Investigation of the pathogenesis and treatment of inflammatory bowel disease that includes ulcerative colitis and Crohn's disease has been of increasing interest over the last decade. Inflammatory bowel disease is a chronic idiopathic inflammatory disease of the gastrointestinal tract affecting significant population numbers in the Western world(Reference Leenen and Dieleman1), and the frequency of which has increased considerably over the past few decades(Reference Loftus and Sandborn2, Reference Bernstein, Blanchard and Kliewer3). The existing therapies not only show limited benefits, but they also have unwanted side effects(Reference Hendrickson, Gokhale and Cho4). Consequently, there is a need for alternative anti-inflammatory agents that are at least equally effective, if not more so, and cause fewer side effects.
Some constituents of edible mushrooms, particularly glucans, have been reported to be useful in the management of a variety of cancer diseases(Reference Wasser5, Reference Sullivan, Smith and Rowan6) and are known to possess immunomodulatory ability. Although glucans have been shown to increase resistance to infections(Reference Williams, Mueller and Browder7), the uptake and biological effects of orally administered glucans are highly controversial(Reference Wu, Han and Bronson8). Most studies performed in animal models that have proposed the use of glucans as pharmaceuticals for the treatment of experimental infections have involved their administration via intravenous, intraperitoneal, subcutaneous, or intramuscular routes(Reference Williams and Browder9). It is only recently that Rice et al. showed that fungal-derived soluble glucans translocate from the gastrointestinal tract into the systemic circulation in normal animals(Reference Rice, Adams and Ozment-Skelton10). The edible mushroom Pleurotus is considered a health food with very high nutritional value; in addition, glucans extracted from Pleurotus species have been shown to exhibit a wide variety of medicinal properties(Reference Lavi, Friesem and Geresh11–Reference Bobek and Galbavy13).
Pleuran is a β-glucan isolated from Pleurotus ostreatus. When pleuran was locally administered, with or without concomitant parenteral pre-treatment, it was effective in reducing colonic damage induced by acetic acid, but only when administered prophylactically by the intraperitoneal, but not the oral route(Reference Nosalova, Bobek and Černa14). Barley or yeast β-glucan also showed an immunostimulatory effect mediated by the activation of neutrophils, macrophages, monocytes and natural killer cells through specific receptors CR3 (CD11b/CD18)(Reference Vetvicka, Thorton and Ross15), and through β-glucan receptor. This was associated with stimulated production of cytokines, such as TNF-α and IL-1, resulting in increased immunological surveillance(Reference Thorton, Vetvicka and Pitman16). We recently identified a novel, low-molecular-weight, hot water-soluble α-glucan produced by the edible mushroom P. ostreatus and showed its potent inhibition of colon cancer cell growth with concomitant apoptosis induction(Reference Lavi, Friesem and Geresh11). The aim of the present study was to assess the effect of orally administered P. pulmonarius glucans on experimentally (dextran sulfate sodium; DSS) induced colitis in mice.
Materials and methods
Preparation of glucan extracts from Pleurotus pulmonarius
Hot water solubles extracts
Powdered fruiting bodies of P. pulmonarius (300 g) was extracted with 3 litres of distilled water at 121°C for 30 min and centrifuged at 13 000 g (10°C for 15 min). Ethanol was added to the supernatant fraction to a final concentration of 50 % (v/v) and stored overnight at 4°C. The float was then taken out and the jelly fraction removed and lyophilised. We refer to this fraction as hot water solubles (HWS).
Mycelium extract
P. pulmonarius was maintained on 2 % (w/v) agar basidiomycete synthetic medium (BSM) and prepared essentially as previously described(Reference Ardon, Kerem and Hadar17). Cultures were incubated in liquid BSM for 5 consecutive days. The resultant mycelia were homogenised and this provided the basis for more homogeneous inoculums after 2 additional days of growing in fresh media. After the inoculation, mycelia were grown for 6 more days. The extracellular medium and biomass were collected at the end of the incubation time. Biomass was washed with distilled water at 80°C. The resulting wash water was collected, frozen and lyophilised, and is designated mycelium extract (ME).
Preparation of HWS and ME glucans was performed essentially as previously described(Reference Kodama, Yamada and Nanba18).
Chemical characterisation of hot water solubles and mycelium extracts from Pleurotus pulmonarius
Glucan content in hot water solubles or mycelium extract
Glucose content (following hydrolysis) showed that the major component for both HWS and ME glucans is glucose. Content of glucan (%) in the HWS and ME preparations was calculated using freeze-dried (1–3 g) HWS and ME extracts. The powder was dissolved in double distilled water and ethanol (99 %) in a ratio of 2:1 and kept at 4°C overnight again as described(Reference Kodama, Yamada and Nanba18). The precipitated glucan was collected, dried at 60°C and its percentage from weighed HWS and ME calculated. The glucan recovered following ethanol precipitation in HWS was 95·6 % and in ME was 93·03 %. In both recovered isolates, glucose content was identical to that of the original preparation, indicating that glucans in HWS and ME are recoverable and provide the majority of the molecules constituting these extracts (HWS and ME).
Magnetic resonance analyses of hot water solubles and mycelium extract polysaccharides
1H-NMR or 13C-NMR spectra of polysaccharides (HWS and ME) dissolved in 2H-labelled water were obtained with a 200 or 500 MHz Bruker NMR spectrometer (DPX-200 or DMX-500, respectively; Bruker Biospin GmbH, Rheinstetten, Germany).
In vivo experimental design
Colitis was induced essentially as previously described(Reference Cooper, Murthy and Shah19), with minor modifications. Female BALB/c mice, a strain known to be susceptible to oral DSS, were purchased from Harlan Laboratories Ltd (Jerusalem, Israel) at the age of 7 weeks. All animals were housed in plastic cages (three or five mice/cage) with free access to drinking water and pelleted basal diet. Mice were kept under controlled conditions of light (12 h light–12 h dark cycle) and temperature (23 ± 2°C). Animals were quarantined for 7 d upon their arrival, and then randomised by body weights into groups 1 to 10 as follows (Fig. 1). Group 1 (control) was provided with a commercial powdered diet (Harlan) and drinking water without DSS for 29 d. Group 2 (DSS) had acute colitis induced by providing DSS in the drinking water for 18 d while consuming the commercial powdered diet. Group 3 (high HWS) was given a daily dose of 20 mg HWS/mouse in the food and drinking water without DSS for 29 d. Group 4 (high ME) was given a daily dose of 20 mg ME/mouse in the food and drinking water without DSS for 29 d. Group 5 (high HWS+DSS) was given a daily dose of 20 mg HWS/mouse in the food from 10 d before DSS treatment until the last day before killing, and DSS in the drinking water for 18 d. Group 6 (low HWS+DSS) was given a daily dose of 2 mg HWS/mouse in the food from 10 d before DSS treatment until the last day before killing, and DSS in the drinking water for 18 d. Group 7 (high ME+DSS) was given a daily dose of 20 mg ME/mouse in the food from 10 d before the DSS treatment until the last day before killing, and DSS in the drinking water for 18 d. Group 8 (low ME+DSS) was given a daily dose of 2 mg ME/mouse in the food from 10 d before the DSS treatment until the last day before killing, and DSS in the drinking water for 18 d. Group 9 (treatment with DSS and then high HWS) was given DSS in the drinking water for 18 d and then a daily dose of 20 mg HWS/mouse in the food for an additional 11 d. Group 10 (treatment with high HWS and then DSS) was given a daily dose of 20 mg HWS/mouse in the food for 11 d and then DSS in the drinking water for an additional 18 d.

Fig. 1 Experimental design. Protocol used for colitis induction by dextran sulfate sodium (DSS) in female BALB/c mice. Mice were given the different glucans orally in their food (at a daily dosage of 2 or 20 mg/mouse; low or high, respectively) from 10 d before the start of DSS administration until the last day before killing, or as shown in group 9 for 11 d after DSS induction (18 d) or in group 10 for 11 d before DSS induction (18 d). HWS, hot water solubles; ME, mycelium extract; (■), DSS in drinking water; (▧), treatment in food; (![]() ), water in food.
), water in food.
Colonic inflammation was induced in mice by administering 3·5 % (w/v) DSS of molecular weight 36 000 to 50 000 Da in the drinking water for 18 d (days 1–18 or days 11–29). Daily water consumption was measured per mouse and found to be similar among all experimental groups. To assess the extent of colitis, body weight, stool consistency, and blood in the stools were monitored. Mice were killed on day 29. Colonic tissues were removed and cleaned. Colon length (caecum to rectum) was measured and divided into several sections for organ culture, histology and RNA isolation (the latter were kept in RNAlater solution; see below).
Animal care and experimental procedures were in accordance with the guidelines of the accredited animal ethics committee of the Hebrew University of Jerusalem.
Macroscopic assessment
Animals were checked daily for the three main clinical symptoms of colitis: body weight changes, stool consistency and faecal occult blood. The colonic damage was quantified by assessing stool consistency and rectal bleeding as previously described by Loher et al. (Reference Loher, Bauer and Landauer20). For stool consistency, 0 points were given for normal, well-formed stools, 2 points for pasty stools and 4 points for liquid stools that were stuck to the anus. Faecal occult blood was determined on a haemoccult sensa card (see Table 1) by two investigators who were blinded to the treatment groups. Bleeding was scored 0 for no blood in haemoccult, 2 points for a positive haemoccult and 4 points for gross bleeding. The sum of these individual scores gave a final mark ranging from 0 for good clinical symptoms to 8 for very ill or severe colitis.
Table 1 Faecal occult blood as determined on a haemoccult sensa card in mice

DSS, dextran sulfate sodium; high, 20 mg/mouse daily; HWS, hot water solubles; ME, mycelium extract; low, 2 mg/mouse daily.
Myeloperoxidase activity
The level of tissue myeloperoxidase (MPO) was determined by a standard enzymic procedure as described previously(Reference Krawisz, Sharon and Stenson21) with minor modifications. In brief, the colonic tissue specimen (50–70 mg) was homogenised on ice in potassium phosphate buffer (pH 6·5) with 0·5 % (w/v) hexadecyltrimethylammonium bromide by three 30 s pulses in a Polytron-type homogeniser. The homogenate was then sonicated for 20 s following each of three 15 min freeze–thaw cycles. The sample was centrifuged at 20 000 g for 30 min at 4 °C, and the supernatant fraction was collected. This sample (100 μl) was added to 2·9 ml phosphate buffer (pH 6·5) containing O-dianisidine hydrocholoride (0·167 mg/ml) and 0·0005 % (v/v) H2O2. The sample's absorbance at 460 nm was measured using a spectrophotometer (UV-1601; Shimadzu, Japan) at 25 °C. The values were standardised using concentrations between 0·019 and 0·625 units purified peroxidase per ml (Sigma-Aldrich, St Louis, MO, USA) dissolved in hexadecyltrimethylammonium bromide buffer. A 1 unit of change in MPO level was defined as the value that can degrade 1 μmol H2O2 per min at 25 °C. The values were then calculated as product concentration (U/ml) divided by mg colon weight.
Determination of TNF-α secretion by colonic tissue in organ culture
Mice were killed and colon tissue samples were collected and placed in ice-cold Dulbecco's modified Eagle's medium. Triplicate 3 mm sections were cut longitudinally and placed in twenty-four-well plates containing medium. Tissues in organ culture were maintained in Dulbecco's modified Eagle's medium containing 0·2 % (v/v) antibiotic–antimycotic solution at 37°C in 5 % CO2. After 24 h, the medium was collected and frozen until further processing. TNF-α was measured by ELISA (Mouse TNF-α ELISA Kit, MAX™ Set (Deluxe); BioLegend, San Diego, CA, USA) according to the manufacturer's protocol.
Histopathological evaluation of colitis
After killing, the length of the colon from the colocaecal junction to the anal verge was measured. Approximately 20 % of the distal third of the colon was removed and preserved in a formalin solution. Histological scoring of fixed, sectioned (paraffin-embedded) and haematoxylin and eosin-stained tissues was performed in a blinded fashion as described by Dieleman et al. (Reference Dieleman, Palmen and Akol22), with slight modifications, as follows: inflammation (0 (none) to 3 (severe)), extent (0 (none) to 3 (transmural)) and crypt damage (0 (none) to 3 (entire crypt and epithelium lost)). The three scores were summed to give a total score (0–9). Grading was performed by two investigators blinded to the treatment groups.
cDNA production and quantitative real-time RT-PCR
Total RNA was extracted from colon tissue specimens that had been preserved in RNAlater solution (Ambion Europe, Huntingdon, Cambs, UK) using 1 ml of Tri Reagent (Sigma-Aldrich) according to the manufacturer's instructions. RNA was further purified using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Purified RNA (5 μg) was used for the RT procedure using SuperScript II RNase H-Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA).
To analyse the expression levels of IL-1β and IL-10 transcripts, relative quantification of gene expression was performed using SYBR Green real-time RT-PCR on an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA). Three specific primers for genes IL-1β (forward, GGACATAATTGACTTCACCATGGAA; reverse, CAGTCCAGCCCATACTTTAGGAA), IL-10 (forward, ATGCTGCCTGCTCTTACTGACTG; reverse, CCCAAGTAACCCTTAAAGTCCTGC) and glyceraldehyde-3-phosphate dehydrogenase (forward, ATGACTCCACTCACGGCAAAT; reverse, TCCCATTCTCGGCCTTGAC) as a control and were used to obtain an amplicon of approximately 100 bp of each target gene. All primers were designed by PrimerExpress software (Applied Biosystems). Real-time PCR mixtures were composed of a 12 pmol concentration of each primer, 12·5 μl SYBR Green PCR master mix (Applied Biosystems), 5 μl cDNA (a 1:10 dilution of the 20 μl cDNA product produced as described) and nuclease-free water to a final volume of 25 μl. Amplification conditions were as follows: 2 min at 50°C, 10 min at 95°C, and then forty-five cycles consisting of 15 s at 95°C and 1 min at 60°C. Total cDNA abundance in the samples was normalised using the glyceraldehyde-3-phosphate dehydrogenase gene as a control. In all experiments, samples were amplified in triplicate, and the average cycle threshold was then calculated and used to determine the relative expression of each gene. Two independent experiments were carried out in the same manner, and the final average and standard error of the relative expression values were calculated. The experimental results were subjected to statistical analyses.
Statistical analysis
All values are expressed as mean values with their standard errors, unless otherwise indicated. Statistical significance was calculated by Student's t test or analysed by one- or two-way ANOVA. Differences were considered significant at P < 0·05.
Results
13C- and 1H-NMR analyses
Linkage within the polysaccharides was analysed by 1H- and 13C-NMR. Since the main monosaccharide found in both polysaccharides was glucose, Fig. 2 represents the main linkage found in the HWS (Fig. 2(A) and (B)) and ME (Fig. 2(C) and (D)) polysaccharide, respectively. The 1H-NMR spectrum of the dissolved HWS polysaccharide showed anomeric carbon peaks at 5·10 and 4·51 parts per million (ppm), characteristic of α- and β-linkages, respectively, at a ratio of about 1:1 (Fig. 2(A)). This was confirmed by the 13C-NMR spectrum (Fig. 2(B)) which showed three anomeric carbon peaks: two at 92·00 and 93·11 ppm characteristic of the α-linked anomeric carbons and a single one at 95·81 ppm, characteristic of a β-linked carbon. Compared with the HWS polysaccharide, results with the ME-derived polysaccharide indicated only an α-glucan linkage, as evidenced by its 1H-NMR spectrum (Fig. 2(C)) showing one peak at 5·09 ppm. This was confirmed with a 13C-NMR spectrum (Fig. 2(D)) showing a single signal at 93·19 ppm.

Fig. 2 (A) 1H- and (B) 13C-NMR of hot water solubles (HWS) polysaccharide dissolved in 2H-labelled water indicates the existence of both α- and β-linked sugars. (C) 1H- and (D) 13C-NMR of mycelium extract (ME) polysaccharide dissolved in 2H-labelled water indicates the existence of only α-linked sugars. ppm, Parts per million.
Macroscopic colitis-related assessment after hot water solubles and mycelium extract administration
Fig. 3(A) summarises the macroscopic scores at three different time points in all experimental groups. Even though differences were observed from 12 to 26 d (the first 14 d of DSS treatment), we show only the last 3 d of the experiment, i.e. days 27–29, since this is when the most significant differences were observed. Administration of HWS and ME at high doses (20 mg/mouse daily) + DSS (groups 5 and 7) resulted in a significant reduction in the scoring of colitis symptoms (3·2 (sem 0·63) and 2·8 (sem 0·49), respectively) relative to DSS-only mice (6·2 (sem 0·67)). The occurrence of these clinical signs was delayed in the treatment groups (i.e. 5, 6, 7 and 8): instead of appearing at 5 d into the DSS treatment, they appeared at 8 d. The above-zero scores in groups 3 and 4 (average of 1·3 (sem 0·31)) was due to the existence of pasty stools in a few mice, apparently due to the high content of ME and HWS polysaccharides in the food.
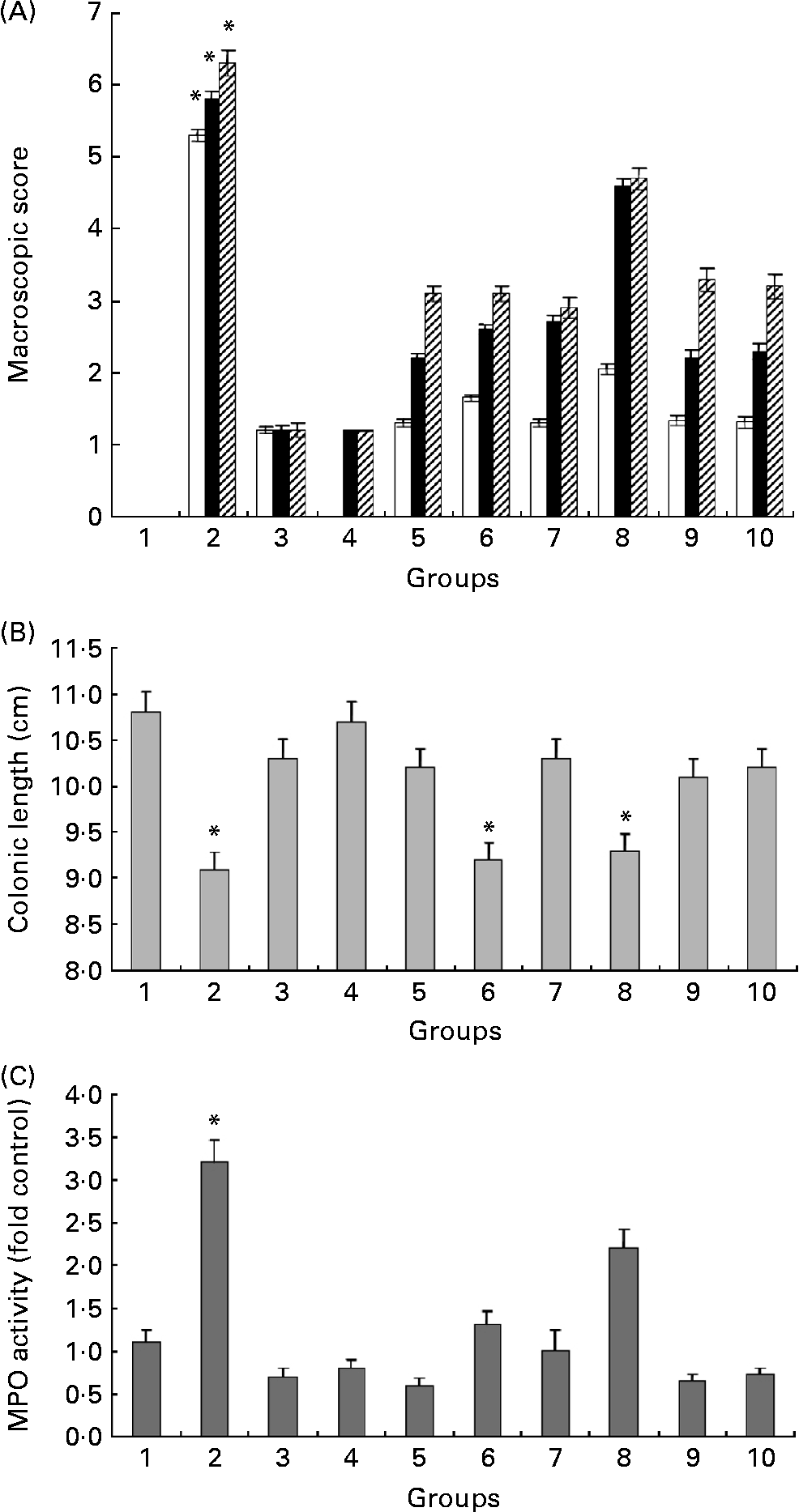
Fig. 3 (A) Effects of hot water solubles (HWS) and mycelium extract (ME) treatments on macroscopic score obtained during the last 3 d of the experiment (□, 27 d; ■, 28 d; ▨, 29 d). The macroscopic score of control group 1 was 0 (data cannot be seen). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from those of all other groups (P < 0·05). (B) Colon length in mice with and without dextran sulfate sodium (DSS)-induced colitis fed HWS and ME in their food for 29 d. Values are means of three or five mice per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of group 1 control mice (P < 0·05). (C) Effect of HWS and ME on myeloperoxidase (MPO) activity in mice subjected to DSS-induced colitis. MPO, an enzyme present in neutrophils, was measured as an index of neutrophil infiltration into the injured tissue. Values are means of three or five mice per group, with standard errors represented by vertical bars. * Mean value was significantly different from those of all other groups (P < 0·05). For group descriptions, see Materials and methods.
Colon length is a useful indication of colitis and was therefore measured as a marker of inflammation (Fig. 3(B)). Following 18 d of treatment with DSS, there was a significant shortening of the colon in the DSS-only group (9·02 (sem 0·76) cm) compared with the high HWS + DSS group (10·14 (sem 0·43) cm) and the high ME + DSS group (10·25 (sem 1·55) cm). The oral administration of low HWS or ME + DSS (groups 6 and 8) did not improve this inflammatory marker compared with DSS treatment alone.
Macroscopic scores and colon lengths in the control high HWS post-DSS group (group 9) and the control high HWS pre-DSS group (group 10) were similar to those in the high HWS + DSS group (group 5).
Effect of hot water solubles and mycelium extract on myeloperoxidase activity and neutrophil infiltration in the colon
The large influx of inflammatory cells was confirmed by measuring the activity of MPO (Fig. 3(C)), an enzyme specific to granulocyte lysosomes and therefore directly correlated to the number of neutrophils. MPO activity rose dramatically in the DSS-only group (group 2), as expected (fourfold compared with the control group). High and low HWS+DSS (groups 5 and 6) as well as high ME+DSS (group 7) oral pre-treatments significantly decreased MPO activity to control levels. The low ME+DSS group (group 8) failed to prevent the increase in MPO following DSS administration. The most effective reduction in MPO activity relative to control mice was in the high HWS + DSS group (25 %), but the reduction was statistically similar in groups 3, 4 and 7; that is, MPO activity was significantly lower in the glucans groups than in the untreated (DSS-only) group. We assume that administration of high concentrations of HWS or ME (without DSS) can reduce MPO activity levels below the basal levels observed in control mice, and may correlate with the ability of these glucans to reduce the expression, release and activity of pro-inflammatory cytokines.
MPO activity in the control high HWS post-DSS group (group 9) and the control high HWS pre-DSS group (group 10) was similar to that in the high HWS + DSS group (group 5).
Histological assessments after administration of hot water solubles and mycelium extract
Histological damage was evaluated by the method described by Dieleman et al. (Reference Dieleman, Palmen and Akol22). Extent of inflammatory colitis was established according to the extent of histological damage and inflammation (Fig. 4(A)–(K)). Fig. 4(L) summarises the damage scores from all treatment groups. The histological index was highest in group 2 (DSS only) with an average score of 8·5 (sem 1) and was significantly higher than in the other treatment groups, excluding group 8 (low ME treatment) with a score of 4·8 (sem 3). Group 7 (high ME group) exhibited the lowest histological index of 1·2 (sem 1·3), and group 5 showed a score of 2·6 (sem 1).

Fig. 4 Representative histological sections of colonic mucosa stained with haematoxylin and eosin showing the anti-inflammatory effects of glucan administration. (A and B) Control, non-dextran sulfate sodium (DSS)-treated mice showing the normal histology of the colon and normal crypt structure (group 1). ↓ , Normal crypt. (C and D) DSS-only mice showing extensive intestinal ulceration with severe inflammatory cell infiltrate in the lamina propria and submucosa (group 2). ← , Damaged crypts. (E and F) Colonic sections of high hot water solubles (HWS) mice (no DSS) and high mycelium extract (ME) mice (no DSS), respectively, showing normal colonic mucosa, submucosa and muscularis propria (groups 3 and 4). (G) High HWS + DSS (group 5) and (H) low HWS + DSS (group 6) showing normal colonic structure and accumulation of goblet cells. A marked reduction in infiltration of inflammatory cells, as well as absence of ulceration, are seen in the treated mice. (I) Colonic section of high ME + DSS (group 7): normal architecture is retained and there is a higher proportion of goblet cells compared with control mice. (J and K) Low ME + DSS showing ulceration and inflammatory reaction involving the mucosa only (group 8). Compensation with connective tissue is shown. Bars = 100 μm. Colonic sections of groups 9 and 10 are identical to those of group 5 (see G). (L) Histology damage score. Extents of inflammation and crypt damage were evaluated and summed to give a total score. The histology damage score for control, HWS and high ME mice (no DSS) is 0. Values are means of three or five mice per group, with standard errors represented by vertical bars. * Mean value was significantly different from that of group 1 control mice (P < 0·05). For group descriptions, see Materials and methods.
Mice with colitis fed HWS or ME had a significantly higher number of goblet cells per crypt than the corresponding non-glucan DSS-only-treated group (Fig. 4 and ), reflecting better maturation of mucin-producing cells than in mice with colitis but without glucan supplementation.
At 18 d of DSS-induced colitis, the DSS-only group showed necrosis, ulceration, gain of goblet cells, and infiltration of neutrophils and macrophages into the colonic mucosal and submucosal layers. In contrast to control mice and all other treatment groups, the colonic mucosal structure of the crypt in the DSS-only group was broken with DSS-induced colonic inflammation, resulting in clear thickening of the mucosal and muscle layers compared with those of control mice (groups 1, 3 and 4) and treatment groups (groups 5, 6, 7 and 8), in which hypertrophy of the mucosal and muscle regions of the colon was not observed. Dietary HWS and ME reversed histological and biochemical alterations in mice with experimental colitis. Histological assessments in the control high HWS post-DSS group (group 9) and the control high HWS pre-DSS group (group 10) were similar to those in the high HWS + DSS group (group 5).
Effect of hot water solubles and mycelium extract on colonic release of the pro-inflammatory cytokine TNF-α
We next examined the effect of the different treatments on release of the pro-inflammatory cytokine TNF-α by ELISA following 24 h in an organ culture system (See Table 2). In the control mice (group 1), TNF-α release amounted to 29·42 (sem 8) pg/ml in contrast to the DSS-only group (group 2), in which the release of the cytokine by the colonic mucosa was almost twofold higher than in control mice (57·08 (sem 11) pg/ml). In all glucan-treated groups (groups 5, 6, 7 and 8), DSS treatment failed to up-regulate the amount of TNF-α released and the difference from the DSS-only group (group 2) was significant (P < 0·05). TNF-α levels in the control high HWS post-DSS group (group 9) and the control high HWS pre-DSS group (group 10) were similar to those in the high HWS + DSS group (group 5).
Table 2 Effect of hot water solubles (HWS) extract and mycelium extract (ME) in vivo treatment on secretion of TNF-α and on IL-1β and IL-10 mRNA transcript levels in mouse colonic mucosa
(Mean values and standard deviations)

DSS, dextran sulfate sodium; high, 20 mg/mouse daily; low, 2 mg/mouse daily.
* Mean value was significantly different from that of the negative control mice (P < 0·05).
† For TNF-α measurement, on the last day of the experiment, mice were killed and colons were placed on Dulbecco's modified Eagle's medium plates. Organ cultures were maintained at 37°C in 5 % CO2 for 24 h. The medium was collected and TNF-α was measured by ELISA.
‡ mRNA levels of the pro-inflammatory cytokine IL-1β and the anti-inflammatory cytokine IL-10 were determined by quantitative real-time RT-PCR (see Materials and methods). The results represent the average ratio of cytokines to glyceraldehyde-3-phosphate dehydrogenase mRNA from three or five mice.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR assays were performed to screen for the effect of treatments on mRNA expression of the cytokines IL-1β and IL-10. The average cellular expression levels of cytokine mRNA were calculated as the ratio between the cytokine and glyceraldehyde-3-phosphate dehydrogenase mRNA levels in the individual samples.
mRNA expression of the pro-inflammatory cytokine IL-1β was significantly increased in mice of the DSS-only group compared with the control group (see Table 2), with a sevenfold increment in average value. Orally administered HWS and ME, at both concentrations, significantly suppressed mRNA levels of IL-1β (Table 2). IL-1β mRNA levels were not significantly different in any of the glucan-treated groups (groups 5, 6, 7 and 8) from the DSS group (group 2). They only differed from the control naive animals (group 1). The level of IL-10 mRNA was significantly higher in the colonic segment of DSS-treated mice (group 2) compared with the corresponding segment in the control group, with a more than 1·5-fold increment in average value. Although the degree of the cytokine IL-10 up-regulation was greater in DSS-treated mice (group 2) than in the other treatment groups (groups 5, 6, 7 and 8), the differences were not significant. No significant correlation between IL-10 mRNA levels and disease activity was observed. IL-1β and IL-10 mRNA levels in the control high HWS post-DSS group (group 9) and the control high HWS pre-DSS group (group 10) were similar to those in the high HWS + DSS group (group 5).
Discussion
In the present study, we assessed the biological activity of glucans extracted from P. pulmonarius cultivated either as fruiting bodies or mycelium. We show that oral administration of either ME or HWS glucans can reduce intestinal inflammation and other symptoms associated with chronic colitis in a mouse DSS model. We clearly demonstrate that glucans from P. pulmonarius significantly ameliorate colonic inflammation induced by DSS, suggesting the clinical potential of these specific mushroom extracts for the treatment of inflammatory bowel disease.
Plant polysaccharides have been previously shown to reduce extensive colonic damage in experimental colitis(Reference Rolandelli, Saul and Settle23–Reference Liu, Wang and Xu25). However, only limited information exists on the effect of supplementing edible mushroom glucans before and during the process of intestinal inflammation.
In the present study, P. pulmonarius glucans were fed orally to mice at daily doses of 2 or 20 mg per mouse. These doses were chosen to approximate the estimated human intake of dietary fibre(Reference Englyst, Quigley and Hudson26). Both dosages of HWS and the high dosage of ME delayed the occurrence of diarrhoea and rectal bleeding and improved macroscopic scores (stools + haemoccult). In addition, high dosages of HWS and ME prevented colon shortening compared with the DSS treatment. Colon shortening is always found in ulcerative colitis patients and can serve as an indirect marker of colonic inflammation(Reference Chung, Yue and To27).
MPO activity, which is directly related to chronic inflammation, was suppressed by HWS and ME, indicating that glucans inhibit the accumulation of neutrophils in the colonic mucosa. MPO activity has been previously shown to be directly related to the number of infiltrating granulocytes, primarily neutrophils, in a model of colonic inflammation(Reference Krawisz, Sharon and Stenson21). Increasing amounts of transmigrating neutrophils induce significant epithelial disruption, resulting in epithelial discontinuities and erosion(Reference Nusrat, Parkos and Liang28). Therefore, it is suggested that interrupted entry of neutrophils into the intestinal wall is involved in the preventive effects of HWS and ME. In addition, histological examinations clearly indicated significant attenuation of inflammation by HWS and ME.
Pro-inflammatory cytokines are known to play an important role in inflammation of the intestinal mucosa(Reference Nakamura, Saito and Kasanuki29). Specifically, increased levels of TNF-α, IL-1β, IL-6 and IL-8 have been reported in ulcerative colitis patients(Reference O'Shea, Ma and Lipsky30–Reference Nielsen, Brynskov and Bendizen34). In the present study, we demonstrate that in DSS-treated mice (colitis-induced, group 2), TNF-α release from colonic tissue samples maintained in organ culture was significantly elevated as compared with normal controls and with the other treatment groups. Even low daily doses (2 mg/mouse) of both HWS and ME were sufficient to significantly attenuate the increase in TNF-α in colon segments. The tissue levels of TNF-α protein were correlated with degree of inflammation and macroscopic score, supporting previous, as well as more recent reports(Reference Akazawa, Sakaida and Higaki35, Reference Olsen, Goll and Cui36). In addition, in line with the present results, recently published clinical reports(Reference Järnerot, Hertervig and Friis-Liby37, Reference Rutgeerts, Sandborn and Feagan38) show that treatment with anti-TNF-α, a specific antibody against TNF-α, induces remission in 30–40 % of ulcerative colitis patients.
Regarding additional cytokines, Okada et al. reported that 1.4-dihydroxy-2-naphthoic acid, an intermediate metabolite of menaquinone biosynthesis, attenuates increases in IL-1β, IL-6 and TNF-α mRNA levels, concomitant with a reduction in mucosal damage(Reference Okada, Tsuzuki and Miyazaki39). Hoentjen et al. reported that a combination of inulin and fructo-oligosaccharides significantly decreases histological inflammation and caecal IL-1β concentration(Reference Hoentjen, Tannock and Dieleman40).
Here we demonstrated a marked increase in mRNA levels of IL-1β, known to be produced primarily by activated macrophages(Reference McAlindon, Hawkey and Mahida41), as measured by quantitative real-time RT-PCR, in control mice. Oral administration of ME and HWS significantly down-regulated the expression levels of IL-1β compared with untreated mice. IL-1β is a key cytokine involved in the activation and production of additional cytokines involved in inflammation. In this context, Akira et al. showed that IL-1 is involved in up-regulating the production of IL-8, IL-6 and TNF-α by macrophages(Reference Akira, Hirano and Taga42). Several studies indicate that glucans isolated from several mushroom strains moderate immunogenic-related activities. In this regard (1 → 3),(1 → 6)-branched glucan isolated from aqueous extract of the fruiting bodies of the edible mushroom P. florida stimulates the phagocytic activity of macrophages(Reference Rout, Mondal and Chakraborty43). In addition, daily feeding of water-soluble polysaccharide from P. citrinopileatus (SPPC) at a dosage of 50 mg/kg to mice developing pulmonary metastatic tumours for 12 d resulted in a significant increase in the number of T cells, CD4+ cells, CD8+ cells and macrophages, compared with mice that were not fed any SPPC(Reference Wang, Hu and Liang44). Together, these findings indicate that the increased production of pro-inflammatory cytokines is probably caused by enhanced number and activity of macrophages.
IL-10 is a pleiotropic cytokine involved in both cell-mediated and humoral immune responses. Furthermore, IL-10 has both anti-inflammatory and pro-inflammatory effects(Reference Melgar, Yeung and Bas45). Anti-inflammatory effects include preventing colitis in severe combined immunodeficiency (SCID) mice and provide further evidence of IL-10's non-redundant role in the functioning of regulatory T cells that control inflammatory responses towards intestinal antigens(Reference Asseman, Mauze and Leach46). These anti-inflammatory effects appear to be executed through inhibition of pro-inflammatory cytokine production by T cells and macrophages(Reference Fiorentino, Zlotnik and Vieira47, Reference Fiorentino, Zlotnik and Mosmann48). Pro-inflammatory actions of IL-10 have been reported in both mice(Reference Wogensen, Huang and Sarvetnick49, Reference Balasa and Sarvetnick50) and man(Reference Furukawa, Becker and Stinn51). In addition, Roller et al. showed that treatment with inulin enriched with oligofructose stimulates IL-10 and interferon-γ production by Peyer's patch cells(Reference Roller, Rechkemmer and Watzl52).
In the present study, IL-10 mRNA levels were increased in the DSS-only mice (group 2), similar to results reported by Niessner & Volk(Reference Neissner and Volk53), who showed increased IL-10 mRNA levels in crude extracts of colon biopsies from ulcerative colitis patients, and of Kucharzik et al. (Reference Kucharzik, Stoll and Lügering54), who reported elevated serum levels of IL-10 in active colitis.
Since DSS and HWS/ME are both carbohydrate compounds (polysaccharides or oligosaccharides), they can affect simultaneously specific β-glucan receptors such as CR3 (CD11b/CD18); thus the DSS-induced colitis model may be related to effects on the uptake of DSS. To overcome this, HWS (high dosage) was given separately from DSS treatment (before treatment with DSS or after treatment with DSS). In both treatment protocols HWS exerted similar effects to those when HWS was provided during 29 consecutive days (as described earlier) in terms of the effect on the pro-inflammatory cytokine TNF-α, histological appearance of colonic samples, histology damage score and mRNA expression of the pro-inflammatory cytokine IL-1β. All values were essentially similar to those received following HWS together with DSS, indicating that the effect is not due to an effect on DSS uptake. Moreover, these control experiments revealed that glucans not only prevent acute colitis but also they can be used as therapeutic treatment following disease outbreak.
In conclusion, the attenuation of colitis following treatment with glucans extracted from mushroom was associated with a reduction in a pro-inflammatory cytokine. Our findings indicate that high doses of both HWS and ME are effective at treating acute DSS-induced colitis and result in improved macroscopic and histological damage scores, decreased MPO activity levels, decreased mRNA levels of the pro-inflammatory cytokine IL-1β and decreased protein levels of TNF-α. We showed that the effective glucans can be extracted from either mushroom fruiting bodies (HWS) or from mycelium produced by fermentation (ME) and that both treatments, when provided orally, are effective in preventing or curing colitis symptoms.
Acknowledgements
This research was partially supported by a grant from Yissum Research Development Company of the Hebrew University of Jerusalem.
I. L., as a PhD student, was in charge of all experiments. D. L. was the technician in charge of glucan preparations. I. P. was the technician in charge of animal experiments. L. N. was the student in charge of animal care. Y. H., as a Ph.D. and M.Sc. tutor and an expert in fungi biology, co-designed and approved all experiments. B. S., as a Ph.D. and M.Sc. tutor and an expert in gastrointestinal tract pathophysiology, co-designed and approved all experiments.
The authors declare no conflicts of interest.








