Iodine deficiency, once endemic in the UK, was eradicated by the concurrent increase in milk-iodine concentration and milk consumption in the post-war years, though neither was aimed at improving human iodine status( Reference Phillips 1 ). From the 1960s, the UK was considered to be an iodine-sufficient country, but data are now emerging that suggest that this may no longer be the case, at least in women of childbearing age( Reference Bath, Walter and Taylor 2 – Reference Lampropoulou, Lean and Combet Aspray 5 ) and pregnant women( Reference Kibirige, Hutchison and Owen 6 – Reference Pearce, Lazarus and Smyth 8 ); indeed, the WHO now classifies the UK as mildly iodine deficient( Reference Andersson, Karumbunathan and Zimmermann 9 ).
Iodine is a key component of the thyroid hormones, which are crucial for brain development, particularly during gestation and early life( Reference Zimmermann 10 ). The WHO iodine recommendation for pregnant women is 250 μg/d, which is considerably higher than the 150 μg/d recommendation for adults. Pregnant women are at a higher risk of developing iodine deficiency as their iodine requirement is higher than that of non-pregnant women. Severe iodine deficiency during pregnancy is well known to cause cretinism and severe mental retardation( Reference Zimmermann 10 ). We have recently found mild-to-moderate iodine deficiency in a large UK cohort of pregnant women and that children of those who have a low iodine status in early pregnancy are more likely to have significantly lower intelligence quotient and reading scores( Reference Bath, Steer and Golding 11 ).
Though it is vital that pregnant women meet their iodine requirements, this is not always achieved even in developed countries such as the USA( Reference Caldwell, Pan and Mortensen 12 , Reference Brough, Jin and Shukri 13 ). Strategies such as salt iodisation programmes that exist in many countries may provide enough iodine for adults but not necessarily for pregnant women( Reference Zimmermann and Andersson 14 ). Therefore, it is important to monitor the iodine status of pregnant women in a population; for this purpose, the WHO recommends collecting spot-urine samples from a group of pregnant women and comparing the median urinary iodine concentration (UIC) with the cut-off value for adequacy (150–249 μg/l)( Reference Secretariat, Andersson and de Benoist 15 ).
The UK has never introduced a national iodine-fortification programme to ensure adequate population iodine intake, as has been done in many countries worldwide( Reference Zimmermann 10 ). Furthermore, advice to pregnant women from the UK Department of Health makes no mention of the need for iodine in pregnancy and nor does it list dietary sources( 16 ). Results from the National Diet and Nutrition Survey suggest that milk (and milk products), fish and eggs are the main dietary sources of iodine (estimated from food diary analysis) in UK adults( Reference Henderson, Irving and Gregory 17 ); certain groups of women may not consume these iodine-rich foods. Not all UK prenatal supplements contain iodine and, in contrast to the situation in the USA, Australia and New Zealand, there is no official recommendation for pregnant women to take an iodine supplement( 18 , Reference Stagnaro-Green, Abalovich and Alexander 19 ).
Iodine deficiency has been demonstrated in studies carried out in pregnant women in Scotland( Reference Barnett, Visser and Williams 7 ), the North East of England( Reference Kibirige, Hutchison and Owen 6 ) and Cardiff( Reference Pearce, Lazarus and Smyth 8 ), but all these studies have limitations: one was only published as an abstract( Reference Barnett, Visser and Williams 7 ), two came out before the publication of the updated WHO iodine requirements for pregnancy( Reference Kibirige, Hutchison and Owen 6 , Reference Barnett, Visser and Williams 7 ), and one reported iodine status of women recruited to a trial( Reference Pearce, Lazarus and Smyth 8 ) who may not be representative of pregnant women in general. There are no data on the iodine status of pregnant women from the South East of the UK or any information on the dietary sources that contribute to iodine status in pregnant women, as none of the previous studies collected dietary data. Furthermore, there is no information on the effect of taking a UK prenatal iodine-containing supplement on iodine status. Therefore, the present study aimed to evaluate iodine status in pregnant women in the South East of the UK and to explore factors that influence iodine status at this critical life stage. On the basis of the sparse UK literature, we hypothesised that women would be iodine deficient by WHO criteria.
Experimental methods
Recruitment of subjects
Pregnant women were recruited consecutively to a cross-sectional study at the time of their first-trimester ultrasound scan (around 12 weeks of gestation) at the Royal Surrey County Hospital, Guildford. Women were not eligible for inclusion if they had a history of thyroid disease or were taking thyroid medication. Owing to budget and time constraints, it was not possible to recruit throughout the year and a decision was made to recruit only in the summer season (July to September 2009). This removed the complication of straddling seasons as milk-iodine content exhibits a seasonal variation( Reference Phillips 1 ) that affects iodine status; other UK studies have found lower UIC in samples collected in summer than in winter( Reference Vanderpump, Lazarus and Smyth 4 , Reference Nelson, Phillips and Morris 20 ).
The participants were asked to provide a spot-urine sample at the ultrasound clinic; all clinics were conducted in the morning, though the timing of sample collection is unlikely to affect the concentration of iodine or creatinine( Reference Wang, Cogswell and Loria 21 ). The participants were also required to complete a short FFQ and a general questionnaire that collected demographic and nutritional information. One of the authors was present during the completion of the questionnaires, giving an opportunity for clarification (e.g. the meaning of ‘iodised salt’). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the NRES Ethics Committee (South East Coast – Surrey, ref 08/H1109/140) and the University of Surrey Ethics Committee (ref EC/2009/39/FHMS). Written informed consent was obtained from all the subjects.
Analysis of dietary intake and nutritional supplement use
The general questionnaire collected details on participant age, smoking status and time since previous delivery (birth in the last 3 years). It also collected information on whether the participant was a vegetarian or vegan and whether she took a prenatal nutritional supplement. The dose of iodine in the supplement, if any, was determined by conducting a shelf-survey of prenatal supplements available in the UK in November 2009.
The forty-six-item semi-quantitative FFQ was designed to obtain detailed information only on foods that were iodine rich or had potential goitrogenic properties. It was intended not to calculate the intake of nutrients, but rather to estimate the quantity of iodine-rich foods consumed (e.g. g of fish/week). The FFQ design was based on the FFQ used in the European Prospective Investigation of Cancer (EPIC) study( Reference Bingham, Welch and McTaggart 22 ). There were nine frequency options for all the foods, except for milk; in the latter case, daily consumption had six frequency options. With the exception of eggs (kept as a frequency coding), all other food items were recoded to the estimated weekly intake (in g). Estimated intake was computed by combining information on the number of weekly portions with the estimated food weights of each portion; medium portion sizes were used( 23 ) and an average portion weight was computed for composite food groups, such as ‘white fish’. The coding of weekly portions was based on that used for the FFQ completed by pregnant women in the Avon Longitudinal Study of Parents and Children (ALSPAC)( Reference Rogers and Emmett 24 ). The weekly portions were as follows: 0 = none/rarely; 0·5 = once a fortnight; 2 = one to four times/week; 5·5 = five to seven times/week; 10 = more than once daily( Reference Rogers and Emmett 24 ).
The individual food items were collapsed into seven food categories: milk (daily intake); seafood (total of processed fish, white fish, oily fish, shellfish and fish roe); meat and poultry (total of all processed meat, red meat and poultry); dairy products (total of all cream, yogurts, butter and cheese); eggs (once/fortnight or less or once/week or more); iodised salt; seaweed; goitrogens (total soya products (soya milk, tofu and other soya products), cruciferous vegetables (broccoli/kale/spring greens, cabbage, Brussels sprouts, cauliflower, turnip/swede, radish) and sweet potatoes). For the purposes of statistical analyses, daily milk consumption was recoded as follows: (1) none; (2) < 140 ml; (3) 140–280 ml; (4) >280 ml. Weekly estimated intake (in g) of seafood, meat/poultry and dairy products was divided into tertiles. For seaweed and iodised salt, the participants were dichotomised into either ‘consumers’ or ‘non-consumers’ due to the low numbers of consumers (n 13 and n 4, respectively).
Laboratory analysis
UIC was measured on a ThermoElemental X-Series ICP-MS (ThermoFisher Scientific) in the Trace Element Laboratory at the University of Surrey. The samples were diluted with an alkaline diluent prepared by dissolving 3·32 g NH4H2PO4 and 1·16 g (NH4)2H2EDTA (Analar grade; Sigma-Aldrich) in deionised water, adding 10·0 ml ammonia solution (specific gravity 0·88) and making up to 1000 ml with deionised water. Using the alkaline diluent, 400 μl of each participant's urine sample were made up to 10 ml. Standard iodine solutions were prepared with potassium iodide solution (Analar grade; Romil Limited) for the construction of calibration curves. To obtain matrix-matched standards, 400 μl of control urine were added to 400 μl of each standard. An internal standard was added to all the samples: rhodium (103Rh) and iridium (192Ir) (both obtained from SPEX Certiprep Limited) were made up in a working standard solution of 1 mg/l (parts per million) in 1 % v/v HNO3 (Trace analysis grade; Fisher Scientific). This solution was made up in a ratio of 1 in 10 with the diluent. In the analysis, 150 μl of this solution were added to every tube. To evaluate the accuracy of the method, certified reference materials were obtained through quality-assurance programme of the Centre for Disease Control, entitled ‘Ensuring the Quality of Urinary Iodine Procedures’ (EQUIP). Our observed mean values for the EQUIP-certified reference materials were as follows: 27·6 (sd 2·3, n 3) for U02 (certified mean 28·7 μg/l, range 20·1–37·3); 47·4 (sd 2·1, n 4) for U05 (certified mean 45·0 μg/l, range 31·5–58·5); 301·8 (sd 7·5, n 4) for U09 (certified mean 296·3 μg/l, range 251·9–340·7); 9·6 (sd 0·8, n 2) for U10 (certified mean 12·2 μg/l, range 8·5–15·9). Urinary creatinine concentration was measured in the Biochemistry Department at the Royal Surrey County Hospital on the ADVIA Chemistry System (Siemens Healthcare) by the Jaffe rate method.
Classification of iodine status
The UK Dietary Reference Values for iodine were published in 1991, and they are outdated as they do not reflect the need for additional iodine during pregnancy( Reference Zimmermann 10 , Reference Delange 25 ). For the present study, we therefore used WHO criteria to assess iodine status in pregnancy( Reference Secretariat, Andersson and de Benoist 15 ). The iodine status of the group was described by comparing the median UIC value with the WHO UIC cut-offs for iodine adequacy in pregnancy( Reference Secretariat, Andersson and de Benoist 15 ). However, these cut-offs cannot be used to identify iodine deficiency in an individual owing to the large intra-individual variation in iodine excretion from day to day in a spot-urine sample; it is often incorrectly assumed that anyone with UIC in a spot-urine sample that is below the cut-off for adequacy (150 μg/l in the case of pregnancy) is iodine deficient, which may well not be the case( Reference Zimmermann 10 ).
Using urinary creatinine concentration in a spot-urine sample to correct UIC for intra-individual variation in daily urine volume produced, we can more closely approach individual iodine status, especially when the age and sex of the individual are taken into account( Reference Knudsen, Christiansen and Brandt-Christensen 26 – Reference Rasmussen, Ovesen and Christiansen 28 ). Therefore, we report iodine status in three ways: as the simple iodine concentration (μg/l), as the iodine:creatinine ratio (μg/g), and as the estimated 24 h iodine excretion (μg/d). The 24 h iodine excretion was estimated by multiplying the iodine:creatinine ratio by the expected daily creatinine excretion, which is 1·23 g/d( Reference Knudsen, Christiansen and Brandt-Christensen 26 ) for our cohort of women aged from 19 to 47 years (on the basis that the value for non-pregnant adults can be used as creatinine excretion is unaltered in pregnancy( Reference Mojtahedi, de Groot and Boekholt 29 , Reference Pahl, Culver and Strong 30 )). To explore relationships between participant characteristics (such as age) and dietary intake (estimated from the FFQ), we used the estimated 24 h iodine excretion value for each participant as a proxy for individual iodine status, though we recognise the limitations of this method.
Statistical analyses
UIC and the estimated 24 h iodine excretion values were not normally distributed and therefore medians with the 25th and 75th percentiles are reported. The estimated 24 h iodine excretion values were log-transformed using the natural logarithm to allow parametric testing. Maternal age was recoded as a categorical variable: 19–34 and 35–49 years. The log-transformed data were subjected to independent t tests or one-way ANOVA to compare two groups or two or more groups, respectively. Spearman's rank correlation was used to explore relationships between continuous data.
Multiple linear regression (using the general linear model in the Statistical Package for Social Sciences) was used to evaluate associations between dietary and demographic factors and the (log-transformed) estimated 24 h iodine excretion; variables that were significantly related to the estimated 24 h iodine excretion in the unadjusted analyses were entered into the model. The model was then used for the calculation of the estimated geometric means with their 95 % CI of the estimated 24 h iodine excretion for each category of milk intake; the geometric means were computed by back-transformation of the adjusted means and 95 % CI of the log-transformed estimated 24 h iodine excretion values.
Significance was set at P< 0·05, and analyses were conducted using the Statistical Package for Social Sciences (version 19.0; SPSS, Inc.).
Results
During the defined study period (July to September 2009), 100 women were recruited to the study. The mean age of the women was 32·4 (sd 4·7) years with a range of 19–47 years. The median UIC was 85·3 μg/l, classifying this group of pregnant women as mildly to moderately iodine deficient( Reference Secretariat, Andersson and de Benoist 15 , Reference Zimmermann 31 ) (Table 1). The median iodine:creatinine ratio (122·9 μg/g) was also low, and the median estimated 24 h iodine excretion value (151·2 μg/d) was considerably less than would have been expected (i.e. 225 μg) if 250 μg iodine (the dietary requirement for pregnant women) had been consumed/d of which 90 % was excreted.
Table 1 Iodine status of pregnant women from the South East of the UK (n 100), reported as iodine concentration (μg/l), iodine:creatinine ratio (μg/g) and estimated 24 h excretion (μg/d)* (Median values with their 25th–75th percentiles)
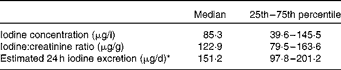
* Age- and sex-adjusted iodine:creatinine ratio on the basis of an expected daily urinary creatinine excretion value of 1·23 g/d( Reference Knudsen, Christiansen and Brandt-Christensen 26 ).
Describing the prevalence of iodine deficiency within the cohort of the present study is challenging. Many authors report the percentage of values below the WHO cut-off of 150 μg/l, which in the present study would be 76 % (or 67 % if using the iodine:creatinine ratio with a cut-off of 150 μg/g). However, this may lead to an overestimation of the extent of deficiency; strictly speaking, the estimated average requirement (EAR), and not the reference nutrient intake, should be used to report the prevalence of deficiency of a nutrient in a population( Reference Allen, de Benoist, Dary and Hurrell 32 ). Hence, it may be more correct to report the percentage with values below the EAR, which we estimated to be 180 μg/d (calculated by converting the pregnancy reference nutrient intake of 250 μg/d to an EAR using the published reference nutrient intake-to-EAR conversion factor for iodine of 1·4( Reference Allen, de Benoist, Dary and Hurrell 32 )). Therefore, the expected 24 h urinary excretion value would be approximately 160 μg/d (assuming a 90 % excretion of the EAR of 180 μg/d). By these calculations, 53 % (n 53) of the women in the present study were found to have an estimated 24 h iodine excretion value below the EAR.
Participant characteristics and iodine status
Table 2 summarises the effect of participant characteristics on maternal iodine status. There was no significant difference in iodine excretion by smoking status, consumption of a vegetarian diet (there were no vegans) or time since last birth (Table 2). The estimated 24 h iodine excretion value was significantly higher in women aged above 35 years than in those aged 19–34 years (P= 0·01; Table 2). We explored the relationship between maternal age and urinary creatinine excretion to investigate whether the higher iodine status at older age was explained by lower creatinine excretion( Reference Haddow, McClain and Palomaki 33 ); we did find a significant negative correlation between maternal age and urinary creatinine concentration (r − 0·21, P= 0·01). To test whether older women consumed more iodine-rich foods, we explored correlations between dietary food groups and maternal age and found a positive association with seafood (r 0·22, P= 0·03) and cheese (r 0·30, P= 0·003) (data not shown).
Table 2 Estimated 24 h iodine excretion* (μg/d) according to participant characteristics (Number of participants, percentages and median values with their 25th–75th percentiles)
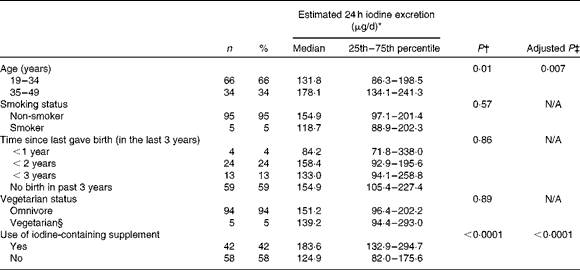
N/A, variable not entered into the multivariate analyses.
* Age- and sex-adjusted iodine:creatinine ratio on the basis of an expected daily urinary creatinine excretion value of 1·23 g/d.
† P value for comparison between the groups from independent t test or ANOVA conducted on log-transformed estimated 24 h iodine excretion data.
‡ Adjusted P value from the general linear model with the following variables: maternal age; supplement use; milk, seafood and egg intake.
§ One woman was classified as a pescatarian, but was excluded from the statistical analysis that compared vegetarians and omnivores.
Use of an iodine-containing nutritional supplement
A total of seventy-five (75 %) participants were taking a nutritional supplement at the time of recruitment (including single folic acid supplements); fifty-one (51 %) women were taking a multivitamin or mineral supplement, but only forty-two (42 %) women were using a prenatal vitamin and mineral formulation that contained iodine. The median content of iodine in the supplements reported by the participants was 140 μg/dose (range 75–150 μg). The iodine status of women who took an iodine-containing supplement was significantly higher than that of those who did not take such a supplement; this relationship was demonstrated both by the simple iodine concentration measure (P< 0·0001) and by the estimated 24 h iodine excretion (P< 0·0001; Table 2). Fig. 1 shows the result obtained in the present study in comparison with the WHO recommended levels for a population of pregnant women( Reference Secretariat, Andersson and de Benoist 15 ). The median UIC of women taking an iodine-containing supplement (111 μg/l) was closer to the WHO adequate range for pregnant women (150–249 μg/l) than was that of women not taking such a supplement (61 μg/l), which was close to the severe-deficiency level ( ≤ 50 μg/l)( Reference Zimmermann 31 ).
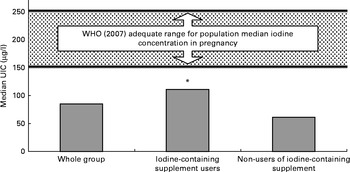
Fig. 1 Iodine status of women by the use of iodine-containing supplements. * Urinary iodine concentration (UIC) was significantly higher in women taking an iodine-containing supplement than in non-users (P< 0·0001; analysis on log-transformed data).
Dietary influences on iodine status
Iodised salt was rarely or never used by 96 % of the women, and there was no significant difference in iodine excretion between consumers and non-consumers (Table 3). There was no difference in iodine status between consumers (n 13) and non-consumers of seaweed (P= 0·23; data not shown). Weekly estimated intake of goitrogens or meat and poultry was not significantly associated with iodine excretion (Table 3). Although iodine excretion was higher in the top tertile of dairy-product intake than in the bottom tertile (Table 3), the difference was not significant (P= 0·10).
Table 3 Estimated 24 h iodine excretion* (μg/d) according to maternal diet (Median values with their 25th–75th percentiles)
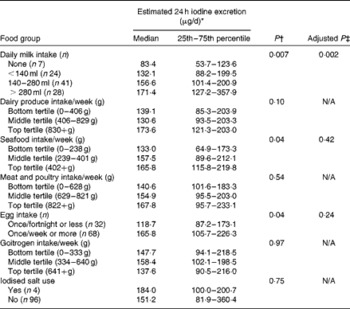
N/A, variable not entered into the multivariate analyses.
* Age- and sex-adjusted iodine:creatinine ratio on the basis of an expected daily urinary creatinine excretion value of 1·23 g/d.
† Results from ANOVA on the log-transformed estimated 24 h iodine excretion data.
‡ Adjusted P value from the general linear model with the following variables: maternal age; supplement use; milk, seafood and egg intake.
By contrast, the intakes of milk, eggs and seafood were positively associated with the estimated 24 h-iodine excretion (Table 3). The difference in iodine status between the categories of milk consumption was highly significant (P= 0·007), and post hoc tests indicated that women who did not consume milk had significantly lower iodine excretion than those consuming more than approximately 280 ml/d (P= 0·008). Consumption of up to approximately 570 ml of goats' milk/d was reported by one woman; she was an outlier with an estimated 24 h iodine excretion value of 1414·0 μg/g. The estimated 24 h iodine excretion differed significantly between the tertiles of seafood intake (P= 0·04), and the post hoc test indicated that iodine excretion was significantly lower in the bottom tertile than in the top tertile (P= 0·03; Table 3). Iodine excretion differed significantly with the frequency of egg consumption (P= 0·04); higher iodine excretion was associated with a greater frequency of weekly egg consumption (Table 3).
In the adjusted analyses (which included significant variables from univariate analyses), the only factors significantly associated with the estimated 24 h iodine excretion were categories of milk consumption (P= 0·002), use of an iodine supplement (P< 0·0001) and maternal age (P= 0·007). There were no significant interactions between the variables. The model explained 31·1 % of the variance in the estimated 24 h iodine excretion (adjusted R 2 0·311); milk 15·1 % (partial η2= 0·151), iodine supplement use 14·5 % (partial η2= 0·145) and maternal age 7·8 % (partial η2= 0·078). The intakes of eggs (P= 0·24) and seafood (P= 0·42) were not significantly associated with iodine excretion in the adjusted model. When all the other factors were controlled for, there remained evidence of a trend of increasing estimated 24 h iodine excretion with increasing daily milk consumption (Fig. 2).
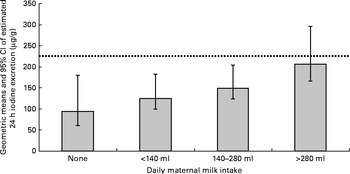
Fig. 2 Adjusted geometric means and 95 % CI of estimated 24 h iodine excretion (computed by back-transformation of the log-transformed 24 h iodine excretion data) by maternal milk intake (variables included in the model: frequency of egg intake; maternal age (years); use of iodine-containing supplement; tertiles of weekly seafood intake (g)). ![]() represents the expected value (225 μg) for 24 h iodine excretion if 250 μg iodine (the dietary requirement for pregnant women) were consumed per d of which 90 % was excreted.
represents the expected value (225 μg) for 24 h iodine excretion if 250 μg iodine (the dietary requirement for pregnant women) were consumed per d of which 90 % was excreted.
Discussion
The present results support our hypothesis that pregnant women in the South East of the UK would have an inadequate iodine status as the median UIC classified the women as mildly to moderately iodine deficient( Reference Secretariat, Andersson and de Benoist 15 , Reference Zimmermann 31 ). The present results, together with those from earlier UK studies( Reference Kibirige, Hutchison and Owen 6 – Reference Pearce, Lazarus and Smyth 8 ), suggest that UK pregnant women are not meeting the higher iodine requirements of pregnancy; this may be having a negative effect on fetal brain development( Reference Zimmermann 10 ). Indeed, the level of deficiency found in the present study (median UIC 85·3 μg/l) is similar to that in an Australian study (in women recruited before mandatory iodine fortification of bread in 2009)( Reference Hynes, Otahal and Hay 34 ) and in our own recent UK study( Reference Bath, Steer and Golding 11 ) (median UIC 81 and 91·1 μg/l, respectively); these studies found associations between iodine status in pregnancy and poorer cognition (intelligence quotient, reading ability and spelling) in children up to the age of 9 years.
As we collected only one urine sample from each woman, we cannot accurately describe the prevalence of iodine deficiency in the present study; ideally, multiple urine samples from each woman should be collected to adjust the iodine concentration distribution for intra-individual variation( Reference Zimmermann and Andersson 14 ). However, while recognising the limitation that one urine sample from each woman may not reflect usual iodine status, we feel that reporting the percentage of women below the EAR threshold (53 %) is a more accurate reflection of the extent of iodine deficiency than the traditional approach of reporting the percentage below the WHO cut-off (150 μg/l)( Reference Secretariat, Andersson and de Benoist 15 ) (which reflects the reference nutrient intake for pregnancy). In our opinion, the methodology for the use of the EAR cut-point method during pregnancy needs further development.
We found that maternal age was positively associated with the estimated 24 h iodine excretion. There are two potential explanations for this: older women consume more iodine-rich foods and urinary creatinine excretion decreases with age (which we found), thus increasing the iodine:creatinine ratio. Data from the 2000/01 Adult National Diet and Nutrition Survey suggest an increased dietary iodine intake with advancing age, as older women had a significantly higher iodine intake (estimated from food diaries) than younger women( Reference Henderson, Irving and Gregory 17 ).
Women who reported taking an iodine-containing supplement had a significantly higher iodine status than those who did not take such a supplement, which is consistent with other research findings in Europe( Reference Alvarez-Pedrerol, Ribas-Fito and Garcia-Esteban 35 – Reference Andersen, Sorensen and Krejbjerg 37 ) and New Zealand( Reference Pettigrew-Porter, Skeaff and Gray 38 ). Health authorities in America, Australia and New Zealand recommend that all pregnant (and lactating) women take a supplement containing 150 μg/d of iodine during pregnancy( 18 , Reference Stagnaro-Green, Abalovich and Alexander 19 ). There are just two trials that supplemented pregnant women with iodine in regions of mild-to-moderate iodine deficiency that included child cognitive outcomes( Reference Berbel, Mestre and Santamaria 39 , Reference Velasco, Carreira and Santiago 40 ); while these suggest a benefit for child cognition, further good-quality evidence from a randomised controlled trial is needed. In the present study, we obtained no data on maternal or neonatal thyroid function or on long-term cognitive outcomes in the offspring that would support benefit (or indeed lack of harm( Reference Rebagliato, Murcia and Alvarez-Pedrerol 41 , Reference Moleti, Di Bella and Giorgianni 42 )) of initiating iodine supplementation in pregnancy. Despite the fact that 51 % of the women in the present study were taking a prenatal multivitamin and mineral supplement, only 42 % were taking a supplement that contained iodine – a reflection of the fact that, at the time, only 67 % of UK prenatal supplements contained iodine and that there is no official UK advice to take an iodine supplement during pregnancy. A higher percentage of women in the present study were taking an iodine-containing supplement than were women in the USA( Reference Perrine, Herrick and Serdula 43 , Reference Gregory, Serdula and Sullivan 44 ), Switzerland( Reference Andersson, Aeberli and Wust 45 ), New Zealand( Reference Pettigrew-Porter, Skeaff and Gray 38 ) or Australia( Reference Charlton, Gemming and Yeatman 46 ). Most UK pregnant women are unaware of the need for iodine( Reference Williamson, Lean and Combet 47 ), so the presence of iodine in the supplement is unlikely to influence choice, but the leading UK prenatal supplement brand does contain iodine. In addition, Surrey is an affluent area( 48 ) and therefore supplement use may be higher than that in other, less-affluent, UK regions( Reference Henderson, Gregory and Swan 49 ).
The finding that a higher intake of milk was related to higher urinary iodine excretion is consistent with the findings of studies in pregnant women in other countries( Reference Alvarez-Pedrerol, Ribas-Fito and Garcia-Esteban 35 , Reference Perrine, Herrick and Serdula 43 , Reference Mian, Vitaliano and Pozza 50 , Reference Brantsaeter, Haugen and Julshamn 51 ). The present results suggest that women consuming more than approximately 280 ml of milk per d had an iodine status greater than the cut-off for iodine adequacy in pregnancy, even after adjusting for other factors. However, it needs to be remembered that this milk-volume value is related to the intake of summer milk (as we recruited participants over the summer), which has a lower iodine content than the winter milk. The inaccuracies of a FFQ for collecting dietary intake data should also be kept in mind.
We have shown a positive association between egg intake frequency and maternal iodine status, although the significance disappeared in the adjusted analyses. This is in contrast to the findings of the UK study of iodine status of teenage schoolgirls, which surprisingly found that a high intake of eggs was associated with low urinary iodine excretion( Reference Vanderpump, Lazarus and Smyth 4 ), though we have previously suggested that failings in the FFQ in that study (ambiguous and unquantified frequency options) resulted in spurious associations( Reference Bath and Rayman 52 ). Iodine status was higher in women in the top tertile of fish intake, but the relationship was no longer significant when adjusted for other dietary factors. The intake of seafood also failed to exhibit associations with the iodine status of pregnant women in Spain( Reference Alvarez-Pedrerol, Ribas-Fito and Garcia-Esteban 35 , Reference Costeira, Oliveira and Ares 53 ) and teenage girls in the UK( Reference Vanderpump, Lazarus and Smyth 4 ). The lack of a strong relationship between fish intake and iodine status (compared with the relationship with the intake of milk, despite its lower iodine concentration) may be explained by the insensitivity of an FFQ (see limitations) and the relatively poor consumption of fish by many UK women( Reference Henderson, Gregory and Swan 49 ). The fact that such a low percentage of pregnant women in the present study reported the use of iodised salt may reflect its poor availability in UK supermarkets( Reference Bath, Button and Rayman 54 ).
The present study was limited by the relatively small sample size; however, a study of adults has estimated that 100 spot-urine samples (if corrected to estimate 24 h iodine excretion) are sufficient to give a population estimate of urinary iodine excretion (within a 95 % CI) with a precision of ± 10 %( Reference Andersen, Karmisholt and Pedersen 55 ). Nevertheless, the stratified analyses in the present study (e.g. by age or dietary pattern) are based on a small sample size and therefore the results should be treated with caution. The other major limitation of the study is the fact that recruitment was conducted only in the summer months and in only one region of the UK (Surrey). Given the known seasonal variation in the iodine content of dairy products( Reference Phillips 1 ), it is likely that the present study represents a worst-case scenario. On the other hand, with Surrey being an affluent county, the women in the present study are likely to have a higher socio-economic status than those in many other UK regions. Thus, results may be biased towards a higher iodine status, as the intake of iodine-rich foods, particularly fish, may be greater in more-affluent women. A large multi-centre study is needed to provide a more comprehensive view of the iodine status of UK pregnant women as Surrey women may not be representative of UK women in general. The evaluation of the impact of diet on iodine status is limited by the inherent limitations of a FFQ. It is perhaps unsurprising that some dietary items failed to exhibit associations with iodine status, given that iodine excretion reflects recent (previous 24–48 h) dietary iodine intake( Reference Rasmussen, Ovesen and Christiansen 28 ) and FFQ evaluate intake over a longer time period. Thus, consumption of foods consumed regularly, such as milk, is more likely to affect the iodine concentration of a spot-urine sample than food items consumed irregularly and in low amounts within the cohort, such as fish; this is likely to be especially true in studies with small sample sizes, such as the present study. We are aware that there are limitations of using a single spot-urine sample for individual iodine status assessment, and although we have attempted to overcome some of these limitations by use of the age- and sex-adjusted iodine:creatinine ratio, this method still has limitations( Reference Konig, Andersson and Hotz 27 ); future studies might explore the use of body weight for further adjustment of the iodine:creatinine ratio and the collection of repeated urine samples for the measurement of iodine status in the same individual( Reference Konig, Andersson and Hotz 27 ).
In conclusion, we have demonstrated iodine deficiency in pregnant women in the South East of the UK – a cause for concern, given the need for adequate iodine for fetal brain development( Reference Zimmermann 10 ). The intakes of milk and iodine-containing supplements were the most important predictors of iodine status in UK pregnant women. Women who follow the current government generic pregnancy healthy eating advice of consuming 2–3 portions of dairy products per d, together with the recommended 1–2 portions of fish per week, should receive an adequate supply of iodine. However, for women who do not consume these iodine-rich foods, an appropriate prenatal iodine-containing supplement is probably appropriate (although kelp supplements should be avoided); indeed, in the present study, we have demonstrated that the use of such a supplement is associated with a higher iodine status. Ensuring adequate iodine intake is likely to be particularly important before pregnancy to ensure that stores of iodine are optimised so that there is no shortfall in early gestation( Reference Moleti, Di Bella and Giorgianni 42 , Reference Moleti, Lo Presti and Campolo 56 ). The dietary advice to UK pregnant women needs to be revised to explain why increased iodine intake during pregnancy is important and good dietary sources should be clearly signposted.
Acknowledgements
The authors are extremely grateful to the women who participated in the study and to the staff of the antenatal clinic at the Royal Surrey County Hospital for support during subject recruitment.
A PhD studentship for S. C. B. from the Waterloo Foundation and Wassen International covered the costs of the study and an MRC Population Health Scientist Fellowship supported S. C. B. during the writing of the manuscript. The Waterloo Foundation, Wassen International and MRC had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: S. C. B., J. W. and M. P. R. designed the study; S. C. B. recruited the participants, facilitated the laboratory analysis, conducted the statistical analyses and prepared the first draft of the manuscript; A. W. and A. T. developed the laboratory method and completed the laboratory analysis; S. C. B. and M. P. R. wrote the manuscript; all the authors approved the final manuscript.
None of the authors has any conflicts of interest to declare.







