Micronutrient deficiencies are known to affect more than 2 billion people globally(1). In Bangladesh, the prevalence of anaemia among adolescent girls varies from 22 to 44 %(2–Reference Ahmed, Hasan and Kabir4) and Fe deficiency is reported to be the primary cause of anaemia(Reference Ahmed, Khan and Islam5–Reference Ahmed, Khan and Banu7). An earlier report noted that nearly half (47 %) of Bangladeshi rural adolescent girls have sub-optimal vitamin A status (serum retinol < 1·05 μmol/l)(8). Dietary surveys also indicate that a large proportion of adolescent girls do not meet their daily requirements for various micronutrients(Reference Jahan and Hossain9, Reference Ahmed, Zareen and Khan10). In a recent study, we also showed concurrent multiple micronutrient (MMN) deficiencies in anaemic adolescent girls in rural Bangladesh(Reference Ahmed, Khan and Banu7).
Intervention studies with micronutrients have shown beneficial effects on growth(Reference Sarma, Udaykumar and Balakrishna11, Reference Hyder, Haseen and Khan12), physical performance(Reference Suboticanec, Stavljenic and Schalh13) and cognitive development(Reference Grantham-McGregor and Ani14, Reference Eilander, Gera and Sachdev15) in schoolchildren and adolescents. Thus, maintaining adequate micronutrient status during adolescence is likely to be crucial for optimal health, including physical and mental performance. In Bangladesh, the first pregnancy is likely to occur before adolescence is completed(Reference Mitra, Al-Sabir and Cross16), and sub-optimal micronutrient status in young Bangladeshi women could be an additional important determinant of adverse pregnancy outcomes.
Coexistence of other micronutrient deficiencies with Fe deficiency is likely to increase the risk of anaemia and limit the haematological response to Fe supplementation(Reference Allen, Rosado and Casterline17). Because Fe deficiency is often accompanied by other micronutrient deficiencies(Reference Ahmed, Khan and Banu7, Reference Ronnenberg, Goldman and Aitken18, Reference Dijkhuizen, Wieringa and West19), a case is increasingly being made for providing MMN supplements instead of Fe–folic acid (IFA) supplements not only for pregnant and lactating women but also for adolescent girls in developing countries to prevent anaemia and micronutrient deficiencies and to improve stores before pregnancy(20, Reference Huffman, Baker and Shumann21). In 1999, the UNICEF, the United Nations University (UNU) and the WHO jointly proposed a formulation for MMN supplements for pregnant women, known as the United Nations International Multiple Micronutrient Preparation (UNIMMAP)(Reference Huffman, Baker and Shumann21). It was also suggested that intermittent supplementation with the UNIMMAP should be promoted for adolescent girls in developing countries(Reference Huffman, Baker and Shumann21).
The Ministry of Health and Family Welfare of Bangladesh endorsed in 2006 a national strategy for the prevention and control of anaemia in Bangladesh developed by a national consultation(22). It identified comprehensive strategies and interventions for, among others, adolescent girls. One of the priority strategies identified was micronutrient supplementation, and IFA supplementation was recommended as the primary intervention. However, considering increasing evidence of the presence of MMN deficiency in this population, the consultation also recommended revising the guidelines if the results of MMN supplementation studies indicated. Currently, the National Nutrition Programme recommends the control of anaemia by providing IFA supplement and anti-helminthic treatment for adolescent girls at the community level by Community Nutrition Promoters(22). However, adolescent girls and non-pregnant women are not yet included in the nationwide operational efforts.
Recently, we reported that long-term (52-week) twice-weekly supplementation with MMN supplement containing a double dose of the UNIMMAP formulation (except folic acid) improved Hb concentration and micronutrient status better than with IFA in Bangladeshi adolescent girls with nutritional anaemia(Reference Ahmed, Khan and Akhtaruzzaman23). However, for a blanket supplementation approach to correct micronutrient deficiency in adolescent girls in Bangladesh, it is important to know the effect of MMN supplementation in non-anaemic girls, too. The present study investigates the effect of long-term (52-week) once-weekly MMN (MMN-1) and twice-weekly MMN (MMN-2) supplementation, using a double dose of all nutrients of the UNIMMAP formulation except for folic acid, and twice-weekly IFA (IFA-2) supplementation, on Hb concentration and micronutrient status of non-anaemic adolescent schoolgirls in Bangladesh. Furthermore, we sought to compare the effect of supplementation at week 26 with that at week 52, within the three supplement groups.
Subjects and methods
Study setting and subjects
The participants were non-anaemic (Hb>120·0 g/l) and non-pregnant girls aged 11–17 years, and were students of five high-schools for girls, in rural Upazilas (sub-districts) of Dhaka, Narayanganj and Narshingdi Districts in Bangladesh. Both the Upazilas and schools were selected purposively based on the willingness of schools to participate and their proximity to Dhaka. The selected Upazilas were not in malaria-endemic areas. Informed written consent was obtained from the participants and their guardians before enrolling in the study. Overall refusal rates were low (10 %). Of the 886 girls screened, 562 girls had Hb concentration>120·0 g/l (non-anaemic) and 324 of them were randomly recruited for the study. Pre-menarcheal girls and those who were identified to be suffering from any disease or taking Fe or multivitamin supplements were excluded from the study. None of the subjects was a beneficiary of the Bangladesh National Nutrition Programme IFA supplementation programme. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and was approved by the Ethical Review Committees of the Bangladesh Medical Research Council, Bangladesh and the University of Queensland, Brisbane, Australia.
The sample size calculation was based on 80 % power and 5 % significance level for two variables: Hb and serum retinol (vitamin A), with the assumption that serum retinol served as a sentinel micronutrient of representativeness of other micronutrients and so served as the basis for calculating the sample size. The sd for Hb in this population is 11·0 g/l, obtained from a previous study(Reference Ahmed, Hasan and Kabir4). Therefore, to detect a difference in Hb of 5·0 g/l between the supplement groups required at least seventy-five girls per group. For serum vitamin A with a sd of 0·33 μmol/l(Reference Ahmed, Khan and Islam5), to detect a difference of 0·175 μmol/l between the supplement groups was calculated to require fifty-seven girls in each group. The expected differences for Hb (5·0 g/l) and serum vitamin A (0·175 μmol/l) used have been found to be biologically significant(Reference Angeles-Agdeppa, Schltink and Sastroamidjojo24). We considered the larger (n 75) of the aforementioned two estimates of sample size, and after including an allowance for dropout of 25 % during the follow-up, each supplement group required 100 girls.
Study design interventions
This study was a randomised, double-blind, controlled trial. Equal numbers of the 324 girls recruited for the study were randomly allocated to one of three supplemented groups: (1) to receive a double UNIMMAP dose of MMN-1 and a placebo; (2) a double UNIMMAP dose of MMN-2; (3) the routine standard dose of IFA-2 for 52 weeks. Randomisation was carried out by using three different letter-coded groups, and the girls were allocated to one of the three letter codes by lottery carried out by an independent researcher at the time of recruitment. The supplement preparations (MMN, IFA and placebo tablets) were identical in appearance. The tablet containers were labelled by the manufacturer (Incepta Pharmaceuticals) with alphanumeric codes assigned by the UNICEF, which were not disclosed to the researchers until preliminary analysis was completed. The preparations were independently analysed by Eurofins Food and Pharma A/S, and the amounts of all ingredients were found within range.
The MMN preparation was the same as the one we had used for the study in anaemic adolescent girls(Reference Ahmed, Khan and Akhtaruzzaman23) in that it contained a double dose of the UNIMMAP formulation of all fifteen micronutrients except folic acid, which was kept at 400 μg (Table 1). The rationale for not doubling the folic acid in the MMN preparation was to maintain parity with the amount of folic acid in the IFA preparation. The formulation recommended in the National Strategy for the Prevention and Control of Anaemia in Bangladesh was used (60 mg Fe and 400 μg folic acid, twice-weekly) in the IFA preparation(22).
Table 1 Composition of the multiple micronutrients (MMN) and iron–folic acid (IFA) preparations*
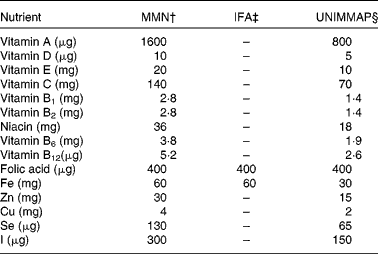
UNIMMAP, United Nations International Multiple Micronutrient Preparation.
* Per tablet.
† MMN (double dose of UNIMMAP formulation except for folic acid); the twice-weekly MMN group received two MMN tablets/week and the once-weekly MMN group received one MMN tablet/week.
‡ Twice-weekly IFA group received two IFA tablets/week.
§ Huffman et al. (Reference Huffman, Baker and Shumann21).
Before the start of the supplementation protocol, all participants were de-wormed with albendazole (400 mg). The girls were again treated with albendazole on the day of blood collection after 26 and 52 weeks of supplementation.
Compliance
The preparations were given to girls on the school premises. The girls swallowed the tablets with water under close supervision of fieldworkers throughout the supplementation period except during the Muslim fasting month of Ramadan (for about 5 weeks) and school holidays (3 weeks) in the second half of the study period. For these periods, the required numbers of tablets were given to the girls in two plastic containers labelled with the codes on the day before holidays and the girls were also given a blank compliance record form containing the dates on which the supplement was to be taken. They were instructed on how and when to take the tablets and how to keep a record of consumption and asked to return the completed compliance forms along with the containers with any unconsumed tablets to the fieldworkers after school reopened.
Data and blood specimen collection
Socio-economic data were collected from parents using a self-administered questionnaire. In the case of illiterate parents, the participants asked their parents and filled in the form. At baseline, trained field staff collected personal and health-related information from the girls, and recorded anthropometric measurements. At baseline, 26 and 52 weeks, 5·0 ml venous blood were drawn from each girl. Details are described elsewhere(Reference Ahmed, Khan and Akhtaruzzaman23). The participant selection process, with the timing of blood collection and reasons for loss to follow-up, are presented in Fig. 1.
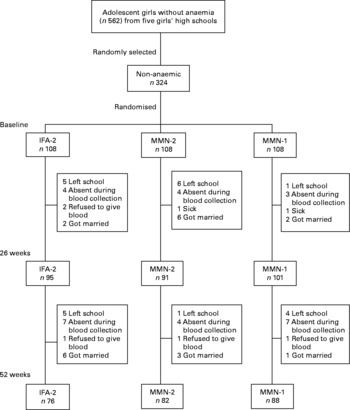
Fig. 1 Selection process for the study participants, timing of blood collection and reasons for loss to follow-up in a randomised, double-blind trial with once- and twice-weekly multiple micronutrients (MMN-1 and MMN-2) and twice-weekly iron–folic acid (IFA-2) supplementation for 52 weeks.
Analytical procedure
Hb concentration was measured by the cyanmethaemoglobin method using a commercial kit (Human Diagnostics). Serum ferritin (SF) concentration was measured by ELISA with a commercial kit (BioCheck, Inc.). Erythrocyte folic acid concentration was determined by a chemiluminescence method using a commercial kit (IMMULITE®, Diagnostic Products Corporation). Serum retinol concentration was determined using HPLC(Reference Ahmed, Hasan and Kabir4). Plasma vitamin C was measured according to Lowry et al. (Reference Lowry, Lopez and Bessey25). Erythrocyte glutathione reductase activation coefficient (EGRAC, a dimensionless number inversely proportional to riboflavin status) was measured according to Vudhivai et al. (Reference Vudhivai, Pongpaew and Praurahong26). Serum C-reactive protein (CRP) was measured by the nephelometric method using a commercial kit (Turbox Plus®; Orion Diagnostica).
Statistical analysis
Statistical analysis was carried out using SPSS (version 17.5; SPSS, Inc.) and SAS (SAS for Windows version 9.2, SAS Institute, Inc.). At baseline, distributions of SF and plasma vitamin C concentrations were skewed and hence these data were log-transformed and then analysed using geometric means and standard deviations. Intention-to-treat analysis was done. Repeated-measures ANOVA was performed using the generalised estimating equations approach within the SAS GENMOD procedure and set up as post hoc contrasts (time), with the χ2 score test based on a generalised score function to compare the concentrations of all biochemical variables at baseline, 26 and 52 weeks, both within and between the three supplement groups. The general linear model, with mean change in concentration as the dependent variable, was used to compare the change over time for each supplement group. The results were adjusted for baseline values for each variable. The values are presented as means and standard deviations.
Anaemia was defined as Hb level < 120·0 g/l(27). Nutrient deficiencies were defined as: SF concentration < 12 μg/l for Fe(28), < 16·5 μmol/l for plasma vitamin C(Reference Jelliffe and Jelliffe29), < 317 μmol/l for erythrocyte folic acid (DPC Diagnostic), < 1·05 μmol/l for serum vitamin A(30) and EGRAC>1·4 for riboflavin(Reference Powers, Bates and Duerden31). Prevalence of anaemia and deficiency of micronutrients in the three supplement groups were compared by the χ2 test or Fisher's exact test, as appropriate. Prevalence of anaemia and micronutrient deficiencies at baseline, 26 and 52 weeks were further compared by logistic regression, with deficiency status (present/absent) as the dependent variable. OR and 95 % CI were estimated by the likelihood ratio method after adjusting for baseline status for each variable.
Results
Information on dropouts
Of the 324 girls enrolled, 246 completed the 52-week protocol: 70·4 % of the IFA-2 group, 75·9 % of the MMN-2 group and 81·5 % of the MMN-1 group. Dropout rates between the groups did not differ significantly. Reasons for dropout were: left school (28·2 %), absent on the day of blood collection (37·2 %), refused to give blood (6·4 %), ill (2·6 %) and got married (25·6 %). Reasons for dropout were not significantly different between the three groups. Baseline anthropometric, socio-economic and biochemical measures of the girls who completed the study protocol were similar to those of the girls who left the study, except for age. Age of the girls who left the study (14·1 (sd 1·3) years) was significantly (P =0·001) higher than that of the girls who completed the study (13·5 (sd 1·1) years).
Compliance with the intervention
For those who completed the 52-week study, the mean number of tablets consumed by the IFA-2 group (94 (sd 9)) was not significantly different from the mean number of tablets consumed by the MMN-2 group (92 (sd 11)). Only 9 % of IFA-2 and 18 % of MMN-2 girls consumed less than 80 % of the total doses of 104 tablets. The mean number of MMN tablets consumed by the MMN-1 group was 46 (sd 7), and 14 % consumed less than 80 % of the total doses of fifty-two tablets.
Baseline comparison
Baseline age, body weight, height, BMI, parents' education level and per-capita family income did not differ among the three supplement groups for those who completed the study protocol (data not shown). At baseline, the concentrations of Hb and the various micronutrients (vitamins A, B2, C and folate), and the prevalence of deficiencies did not differ significantly between the three supplement groups, except for serum vitamin A concentration of the MMN-1 group, which was significantly lower than that of the MMN-2 group (Table 2), and for folate deficiency, which was higher in the MMN-1 and MMN-2 groups than in the IFA-2 group (Table 4).
Table 2 Effect of once- or twice-weekly multiple micronutrients (MMN-1 or MMN-2) supplementation and twice-weekly iron–folic acid (IFA-2) supplementation on Hb, circulating micronutrient concentrations and erythrocyte glutathione reductase activation coefficient (EGRAC) at weeks 26 and 52 in non-anaemic adolescent girls who completed the study protocol* (Mean values and standard deviations)
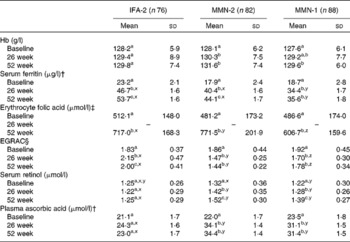
a,b,c Mean values within a column (in each supplement group) with unlike superscript letters were significantly different (P <0·05).
x,y,z Mean values across the rows for each of the listed micronutrients (at each time point)) with unlike superscript letters were significantly different (P <0·05).
* Repeated-measures ANOVA, with time as the repeated measures, was carried out.
† Values were represented as geometric means and standard deviations.
‡ Analysis was not done at 26 weeks.
§ EGRAC (inversely proportional to riboflavin, vitamin B2 status).
C-reactive protein values
Prevalence of high serum CRP concentration, a marker for sub-clinical infection, was low (2·7–7·0 %) in all three groups and there was no difference between the three supplement groups at baseline, 26 or 52 weeks.
Effect on Hb and anaemia
Hb concentration increased significantly in the MMN-2 group at week 26, and at week 52 in the MMN-1 group, but remained unchanged in the IFA-2 group (Table 2). After adjusting for baseline values, mean changes in the Hb concentration did not differ between the supplement groups at week 26 (Table 3). At week 52, the mean change in Hb concentration in the MMN-2 group was 2·0 g/l higher than in the IFA-2 group and the difference approached statistical significance (P =0·07).
Table 3 Changes in Hb, circulating micronutrient concentrations and erythrocyte glutathione reductase activation coefficient (EGRAC) values after 26 and 52 weeks of once- and twice-weekly multiple micronutrients (MMN-1 and MMN-2) supplementation and twice-weekly iron–folic acid (IFA-2) supplementation in the non-anaemic girls who completed the study protocol* (Adjusted mean values and standard deviations)
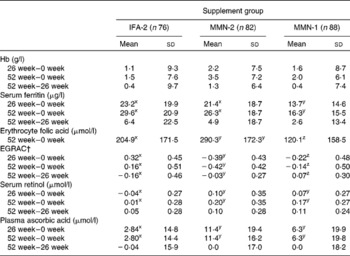
x,y,z Mean values across the rows for each of the listed micronutrients (at each time point) with unlike superscripts letters were significantly different (P <0·05).
* Based on the general linear model with change in level as the dependent variable, assumed to be normally distributed. Adjusted for baseline value for each variable.
† EGRAC (inversely proportional to riboflavin, vitamin B2 status).
At week 26, 3·7 % of the girls in the MMN-2 group and 14·5 % of the girls in the IFA-2 group had developed anaemia (P =0·05). The girls in the IFA-2 group were more likely to be anaemic compared with the girls in the MMN-2 group at week 26 (OR, 5·1, 95 % CI 1·3, 19·5; P =0·018; Table 4). About 10 % of the girls in the MMN-1 group developed anaemia at week 26, which then decreased to 3·4 % at week 52. At this time (week 52), the prevalence of anaemia in the MMN-1 and MMN-2 groups was lower than in the IFA-2 group; however, the difference was not statistically significant.
Table 4 Prevalence of anaemia and various micronutrient deficiencies at baseline, and at 26 and 52 weeks of supplementation in subjects by supplement group, and the estimates of OR for the prevalence of anaemia and various micronutrient deficiencies (Odd ratios and 95 % confidence intervals)
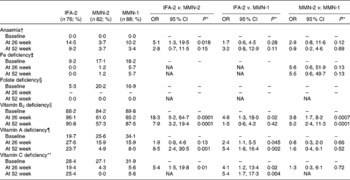
IFA-2, twice-weekly iron–folic acid; MMN-2, twice-weekly multiple micronutrients; MMN-1, once-weekly multiple micronutrients; NA, model could not be fitted due to small frequencies.
* Based on logistic regression and adjusted for baseline values.
† Hb level < 120·0 g/l.
‡ Serum ferritin < 12·0 μg/l.
§ Erythrocyte folic acid < 317 μmol/l.
∥ Erythrocyte glutathione reductase activation coefficient>1·4.
¶ Serum retinol (vitamin A) < 1·05 μmol/l.
** Plasma vitamin C < 16·5 μmol/l.
Effect on serum ferritin and iron deficiency
SF concentration increased significantly in all three supplement groups at week 26, which increased further at week 52 except for the MMN-1 group (Table 2). The mean changes in SF concentration in the IFA-2 and MMN-2 groups were not significantly different at weeks 26 and 52, but was significantly lower in the MMN-1 group than in the IFA-2 and MMN-2 groups at both time points (Table 3). The prevalence of Fe deficiency decreased significantly in all three groups at weeks 26 and 52 (Table 4).
Effect on folic acid status
Erythrocyte folic acid concentration increased significantly in all three groups at week 52 (Table 2). The mean change in the erythrocyte folic acid concentration in the MMN-2 group was significantly greater than in the IFA-2 group, whereas it was significantly less in the MMN-1 group than in either the IFA-2 or MMN-2 group (Table 3). At week 52, the prevalence of folate deficiency reduced to zero in all three groups (Table 4).
Effect on vitamin B2 status
Mean EGRAC (inversely related to riboflavin status) in the MMN-1 and MMN-2 groups decreased significantly but increased in the IFA-2 group at week 26 (Table 2). The EGRAC did not differ significantly at week 52 from that at week 26 in any of the groups, except in the IFA-2 group. The mean changes (decrease) in EGRAC in the MMN-1 and MMN-2 groups were significantly different from the mean change (increase) in the IFA-2 group, both at weeks 26 and 52, i.e. riboflavin status improved in both MMN groups after supplementation. However, the mean change (decrease) in EGRAC in the MMN-1 group was significantly lower than in the MMN-2 group at both time points (Table 3). The prevalence of vitamin B2 deficiency decreased significantly in the MMN-2 group both at weeks 26 and 52, while it increased in the IFA-2 group (Table 4).
Effect on vitamin A status
Serum vitamin A concentration in the MMN-2 and MMN-1 groups increased significantly at both weeks 26 and 52, while it remained unchanged in the IFA-2 group (Table 2). The mean changes in serum vitamin A concentration in the MMN-2 and MMN-1 groups were significantly greater than in the IFA-2 group at both time points (Table 3). The prevalence of vitamin A deficiency decreased significantly in both the MMN-1 and MMN-2 groups at week 26 followed by a further decrease in prevalence at week 52 (Table 4).
Effect on vitamin C status
Plasma vitamin C concentration increased significantly in the MMN-1 and MMN-2 groups at week 26, which remained unchanged at week 52. No change in plasma vitamin C concentration was observed in the IFA-2 group (Table 2). The mean changes in plasma vitamin C concentrations in the MMN-2 and MMN-1 groups were significantly greater than in the IFA-2 group at both time points (Table 3). The prevalence of vitamin C deficiency decreased significantly in the MMN-2 and MMN-1 groups at week 26, with a further decrease in prevalence at week 52 only in the MMN-2 group (Table 4).
Discussion
The present study shows that concurrent deficiencies of several micronutrients are common in this population, supporting the previous findings in Bangladeshi adolescent girls(Reference Ahmed, Khan and Banu7, Reference Jahan and Hossain9, Reference Ahmed, Zareen and Khan10). Overall, there was a small increase in mean Hb concentration in the girls receiving MMN-1 or MMN-2 and IFA-2 supplements for a period of 1 year (52 weeks). This is not surprising, as the girls were not anaemic. A study in non-anaemic Mexican women reported no increase in Hb concentration after supplementation with either MMN or Fe only(Reference Moriarty-Craige, Ramakrisnan and Neufeld32). When compared with the supplement groups, the mean increase in Hb concentration in the MMN-2 group was 2·0 g/l greater than in the IFA-2 group at week 52, and after adjustment for baseline Hb, the difference between the two groups was not statistically significant (P =0·07). However, prevalence data reveal that significantly fewer girls in the MMN-2 group developed anaemia than in the IFA-2 group after 26 weeks of supplementation, which could have programmatic implications. Additionally, the odds of developing anaemia in the girls receiving IFA-2 was 5·1 times greater than the odds of developing anaemia in the girls receiving MMN-2 after 26 weeks (P =0·018). Although not significant, after 52 weeks, the odds of developing anaemia among the girls in the IFA-2 group was 2·8 times greater than the girls in the MMN-2 group (P =0·15). The results indicate that MMN-2 supplementation may not bring a considerable additional benefit compared with receiving IFA supplements twice a week in improving Hb concentration in this population, whether for 26 or 52 weeks. However, supplementation with MMN supplements can provide an additional benefit over IFA supplements in preventing anaemia in non-anaemic Bangladeshi adolescent girls whose dietary intake of nutrients is inadequate. In the earlier report on Bangladeshi adolescent girls with nutritional anaemia(Reference Ahmed, Khan and Akhtaruzzaman23), we found a small (2·4 g/l) but significantly (P = 0·045) greater increase in Hb by MMN-2 supplementation than by IFA-2 supplementation at week 26. A recent systematic review of MMN supplementation in children also found that addition of MMN to Fe supplements resulted in a marginal improvement in Hb response when compared with an Fe supplement alone(Reference Gera, Sachdev and Nestel33).
Although there is no single ideal biological measure for Fe status, SF concentration has been widely used as a marker of body Fe stores and/or Fe status in nutrition surveys and clinical assessment(34). In the present study, we also used SF concentration as a measure of Fe status. A significant proportion of the girls in this study were Fe-deficient in varying degrees, with 15 % having low Fe reserves at recruitment. At week 52, a significant increase in SF concentration and a substantial reduction in the prevalence of Fe deficiency were observed in both the IFA-2 and MMN-2 groups. The girls in the MMN-2 and IFA-2 groups consumed a maximum cumulative dose of 6240 mg of Fe over a 1-year-period (52 weeks), which would have contributed 57 % of their RDA for Fe (30 mg/d), assuming that they ate a largely vegetarian diet(35). Further, there was a significantly lesser increase in SF and slightly greater prevalence of Fe deficiency in the MMN-1 group than in the IFA-2 and MMN-2 groups – indicating a greater impact of MMN-2 and IFA-2 supplementation compared with the MMN-1 supplementation on Fe status, which was not unexpected as the MMN-1 group got half the dose the two other groups were given. Similar results were also observed in anaemic adolescent girls(Reference Ahmed, Khan and Akhtaruzzaman23). Given that SF levels increase in the presence of sub-clinical infection or inflammation(Reference Thompson, Milford-Ward and Whicher36), the SF data were also analysed after controlling for serum CRP concentrations, and the results were not different from that of the unadjusted findings (data not shown). Thus, it is reasonable to suggest that long-term, MMN-2 or IFA-2 supplementation with 60 mg doses of Fe can prevent Fe deficiency effectively and increase body storage of Fe.
Although the overall effect size of either IFA or MMN supplements on Hb concentration in the present study was small over the period of 52 weeks, when compared between the two time periods, the increase in Hb and SF concentrations in all supplement groups in the first 26-week period was relatively greater than the increase in the second 26-week period, similar to what was observed in anaemic girls(Reference Ahmed, Khan and Akhtaruzzaman23). Another supplementation study using an MMN-fortified beverage for 12 months in Bangladeshi rural adolescent girls reported similar findings on Hb and SF levels(Reference Hyder, Haseen and Khan12). One explanation could be that the rate of Fe and other micronutrient absorption and Hb synthesis was higher at the beginning, when the deficiency was greater than in the later part of the study, when concentrations had already reached their optimal levels. Another explanation could be lower compliance during the 8-week holidays in the second 26-week period of the trial, when the girls themselves consumed the supplements at home.
In the present study, we also examined the effect of MMN supplements on the status of other micronutrients. As expected, the mean increase in erythrocyte folic acid in the MMN-1 group was significantly lower than in the MMN-2 and IFA-2 groups. Although both the IFA-2 and MMN-2 groups were given the same dose of folic acid (400 μg) for 52 weeks, the mean increase in erythrocyte folic acid was significantly higher in the MMN-2 than in the IFA-2 group, a finding similar to what was observed in our earlier studies in anaemic girls(Reference Ahmed, Khan and Akhtaruzzaman23, Reference Ahmed, Khan and Akhtaruzzaman37). This suggests an influence of one or more of other micronutrients present in the MMN preparation on uptake of folic acid.
Further, there was a significant improvement in riboflavin status, reflected by overall EGRAC, in the MMN-2 group, whereas this did not happen in the IFA-2 group. Nevertheless, many of the girls apparently remained deficient at the end of the trial, similar to the findings that were observed in the anaemic girls(Reference Ahmed, Khan and Akhtaruzzaman23). More frequent supplementation or a higher dose was probably needed to reduce riboflavin deficiency in this population. There were also significantly greater improvements in vitamin A and vitamin C status in both the MMN-1 and MMN-2 groups than in the IFA-2 group. The OR indicate that the girls who were supplemented MMN-1 or MMN-2 were significantly less likely to be deficient in riboflavin or vitamins A and C than were the girls supplemented IFA-2. However, the impact was much higher in the MMN-2 than in the MMN-1 supplemented groups. Given that the food habit of these girls is unlikely to change, supplementation with MMN could be an effective measure to improve micronutrient status.
Any incremental effect of MMN supplement on Hb and the status of the other measured micronutrients was marginal beyond 26 weeks. One possibility is that a time span of 26 weeks was a sufficient period of treatment to rectify any deficits. The other possibility is that continued supplementation may be required to sustain the impact on Hb and other micronutrient status. However, this can only be speculated at this stage, since there was no group in this study to compare the change in micronutrient status after withdrawal of supplementation. Further studies are needed to see whether status deteriorates after withdrawal of supplementation. However, other studies have found an attenuation of supplementation effect over time; so we think the important programmatic, and resource, implications are that such supplementation should generally be done for a period of 26 weeks.
A common problem with Fe supplementation programmes is poor compliance due to the side effects of Fe. However, as reported earlier(Reference Ahmed, Khan and Akhtaruzzaman23), the 60 mg dose of Fe (as ferrous fumarate) used in the supplement preparation was well tolerated by the girls. Poorer compliance has been found with cheaper multi-micronutrient supplements in adolescent girls in an Indonesian study (Bloem, personal communication). Consequently, considerations of cost-effectiveness would need to include any additional costs associated with added micronutrients beyond IFA, as well as the quality of the supplement. The supplements used in the present study were specially prepared and so costing was not done. However, the cost of a multivitamin tablet in Bangladesh is about Taka 1·5 each, compared with Taka 0·18 for each IFA tablet, and while bulk purchasing by the Government or through the UNICEF system would substantially reduce the cost, any increased costs would need to be factored in by the Government when making decisions on supplementation to adolescent girls, a currently neglected group. However, this study was looking at the physiological impact rather than cost-effectiveness, which would require further work before recommendations can be made by the appropriate authorities.
The present study has generated a number of important findings which include: first, twice-weekly supplementation with either IFA or MMN can prevent Fe deficiency effectively. Second, MMN-2 supplementation can substantially prevent other micronutrient deficiencies. Third, the impact of giving MMN-1 supplements on micronutrient status (except for vitamin C) is less than MMN-2 supplements. Fourthly, while long-term MMN-2 supplements can provide only a marginal additional benefit in improving Hb concentration compared with giving IFA supplements twice a week, this significantly reduces the risk of anaemia. It also appears that supplementation beyond 26 weeks has only a limited additional benefit.
In conclusion, this study suggests that the addition of other micronutrients to IFA supplements, given twice-weekly, in non-anaemic Bangladeshi rural adolescent girls can improve their micronutrient status effectively. Further, it merits consideration that MMN-2 supplementation significantly reduced the risk of anaemia in the girls, who were not anaemic at the beginning. To decide whether MMN should be recommended for these girls as a public health programmatic approach, further research is needed to assess whether MMN supplementation has any added health and development benefits over IFA supplementation.
Acknowledgements
The authors' contributions to the present study were as follows: F. A. took the lead in study planning and design, provided guidance on the data collection and wrote the manuscript. M. R. K. supervised the blood collection, laboratory analysis and contributed to the writing of the manuscript. R. K., M. A. and B. N. supervised the field work and data collection. C. P. B. was responsible for laboratory analysis. G. W. contributed to the study design, statistical analysis of the data and interpretation. I. D.-H. contributed to the study design and writing of the manuscript. F. A. had primary responsibility for the final content. All the authors read and approved the final manuscript. None of the authors had any conflict of interest. The study was supported by the UNICEF (Dhaka and New York).







