Postoperative pericardial tamponade is defined as a complication that may lead to multiple organ failure by causing low cardiac output syndrome with an increase in ICU and in hospital stay as well as morbidity and mortality.Reference You, Shim and Hong1 Even trace amounts of pericardial collection especially in the posterior pericardium may lead to haemodynamic disturbance due to compression of the left atrium and the left ventricle. Neonates and infants may be at high risk for postoperative haemorrhage due to increased haemodilution after cardiopulmonary bypass circuit priming and also because of the immaturity of the coagulation systems or coagulation deficiencies.Reference Guzzetta, Allen, Wilson, Foster, Ehrlich and Miller2,Reference Wolf, Maher, Kanter, Kogon, Guzzetta and Mahle3 Furthermore, complex cardiac surgical procedures, systemic hypothermia, and extensive suture lines may also contribute to postoperative bleeding.Reference Wolf, Maher, Kanter, Kogon, Guzzetta and Mahle3 A posterior left pericardiotomy, which was first described by Mulay et al. in 1995, allows prolonged drainage of pericardial fluid into the left pleural space.Reference Mulay, Kirk, Angelini, Wisheart and Hutter4 Since then, several studies revealed that posterior pericardiotomy reduces the incidence of pericardial effusion after adult cardiac surgical procedures, with only few contradictory reported results.Reference Gozdek, Pawliszak and Hagner5 Readmission requirement after cardiac surgery with an incidence of 1.1% is a well-known morbidity after cardiac surgery.Reference Elias, Glatz and O’Connor6 Posterior pericardiotomy in adults has been mentioned in several studies to in order to prevent mediastinal blood and effusions in the pericardium to become significant and lead to pericardial tamponade.Reference Gozdek, Pawliszak and Hagner5 We considered the posterior pericardiotomy as a factor that may remarkably reduce the incidence of early and late pericardial tamponade after paediatric cardiac surgical procedures. Since there is limited evidence and gaps in the literature regarding the use of posterior pericardial window in the paediatric population, we aimed to present our experience with creation of a posterior pericardial window following congenital cardiac surgical procedures to evaluate the effectiveness of the posterior pericardial window in preventing pericardial tamponade following paediatric cardiac surgical procedures.
Materials and methods
This retrospective and single-centre study was approved by the local Ethics Committee with the decision number 03/01/2023-001 and conducted in accordance with the principles of the Declaration of Helsinki. The primary outcome of this study was determined as preventing the early and late pericardial tamponade by surgically creating a posterior pericardial window that effectively drains the pericardial space to the left thoracic cavity after congenital cardiac surgical procedures. A total of 229 patients who had undergone congenital cardiac surgical procedures via a median sternotomy in our centre between June 2021 and January 2023 were analysed retrospectively. A posterior pericardial window was created in all patients. Patients operated on without cardiopulmonary bypass support were excluded from the study group due to the fact that lifting the heart for creating a posterior pericardiotomy would not be safe without cardiopulmonary bypass support. Patients who were operated on via a thoracotomy incision were also excluded. Informed consents were obtained from the participants. Regarding the fact that no tamponade was encountered in the study group, comparative subgroup analysis was not made.
Surgical technique
The posterior pericardiotomy was surgically created at the beginning of the surgical
procedure after the cardiopulmonary bypass was initiated. After the heart was lifted very firmly, the anatomical structures such as the phrenic nerve, the diaphragm, and the left inferior pulmonary vein are identified carefully. After holding and elevating the pericardium with a long curved console clamp (thus moving away from oesophagus and descending aorta), the pericardiotomy is performed by a long-tipped electrocautery device. During this procedure, only the cutting function of the electrocautery device is used in order to avoid any thermal damage to the phrenic nerve. In neonates and infants, pericardiotomy is performed in the form of an equilateral triangle with a length of 2 cm on each side and 3 cm for the childhood patients, respectively (Fig. 1). Creation of an adequate area that would provide effective drainage from the pericardial space to the left hemithorax without causing cardiac herniation as pointed out by Yorgancioglu et al. previously (Fig. 2).Reference Yorgancioglu, Farsak, Tokmakoglu and Gunaydın7 The pericardium was partially closed in all patients except those who were left to delayed sternal closure due to haemodynamic instability or coagulopathy. Primary closure was also not feasible for the patients whose sections of the pericardium was harvested for use in cardiac repair. Further, in patients who did not have suitable pericardial tissue for closure after re-do surgery, the pericardium was left open. A curved chest tube was placed and positioned at the posterolateral pericardiophrenic sinus. Regardless of the posterior pericardiotomy and the left thoracic tube insertion, a straight anterior mediastinal chest tube was also inserted in every patient. In patients who underwent bidirectional Glenn, Fontan and Senning procedures, additionally, the right pleura was opened and an additional curved drain was placed in the right hemithorax. During the postoperative course, continuous suction was applied to all drains in the ICU follow-up. The assessment of pericardial effusion accumulation was performed daily by two-dimensional echocardiography, whilst the patient was followed-up in the ICU. After the patient was taken to the wards, two more echocardiographic evaluations were performed until the patient was discharged. The first outpatient clinic visit was scheduled at the seventh day after discharge; at that time two-dimensional echocardiographic evaluation was performed. Thirty days after discharge was choosen as the cut-off for echocardiographic surveillance for late tamponade. Late pericardial tamponade was defined as a cardiac tamponade presenting between the seventh and thirtieth postoperative day. Late pleural drainage was defined as a pleural effusion or chest tube insertion after removing the intraoperatively chest tubes. Pacer wires were routinely removed before discharge. During the postoperative period, intravenous heparin was administered in three to four divided injections per day, and the dose was adjusted according to the activated clotting time levels. Oral acetylsalicylic acid was administered at 5 mg/kg/day beginning from the first postoperative day. As indicated and necessary, patients received oral warfarin therapy in addition to oral acetyl salicylic acid therapy.
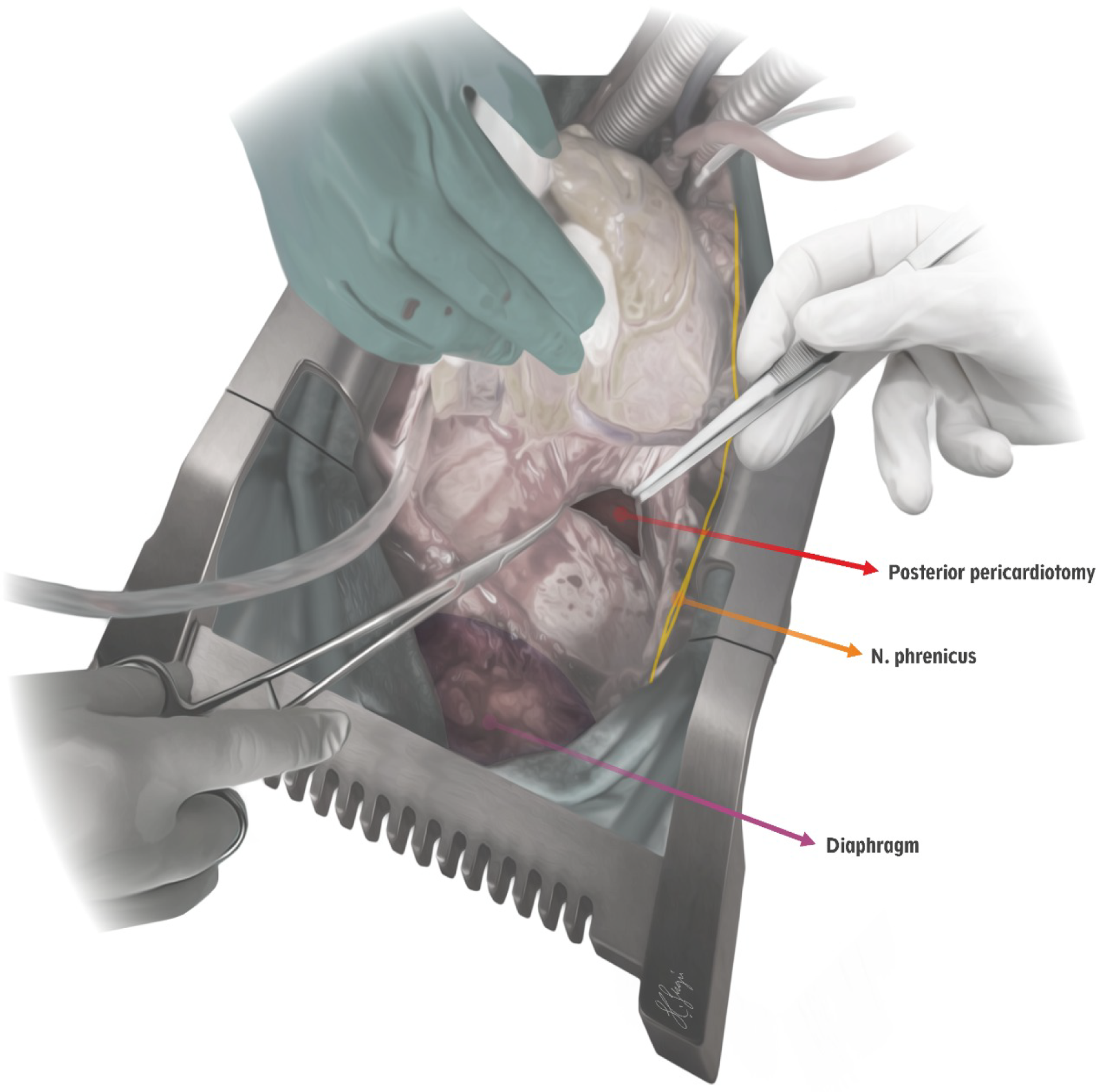
Figure 1. Illustration of the posterior pericardiotomy procedure. The posterior pericardiotomy in the form of an equilateral triangle is configured to be located at a safe distance from the phrenic nerve and above the diaphragm.
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 20.0 software (IBM Corp., Armonk, NY, USA). Continuous data and variability measures were expressed in mean ± standard deviation, central tendency measures were expressed in median (minimum-maximum), while categorical data were expressed in number and frequency.
Results
Among the 229 patients, 135 were male (58.9%) whereas 94 (41.1%) were female. Mean age and body weight were 24.2 ± 26.7 months (range 5 days-12 years) and 10.2 ± 6.7 kg (range 2.3-57 kg), respectively. Eight (3.5%) of the patients were neonates where 109 (47.6%) were infants and 112 (48.9%) were in childhood. All of the patients were operated with cardiopulmonary bypass support for underlying cardiac malformations. Fifty-two (22.7%) re-do operations were performed for staged repairs of complex defects or replacement of degenerated or outgrown prosthetic material. Twenty-six (11.3%) bidirectional Glenn, 17 (7.4%) Fontan completion, 6 (2.6%) surgical repair for tetralogy of Fallot, and 3 (1.3%) aortic surgery were categorised in re-do operations with no pericardial tamponade development postoperatively. Six (2.6%) patients underwent deep hypothermic circulatory arrest during the entire surgical procedure. Delayed sternal closure was performed electively in 9 (3.9%) patients due to haemodynamic instability or severe coagulopathy. None of these patients with delayed sternal closure developed pericardial tamponade. Of the 229 patients evaluated in the study group, seven needed extracorporeal membrane oxygenation (ECMO) support in the postoperative period. None of the patients requiring ECMO support developed pericardial tamponade during or after ECMO support. The demographic, operative, and postoperative data of the patients are summarised in Table 1.
Table 1. The demographic, operative, and postoperative data of the patients.

AC = aortic clamping; CM = centimetres; CPB = cardiopulmonary bypass; DHCA = deep hypothermic circulatory arrest; ECMO = extracorporeal membrane oxygenation; ICU = intensive care unit; KG = kilograms; LOHS = length of in-hospital stay; MIN = minutes; RACHS-2 = risk stratification for congenital heart surgery.
Details of the surgical procedures are summarised in Table 2. Reopening of the sternum due to surgical site bleeding was carried out in 6 (2.6%) patients in the early postoperative period. One (16.6%) of these patients was a neonate, three (49.8%) were infants and two (33.3%) were in childhood. Considering the Risk Stratification for Congenital Heart Surgery (RACHS-2) categories of patients undergoing surgical re-exploration for bleeding; four patients were in category 3, one patient was in category 2, and one patient was in category 6. Types of repair was central aortopulmonary shunts in 2 patients, intra-extra cardiac Fontan completion in 1 patient, double outlet right ventricle repair in 1 patient, tetralogy of Fallot repair in 1 patient, and Damus–Kaye–Stansel procedure in 1 patient whom underwent surgical re-exploration for bleeding. None of the patients who underwent surgical re-exploration for bleeding developed pericardial tamponade. The number of patients who did not undergo post operative surgical re-exploration for bleeding but were under close follow-up due to prolonged haemorrhagic drainage was 26 (11.3%). The mean blood loss was 18.3 ± 8.7 ml/kg/hour for this group. However, pericardial tamponade did not develop either, in the group of patients who were closely followed-up due to prolonged haemorrhagic drainage but did not require surgical re-exploration. Among the 197 (86.1%) patients remaining, the mean blood loss was 7.1 ± 2.6 ml/kg/hour. Late pleural drainage was encountered in two patients in whom chylothorax was the diagnosis. Surgical intervention was considered in these patients with a persistently high volume of chylous output and a prolonged leak. None of the patients who underwent posterior pericardiotomy developed diaphragmatic paralysis. The overall mortality was 3 (1.3%) in the study group. The mean follow-up period was 18.5 ± 5.7 months (range 4-20 months). We did not encounter any early or late pericardial tamponade in this patient population.
Table 2. Details of the surgical procedures.

ASD = atrial septal defect; ASO = arterial switch operation; CAVSD = complete atrioventricular septal defect; DORV = double outlet right ventricle; TAPVC = total anomalous pulmonary venous connection; ToF = tetralogy of Fallot; VSD = ventricular septal defect.
Discussion
Pericardial effusion may result in increased intrapericardial pressure within the pericardial cavity, which may eventually lead to haemodynamic disturbance, clinically termed as tamponade.Reference Adrichem, Le Cessie and Hazekamp8 This clinical scenario may end up with untoward complications in the
ostoperative period such as hypotension, acute renal failure, arrhythmia, prolonged ICU follow-up, and even mortality. A surgically created left pericardiopleural window has been reported in literature as a safe and reproducible method in adult cardiac surgery.Reference Conti9 However, such a preventive technique has not been analysed in patients undergoing congenital cardiac surgery and to the best of our knowledge this is the first study to evaluate the effectivity of posterior pericardiotomy after paediatric cardiac surgical procedures in order to prevent pericardial effusion and/or tamponade. On the other hand, cardiopulmonary bypass use, right-sided heart defects, a longer duration of continuous positive airway pressure ventilation postoperatively, postpericardiotomy syndrome, older age at surgery, and a higher body surface area have been identified as risk factors for the development of pericardial effusion and/or tamponade after paediatric cardiac surgery.Reference Adrichem, Le Cessie and Hazekamp8
Pericardial collection mainly conveyed via mediastinal drainage tubes after cardiac surgical procedures; nonetheless, the posterior pericardial wall may be speculated as an unprotected area, which may cause pericardial effusion to develop septae with an appearance of adhesions to prevent shed blood drainage.Reference Kaya, Iyigun and Yazici10 In addition, the use of postoperative anticoagulant medication may result in subsequent persisting fluid collection.Reference Erdil, Nisanoglu, Kosar, Erdil, Cihan and Battaloglu11 Placement of posterior pericardial drainage tubes was proposed by several cardiac surgeons with certain drawbacks such as induced arrhythmias and tube occlusion.Reference Kaya, Iyigun and Yazici10,Reference Moss, Miller and Jensen12 Nevertheless, the posterior pericardial drains might cause an enclosed gap and make the drainage insufficient due to the pericardial adhesions in between the posterior-inferior surface of the heart and the diaphragm.Reference Eryilmaz, Emiroglu and Eyileten13 Despite the fact that the pericardial and mediastinal collections are drawned out with closed-suction systems, it is a well-known fact that the drainage tubes we prefer in the paediatric patient group are more likely to obstruction due to their small diameter.
Inflammatory process is another pathophysiological mechanism for postoperative late pericardial effusions after cardiac surgical procedures. The most common speculated theory of postpericardiotomy syndrome and postoperative late pericardial effusion is an immune-mediated inflammatory process which involves the pericardium.Reference Lehto, Gunn, Karjalainen, Airaksinen and Kiviniemi14. Whilst the severity of postpericardiotomy syndrome varies, life-threatening cardiac tamponade may develop. Most recent studies have provided evidence of postpericardiotomy syndrome in 3–43% of children with tamponade occuring in 1–2%.Reference Elias, Glatz and O’Connor6,Reference Heching, Bacha and Liberman15 Elias et al. presumed that postpericardiotomy syndrome due to pericardiotomy and right atriotomy combined with chronic volüme overload may be speculated as a risk factor for late pericardial effusion and tamponade.Reference Elias, Glatz and O’Connor6 Contrary, there have been conflicting reports in the literature.Reference Dalili, Zamani and Aarabi-Moghaddam16 In our clinical experience, we observed that a posterior pericardial window is an effective method in order to prevent pericardial tamponade due to postpericardiotomy syndrome and related late pericardial effusion.
One of the issues that is debated in literature is whether the respiratory mechanics will be affected when the left pleural cavity of all patients is opened. Although a study examining the effects of pleural integrity on respiratory functions in patients undergoing paediatric cardiac surgery has not been conducted yet, Rezk et al. stated that preservation of pleural integrity has beneficial effects on pulmonary function and has fewer associated pulmonary complications after coronary bypass surgery. On the other hand, Iskesen et al. confirmed preservation of pleural integrity does not have any beneficial effect on respiratory functions after adult cardiac operations.Reference Rezk, Elgazzar, Abo Youssef, Emeraa, Elkafoury and Moussa17 Posterior pericardiotomy may also result significantly reductions in the duration of postoperative mechanical ventilation.Reference Iskesen, Kurdal, Yildirim, Cerrahoglu and Sirin18 Maze et al. also mentioned that the durations of suffusing of both the pericardial and mediastinal drains were significantly shorter in the posterior pericariotomy group, whilst the left pleural drainage occured more frequently in the non-posterior pericardiotomy group.Reference Sirch, Ledwon, Püski, Boyle, Pfeiffer and Fischlein19 In our experience, we did not observe or encounter any prolonged mechanical ventilation nor reduction in the duration of postoperative mechanical ventilation related to opening of the left pleura. However, it may not be correct to draw this conclusion from our study with regard to different parameters affecting the duration of mechanical ventilation in the paediatric population.
Sen et al. asserted that the pericardial effusion might most frequently be localised around the right atrium and the right ventricle to cause compression; hence, they performed right pericardial window in 120 adult cardiac surgical procedures.Reference Maze, Tokui and Murakami20. They observed that opening right pericardial window in adult cardiac surgical patients was more effective to prevent cardiac tamponade, pericardial effusion, and related postoperative complications than drainage tubes located in the posterior pericardium without opening both pleural cavity. Contrary, they found no statistical difference in between the rates of pneumothorax, pneumonia, pleural effusion, and extubating times. They stated about the easier compression theory to the right atrium and the right ventricle within less pericardial hematoma or effusion compared to the left atrium and ventricle. Although late pericardial effusion may be more common around the right heart, our preferred location for the window is the deepest corner of the pericardial cavity in which the fluid should accumulate under the influence of gravity and pass through the left pericardio-pleural window easily. On the other hand, we think that the right pericardio-pleural window may not drain the effusion accumulated around the left ventricle and the apex, which is the deepest anatomical structure of the pericardium.
Cakalagaoglu et al. performed posterior pericardiotomy on 50 adult cardiac surgical procedures in a case series of 100 patients. In this randomised controlled trial, they found that moderate, large, and very large pericardial effusions before discharge was statistically significantly higher in patients who were included in the non-posterior pericardiotomy group. At the same time, they stated that they encountered late pericardial tamponade in a total of 6 patients in the non-posterior pericardiotomy group. None of the patients who underwent posterior pericardiotomy developed either early or late pericardial tamponade.Reference Sen, Aydin and Iyigun21
Postoperative pericardial effusion after cardiac surgical procedures is harmful with a reduce in both cardiac output and the intensity of ventricular wall motion. This process may lead to either early or late pericardial tamponade, resulting in emergent surgery or postoperative cardiac arrest, and increasing medical costs.Reference Cakalagaoglu, Koksal and Baysal22,Reference Lazaros, Vlachopoulos, Lazarou, Tousoulis and Tsioufis23
Zhe-an Shen et al. mentioned about some drawbacks of posterior pericardiotomy. According to their meta-analysis, there was lack of coagulation function of the posterior pericardiotomy group compared to the control group. They stated that activation of prothrombin factor after pericardial incision may be the main reason to result in stronger coagulation function with a potentially fatal complication for patients after cardiac surgery. Nevertheless, it is also reported that after posterior pericardiotomy, patients may have complications such as pericarditis, pleurisy, and even right heart failure.Reference Vakamudi, Ho and Cremer24,Reference Shen, Hou and Yu25,Reference Gaudino, Sanna and Ballman26
In conclusion, we speculate that a posterior left pericardiotomy is a safe, simple, non-complicating, and effective method in paediatric cardiac surgical procedures to prevent cardiac tamponade. Posterior left pericardiotomy may bear numerous benefits by inducing both pericardial and mediastinal effusions into the left pleural cavity with excreting the inflammatory cells from the pericardial and mediastinal spaces. We strongly believe that especially draining the retained shed blood to the left pleural cavity in the early postoperative period hinders the possibility of early cardiac tamponade. By this pathway whether serous or sero-sanguinous fluid occurs in the late postoperative period, creating a posterior left pericardiotomy also may prevent late cardiac tamponade.
Limitations
The main limitations of this study are the lack of blinding and the retrospective nature of the study. Another limitation is that the study was conducted at a single-centre and consequently may contain provincial tendency which limits generalisation. Additional prospective randomised controlled studies to compare the groups in which posterior pericardiotomy is performed and not applied may help to provide further insight about reassuring the prevention of either early or late pericardial tamponade. Further, the long-term effects of this technique have to be investigated with future research.

Figure 2. Volume rendered 3-dimensional intraoperative view of the posterior pericardiotomy. The posterior pericardiotomy procedure is performed safely while the patient is on cardiopulmonary bypass, especially before the surgical procedure was initiated. Left inferior pulmonary vein is clearly identified.
Acknowledgements
The authors would like to thank Sabri Cagri Sezgin for illustration of the surgical technique and recreating the volume rendered 3-dimensional intraoperative view.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the Helsinki Declaration of 1975, as revised in 2008.







