) was lower than the 88 % observed by Greger et al.( Reference Greger, Zaikis and Abernathy 28 ) in adolescent girls consuming similar dietary Zn (11·5 mg/d) and corresponds to an apparent absorption of 2·53 mg/d (Table 2). This absorption value is similar to the 2·6 mg/d estimated by King and Turnlund( Reference King and Turnlund 29 ) for Zn intakes of 7·40–9·75 mg/d. Taking into account mineral intake, the fractional absorption was estimated to be 21·82 % (Fig. 2). This result was higher than those reported from other balance studies performed in man( Reference Wada, Turnlund and King 30 , Reference Wood and Zheng 31 ) although it did not reach the 40 % of Zn digestibility proposed by Fairweather-Tait( Reference Fairweather-Tait 32 ) for Western-type diets. Since urinary losses represent a minor way of Zn excretion, Zn retention was only slightly lower than absorption (2·15 mg/d), and thus Zn bioavailability was 18·5 %. In accordance with the findings of other authors, dietary Zn levels correlated with faecal Zn losses (r = 0·4766, P = 0·0289)( Reference Greger, Zaikis and Abernathy 28 ) and, moreover, with urinary Zn losses (r = 0·5312, P = 0·0132).
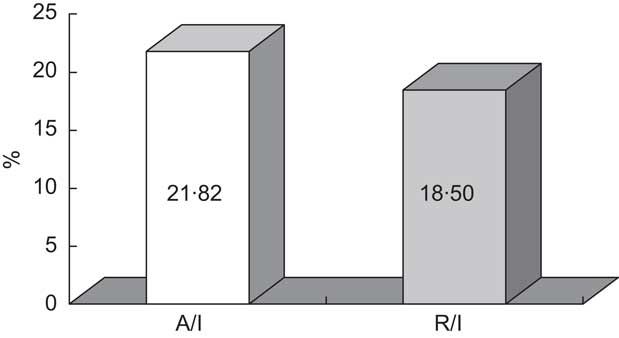
Fig. 2 Mean fractional absorption (%A/I) and utilization efficiency (%R/I) of zinc in the experimental diet consumed by Spanish male adolescents aged 11–14 years (n 20)
Needs for absorbed Zn may be calculated according to mineral losses and requirements for growth, considering an average accretion for new tissue formation of 10 g/d( 1 ). The overall Zn requirement by the participants in the present assay would thus be 3·0 mg/d, taking into account their age and weight. The real average Zn absorption, as mentioned above, was 2·53 (sd 0·55) mg/d with five of the adolescents in our study meeting the calculated needs and the remainder failing to do so. The relatively low Zn absorption may be attributed to a low intake, as has been indicated before in relation to Spanish recommendations. In addition, however, it may be due to a low bioavailability of dietary Zn. As mentioned above, it is well known that Zn bioavailability is decreased by high levels of dietary phytate, which is present in vegetables, cereals and legumes. The Mediterranean diet is characterized by a high content of these types of foods, which increases phytate consumption and, therefore, might affect dietary Zn bioavailability. According to Brown et al.( Reference Brown, Wuehler and Peerson 7 ), the Zn absorbable from the diet may be estimated by calculating the phytate:Zn molar ratio; it is accepted that diets with a phytate:Zn ratio >15 have relatively poor Zn bioavailability, those with a ratio between 5 and 15 have medium Zn bioavailability, and ratios <5 imply relatively good Zn bioavailability. According to the range of phytate contents reported by Brown et al.( Reference Brown, Wuehler and Peerson 7 ) for the different sources of food and taking into account the food consumption among the participants of the present assay, the phytate:Zn ratio in our study ranged from 2 to 11, which would correspond to a high–moderate Zn bioavailability diet. The maximum value is comparable with the overall range of phytate:Zn molar ratio reported by Wuehler et al.( Reference Wuehler, Peerson and Brown 33 ) for Western Europeans (10·6) and with the mean value of 10·1 observed in Koreans consuming varied diets but rich in vegetables, legumes, grains and cereals( Reference Joung, Nam and Lee 34 ). Based on the phytate:Zn ratio calculated in the present assay, a proportion of 30–45 % of dietary Zn available for absorption would be expected( 35 ). This estimation does not correspond to the real percentage of absorption found among the adolescents in our study (Fig. 2). Moreover, no correlation was observed between the minimum or maximum values of the ratio and Zn absorption. Thus, in addition to the dietary phytate content, other factors must have negatively affected mineral absorption.
Certain components of the diet, such as fibre, Ca, P, Fe and Cu, have been considered to decrease Zn bioavailability. In the present study, no correlation between dietary fibre and Zn absorption was observed but fibre correlated with the content of cereals (r 2 = 0·6232, P = 0·0035) and vegetables (r 2 = 0·5326, P = 0·0129), which, as mentioned, are rich sources of phytate. In this line, Lönnerdal( Reference Lönnerdal 11 ) reported that the negative effect of fibre on Zn absorption is usually due to the fact that most fibre-containing foods also contain phytate. Although Spencer et al.( Reference Spencer, Framer and Clemontain 36 ) did not observe any effect of Ca and P on Zn metabolism in man, Wood and Zheng( Reference Wood and Zheng 31 ) suggested that high-Ca diets can reduce net Zn absorption and balance and increase Zn requirements in adults. Lönnerdal( Reference Lönnerdal 11 ) indicated that this negative effect of Ca only takes place in phytate-containing foods since Ca accentuates the decreased Zn bioavailability caused by phytate, by reinforcing the complexing of Zn with phytate and consequently inhibiting Zn absorption. In the present study, Ca intake was correlated with cereal consumption, which could enhance the phytate's inhibitory effect on Zn absorption. No association was observed between dietary P, Fe and Cu and Zn metabolism. On the other hand, it is known that animal protein may increase the overall absorption of Zn from the diet and seems to counteract the negative effects induced by high intakes of phytic acid( Reference Lönnerdal 11 ). The lower intake of meat products among the participants in the present study, with respect to their habitual diet, proposed as one of the changes needed to meet the characteristics of the Mediterranean diet, may have contributed to Zn absorption being lower than expected.
Another factor to be considered as influencing Zn absorption is the dietary content of Maillard reaction products (MRP). These compounds are formed in heat processes such as frying( Reference Delgado-Andrade, Seiquer and Haro 37 ), a common culinary technique in the Mediterranean diet, and, thus, they are usually present in this diet( Reference Delgado-Andrade, Morales and Seiquer 38 ). Animal balance studies have shown that feeding rats with diets containing the MRP model induces significant reductions in Zn absorption and retention (both net and fractional), compared with animals fed control diets( Reference Navarro, Aspe and Seiquer 39 ). Therefore, the presence of MRP in the experimental diet may have been a negative factor for dietary Zn utilization.
In addition to the dietary factors mentioned previously, it should be taken into account that Zn absorption may also be regulated by Zn needs for growth; i.e. an adequate growth rate means that sufficient Zn has been absorbed. Thus, Zn absorption must be examined in relation to Zn biomarkers and the participants’ growth rate.
Biomarkers of Zn status
Although deficient Zn intake may be related to decreased serum Zn levels, the latter were within normal ranges in the participants in the present assay, according to Galdó and Cruz( Reference Galdó and Cruz 40 ) (75–150 μg/dl; Table 3), and similar to the data reported by Kara et al.( Reference Kara, Gunay and Cicioglu 41 ) and Voskaki et al.( Reference Voskaki, Arvanitidou and Athanasopoulou 42 ) in male adolescents. Different correlations have been observed between serum Zn and age, height, weight and BMI( Reference Voskaki, Arvanitidou and Athanasopoulou 42 , Reference Arvanitidou, Voskaki and Tripsiasis 43 ). Moreover, as many dietary factors may influence Zn absorption, some authors have also studied the relationship between Zn status and nutritional habits. In this sense, Voskaki et al.( Reference Voskaki, Arvanitidou and Athanasopoulou 42 ) found a strong correlation between Zn serum levels and meat and milk consumption in a Greek population, while no significant association was found with the consumption of fish, green vegetables, legumes, eggs or fruits. In the present study, no significant correlation was observed between serum Zn and age, height, weight or BMI, probably because the serum Zn values were within the normal range according to the age of the participants and their relatively homogeneous anthropometric characteristics. In a similar way, Urbano et al.( Reference Urbano, Vitalle and Juliano 44 ) found no correlation between BMI and serum levels of Zn in male and female adolescents. Serum Zn was correlated with the consumption of meat (r 2 = 0·4595, P = 0·0361) and cereals (r 2 = 0·4744, P = 0·0298), probably because these foods are the most important sources of Zn, which demonstrates the relationship between Zn status and nutritional habits. Similarly, Gibson et al.( Reference Gibson, Heath and Limbaga 12 ) showed that women who included red meat in their diet had a better biochemical Zn status (based on serum Zn) than did women who avoided eating red meat.
Table 3 Biomarkers of zinc status among Spanish male adolescents aged 11–14 years (n 20)

SOD, superoxide dismutase.
Zn in blood cells and hair also may be used to assess body Zn status, although, according to different authors, these Zn levels may be imperfect indicators of individual functional impairment related to Zn deficiency or during the Zn depletion phase( Reference Gibson, Hess and Hotz 3 ). No Zn reference values have been established for the erythrocytes or hair in children( Reference Schlegel-Zawadzka, Zachwieja and Huzior-Baajewicz 45 ) but the average Zn content in erythrocytes among the adolescents in our study (11·07 μg/ml; Table 3) was similar to the values reported by Schlegel-Zawadzka et al.( Reference Schlegel-Zawadzka, Zachwieja and Huzior-Baajewicz 45 ) in healthy 11-year-old boys in southern Poland. In the same way, the Zn levels in hair measured in the present study (Table 3) coincide with those reported in other studies with adolescents( Reference Schlegel-Zawadzka, Zachwieja and Huzior-Baajewicz 45 , Reference Zachwieja, Chlopicka and Schlegel-Zawadzka 46 ).
Other biomarkers of Zn status include various metalloenzymes, such as SOD, because Zn is found in the structure of this enzyme. It has been reported that Zn deficiency lessens SOD activity, while Zn supplementation increases SOD values( Reference Kara, Gunay and Cicioglu 41 ). Thus, Zn deficiency could negatively affect defence mechanisms against oxidative stress, as SOD is one of the main enzymes involved in the elimination of free radicals( Reference Valko, Morris and Cronin 47 ). No reference values have been reported for SOD in the adolescent population, although the data in the present study (Table 3) are similar to those observed by other authors in children( Reference Liu, Jiang and Shu 48 ). An almost significant correlation between SOD and Zn absorption and retention was observed (r 2 = 0·4415, P = 0·0584; r 2 = 0·4406, P = 0·0590), which supports the association between this parameter and Zn status.
Since all of the biomarkers of Zn status were within normal values for this population, no deficiency signal may be deduced. Moreover, the participants’ increase in height during the experimental period was 0·7 cm, equivalent to a growth of 4·5 cm/6 months, a figure that exceeds the 80th percentile reported by Carrascosa Lezcano et al.( Reference Carrascosa Lezcano, Ferrández García and Fernández Ramos 49 ) for boys aged 12·5–13·0 years and is close to the 90th percentile indicated by Hernández( Reference Hernández 50 ) for boys of the same age. These findings support the view that the adolescents studied presented adequate growth. The importance of Zn for growth and the suitability of indicative parameters of Zn status suggest that the amount of Zn absorbed was sufficient, at least during the experimental period.
Limitations of the present study may be the relatively low sample of participants and the limited duration of the assay. Therefore, without discarding the need for studies covering these aspects to verify the present results and to confirm that a diet based on Mediterranean patterns enables suitable Zn utilization during adolescence, the results of our study suggest, in accordance with Urbano et al.( Reference Urbano, Vitalle and Juliano 44 ), that current Zn Spanish recommendations could be unnecessarily high.
In summary, although a diet based on Mediterranean patterns is associated with factors which can affect Zn absorption, its consumption in adequate amounts allows Zn status to be maintained during adolescence. This finding could provide a basis for further, long-term study.
Acknowledgements
Financial support for the present study was made available through a project of the Spanish Ministry of Science and Innovation. The authors declare that there are no conflicts of interest. I.S. and M.P.N. designed and supervised the study and were responsible for the data collection and the interpretation of the results; M.M. helped design the protocol, participated in the selection of the adolescents and collection of the data and performed the chemical analysis; all the authors contributed to write the manuscript. The authors are indebted to the twenty adolescents who participated in the study.







