Introduction
Terrestrial gastropod molluscs (slugs and snails; Mollusca: Gastropoda) play an important role in the environment. Most species are beneficial decomposers, feeding on decaying organic matter and fertilising soil (Barker Reference Barker2001; Meyer et al. Reference Meyer, Ostertag and Cowie2011). However, some species are adapted to home gardens and cultivated fields, causing substantial damage to quality and yield of many horticultural plants and field crops (Halwart Reference Halwart1994; Joshi Reference Joshi, Vreysen, Robinson and Hendrichs2007; Podgornaya et al. Reference Podgornaya, Didenko, Vasilchenko, Kashchits and Mishchenko2020; Abd El-Halim et al. Reference Abd El-Halim, Ali, El-Sayed and Ali2021). Moreover, some species passively transmit spores of plant pathogens such as Sclerotinia trifoliorum, a fungus that infects forage legumes and causes stem rot and mold crown (Shakeel & Mowat Reference Shakeel and Mowat1992). As intermediate hosts of a range of helminth parasites and combined with their dispersal capacity, some gastropod molluscs have become a veterinary and medical concern. They may be able to spread zoonoses not only to pets, livestock, and wildlife, but also to humans (Spratt Reference Spratt2015; Giannelli et al. Reference Giannelli, Cantacessi, Colella, Dantas-Torres and Otranto2016; Ramos-de-Souza et al. Reference Ramos-de-Souza, Maldonado, Vilela, Andrade-Silva, Barbosa, Gomes and Thiengo2021). It is estimated that more than 300 million people suffer from diseases caused by gastropod-borne helminths (Giannelli et al. Reference Giannelli, Cantacessi, Colella, Dantas-Torres and Otranto2016). For instance, the gastropod nematode Angiostrongylus cantonensis Chen, 1935, is the primary cause of human eosinophilic meningoencephalitis in many parts of the Indo-Pacific region (Barratt et al. Reference Barratt, Chan, Sandaradura, Malik, Spielman, Lee, Marriott, Harkness, Ellis and Stark2016; Pandian et al. Reference Pandian, Najer and Modry2023).
Until recently, a common practise to control gastropod molluscs has been the use of chemical molluscicides, including both inorganic salts and organic molluscicides (Kumar Reference Kumar2020; Zheng et al. Reference Zheng, Deng, Zhong, Wang, Guo and Fan2021). However, a significant breakthrough was made with the discovery of the slug-associated nematode Pellioditis (=Phasmarhabditis) hermaphrodita (Schneider 1859) Andrássy 1983 as a biological control agent, an alternative to molluscicides (Wilson et al. Reference Wilson, Glen and George1993; Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007; Tandingan De Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014; Pieterse et al. Reference Pieterse, Malan and Ross2017). The infective dauer juveniles of P. hermaphrodita penetrate and infect slugs in the area beneath mantle surrounding the shell, often causing disease with the characteristic symptom of swelling of the mantle (Wilson et al. Reference Wilson, Glen and George1993). The infection usually leads to death of the slug within a few days. The juveniles develop into adults and a new generation of dauer juveniles released from the slug cadaver spread into soil and infect new hosts. The remarkable efficacy of P. hermaphrodita as a biological control agent has led to a search for other species of Pellioditis suitable for use as biological control agents. Currently, two products are marketed – Nemaslug® launched in 1994, which is P. hermaphrodita formulated with the bacterial associates, Moraxella osloensis (Moraxellaceae) or Psychobacter spp. (Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007; Sheehy et al. Reference Sheehy, Cutler, Weedall and Rae2022), and Nemaslug 2.0® launched in 2022, which is P. californica Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine, & De Ley Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016 also formulated with M. osloensis (Stenberg et al. Reference Stenberg, Melby, Magnusson, Nielsen, Rydning, Wendell, Alsanius, Krokene, Thomsen, Wright and Rafoss2021). The former is commercially available throughout Europe, whereas the latter is available in England, Scotland, and Wales (Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007; Mc Donnell et al. Reference Mc Donnell, Howe and Denver2023).
Besides Pellioditis, slugs and snails also host members of many other nematode genera as either definitive (parasitic), intermediate, or paratenic (phoretic) hosts (Sudhaus Reference Sudhaus2018). These nematodes are known to belong to as many as eight families including Agfidae, Alaninematidae, Alloionematidae, Angiostomatidae, Cosmocercidae, Diplogasteridae, Mermithidae, and Rhabditidae (Pieterse et al. Reference Pieterse, Malan and Ross2017). In recent years, surveys focused in Canada, the USA, Europe, Africa, Australia, and New Zealand substantially increased the knowledge of diversity of gastropod mollusc-associated nematodes (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019; Antzée-Hyllseth et al. Reference Antzée-Hyllseth, Trandem, Torp and Haukeland2020; Brophy et al. Reference Brophy, Howe, Denver and Luong2020). In Belgium, an exploratory survey in two provinces (East and West Flanders) in the northern part of the country revealed the presence of one new and six known slug-parasitic nematode species (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019). To obtain additional information on diversity of gastropod mollusc-associated nematodes in Belgium and to specifically characterise Pellioditis populations found in the country, a follow-up survey was conducted in the current study.
Materials and methods
Slug and snail collection and identification, nematode extraction
Slugs and snails were collected from forests, parks, botanical gardens, and nature reserves at 13 localities in Belgium (Figure 1 shows locations) from September 2020 to May 2021. Purposive, non-probability sampling (Bhardwaj Reference Bhardwaj2019) was done based on the animal’s selectivity towards favourable micro-habitats (such as moist, cool spaces underneath leaf litter and in between rock rubbles, pieces of wood, etc.). Identification of the slugs and snails was based on morphology (Kerney et al. Reference Kerney, Cameron and Riley1979; Mc Donnell et al. Reference Mc Donnell, Paine and Gormally2009; Thomas et al. Reference Thomas, Mc Donnell, Paine and Hardwood2010). The collected slugs and snails were placed in plastic containers lined with tissue paper and stored at 4°C. The tissue paper was regularly sprayed with water to retain moisture and the slugs and snails were fed with spinach (Spinacia oleracea L.). Before dissection, the molluscs were thoroughly washed with tap water to remove debris and nematodes adhering on body surfaces. Individual molluscs were dissected out on glass Petri plates. Nematodes detected in individual molluscs were removed using a needle and transferred into a glass embryo dish containing diluted M9 buffer solution (6 g Na2HPO4, 3 g KH2PO4, 5 g NaCl, 1 L H2O, 1 mL of 1 M MgS04; dilution: nine parts of the M9 buffer solution to one part H2O). Some specimens were then used for DNA extraction, and the remaining were used for morphological analysis as described in the later sections.

Figure 1. Map of the locations where snails and slugs were sampled in Belgium (n = 13). The numbers in parentheses indicate the number of sub-locations within a larger locality.
Nematode morphological analysis
Nematode morphological and morphometric characterisations were done using both live and fixed specimens mounted on glass slides. Temporary mounts of live nematodes were prepared as described in Singh et al. (Reference Singh, Karssen, Couvreur, Subbotin and Bert2021), and some Pellioditis specimens were recovered from slides for culturing on plates as described further. For making permanent mounts of nematodes, they were killed and fixed in a microwave oven for 3 – 5 sec in a few drops of 4% formaldehyde buffered in phosphate-buffered saline + 1% glycerol and kept at 4°C for 24 – 48 h before they were processed to anhydrous glycerine for light microscopy (Seinhorst Reference Seinhorst1959 as modified by De Grisse Reference De Grisse1965). Nematodes were examined, measured, and photographed using an Olympus BX50 DIC microscope equipped with a ToupTek UCMOS05100KPA USB microscope camera with an eyepiece adaptor and ToupView software.
For scanning electron microscopy, fixed specimens were first washed in 0.1M phosphate buffer (pH = 7.5) and dehydrated in a graded series of ethanol solutions, critical-point-dried with liquid CO2, mounted on stubs with carbon tabs (double conductive tapes), coated with gold of 25 nm, and photographed with a JSM-840 EM (JEOL) at 12 kV (Nguyen et al. Reference Nguyen, Trinh, Couvreur, Singh, Decraemer and Bert2019).
Ex vivo culturing of Pellioditis californica and P. hermaphrodita
Ex vivo cultures of P. californica and P. hermaphrodita were established and maintained in Petri dishes (10-cm diameter) filled with humus agar (4 g agar, 130 mL humus, 60 μL cholesterol, 270 mL bi-distilled H2O) (Grootaert & Maertens, Reference Grootaert and Maertens1976). Each Petri dish was inoculated with one hermaphrodite together with lung tissue of the slug or snail (as food source) from which the nematode was extracted, and the Petri dishes were incubated at 15°C.
Nematode molecular analysis
Before DNA extraction, nematode morphological vouchers were prepared as described in Singh et al. (Reference Singh, Karssen, Couvreur, Subbotin and Bert2021). Vouchered nematodes were picked from the temporary mounts, cut into pieces in distilled water, and transferred to 200-μL Eppendorf tubes containing 20 μL of worm lysis buffer (50 mM KCl, 10 mM Tris at pH 8.3, 2.5 Mm MgCl2, 0.45% NP 40 [Tergitol Sigma], 0.45% Tween 20) followed by incubation at –20°C (10 min), adding of 1 μL proteinase K (2.5 mg/mL), incubation at 65°C (1 h) and 95°C (10 min), and ending by centrifugation at 14,000g for 1 min. Amplification of the partial sequences of 18S of ribosomal DNA (rDNA) was done using the primer pair SSU18A: 5′-AAA GAT TAA GCC ATG CAT G-3′/SSU26R: 5′-CAT TCT TGG CAA ATG CTT TCG-3′ (Mayer et al. Reference Mayer, Herrmann and Sommer2007) following the thermal profile of 94°C for 5 min, 35x (94°C for 1 min, 52°C for 1.5 min, and 68°C for 2 min), 68°C for 10 min and a final hold at 4°C. The D2-D3 expansion segment of 28S rDNA was amplified using the primer pair 391F: 5′-AGC GGA GGA AAA GAA ACT AA-3′/501R: 5′-TCG GAA GGA ACC AGC TAC TA-3′ (Nadler et al. Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007) following the thermal profile of 94°C for 4 min, 42x (94°C for 30 sec, 53°C for 30 sec, and 72°C for 1 min), 72°C for 5 min, and a final hold at 12°C (Nadler et al. Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007). For the internal transcribed spacer region of rDNA (ITS1, ITS2, 5.8S), the primer pair Vrain2F: 5′-CTT TGT ACA CAC CGC CCG TCG CT-3′/Vrain2R: 5′-TTT CAC TCG CCG TTA CTA AGG GAA TC-3′ was used (Vrain et al. Reference Vrain, Wakarchuk, Levesque and Hamilton1992) following the thermal profile as described in Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019). Finally, for amplification of the COI region of mitochondrial DNA, the primer pair JB3: 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′/JB4.5: 5′-TAA AGA AAG AAC ATA ATG AAA ATG (Bowles et al. Reference Bowles, Blair and McManus1992) was used following the thermal profile of 94°C for 3 min, 34x (94°C for 30 sec, 45°C for 30 sec, and 72°C for 1 min), 72°C for 10 min, and a final hold at 4°C. An additional primer pair for COI was also used (i.e., COIF1: 5′-CCT ACT ATG ATT GGT GGT TTT GGT AAT TG-3′/COI-R2: 5′-GTA GCA GCA GTA AAA TAA GCA CG-3′) (Kanzaki & Futai Reference Kanzaki and Futai2002) with thermal profile from Etongwe et al. (Reference Etongwe, Singh, Bert and Subbotin2020). The polymerase chain reaction products were purified according to Singh et al. (Reference Singh, Karssen, Couvreur and Bert2020) and sent for sequencing to Macrogen (https://dna.macrogen.com).
Phylogenetic analysis
All the received forward and backward sequences were assembled using Geneious 10.0.9 (https://www.geneious.com) to generate contigs, and BLAST search was performed using the newly obtained sequences to collect closely related species sequences from GenBank. The partial sequence of 18S rDNA and the D2-D3 expansion segment of 28S rDNA were used to analyse the phylogenetic relationships of the nematode species detected in this study. All the generated sequences and the sequences of closely related species from GenBank were separately aligned for each gene segment using MUSCLE with default parameters, followed by manual trimming of the poorly aligned ends. Phylogenetic trees were generated using the GTR + I + G nucleotide substitution model. Markov chains were set with 1 × 106 generations, four runs, 20% burn-in, and subsampling frequency of 500 generations (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001).
Results
In total, 319 gastropods were collected from 13 localities in Belgium, and nine slug and snail species were identified (Table 1). The number of slug and snail species found per locality varied from one (five localities) to three (five localities). Arion vulgaris was the most commonly found mollusc species in this study (at eight localities). About 20% of the total gastropods collected were found parasitized by 11 nematodes species (Table 2), and the majority of the gastropod species contained nematodes (Table 2); only in Cepaea nemoralis and Oxychilus alliarus, no nematodes were found. The highest prevalence of nematodes was observed in C. aspersum (60%) followed by A. vulgaris (34.8%), Limax maximus (28.6%), and Cepaea sp. (20%). The highest numbers of nematode taxa were isolated from the slug species, A. vulgaris (eight) followed by L. maximus and A. hortensis (four each). Co-infections of more than one nematode species were observed in eight of the 319 (2.5%) slugs and snails collected (i.e., in all the species except in Ambigolimax valentianus, Cepaea sp., and L. maximus).
Table 1. Slug and snail species, and associated nematode species collected from 13 localities in Belgium from September 2020 to May 2021

Table 2. Number and point prevalence of nematode species associated with specific slug and snail species collected from 13 localities in Belgium from September 2020 to May 2021
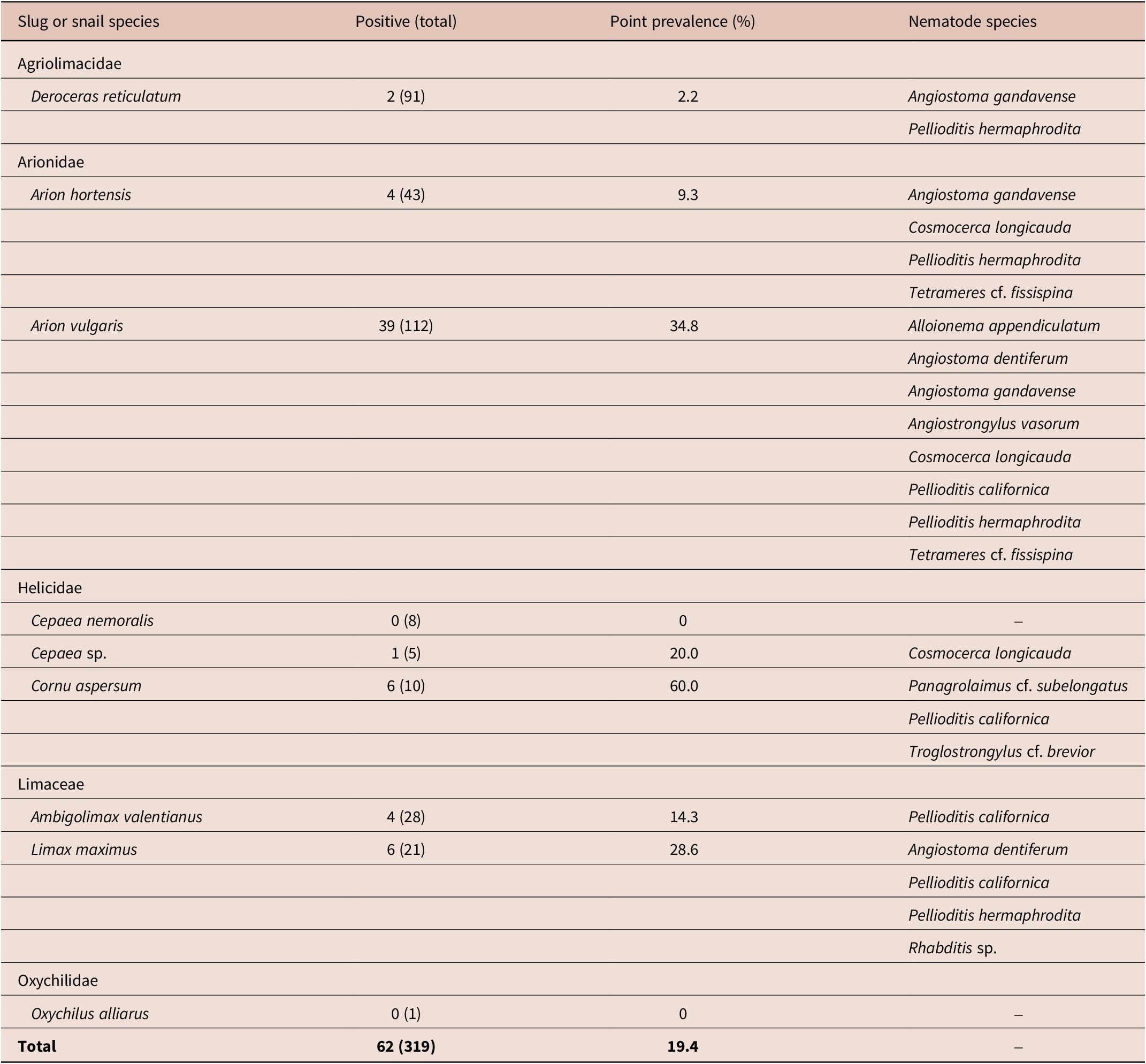
The 11 nematode species that were isolated are Alloionema appendiculatum Schneider, 1859, Angiostoma dentiferum Mengert, Reference Mengert1953, Angiostoma gandavense Singh, Couvreur, Decraemer & Bert, Reference Singh, Couvreur, Decraemer and Bert2019, Angiostrongylus vasorum Baillet, 1866, Cosmocerca longicauda Linstow, 1885, Panagrolaimus cf. subelongatus, P. californica, P. hermaphrodita, Rhabditis sp., Tetrameres cf. fissispina, and Troglostrongylus cf. brevior. The identification of the seven confirmed nematode species were based on morphology, morphometrics, and molecular data; however, comprehensive characterisations of only P. californica and P. hermaphrodita are presented in this paper. The four unconfirmed nematode species were based on limited morphological and molecular data. Three of the nematode species (i.e., A. gandavense, P. californica, and P. hermaphrodita were found respectively at three, five, and eight localities), whereas the remaining nematode species were found at either one or two localities (Table 1), and at three localities, no nematodes were found in the collected slugs and snails (47 specimens). Pellioditis was the most commonly found nematode genus (at nine localities). Most of the nematode co-infections consisted of two nematode species; however, in one A. vulgaris specimen, a co-infection of three nematode species (A. vasorum, P. hermaphrodita, and Tetrameres cf. fissispina) was observed.
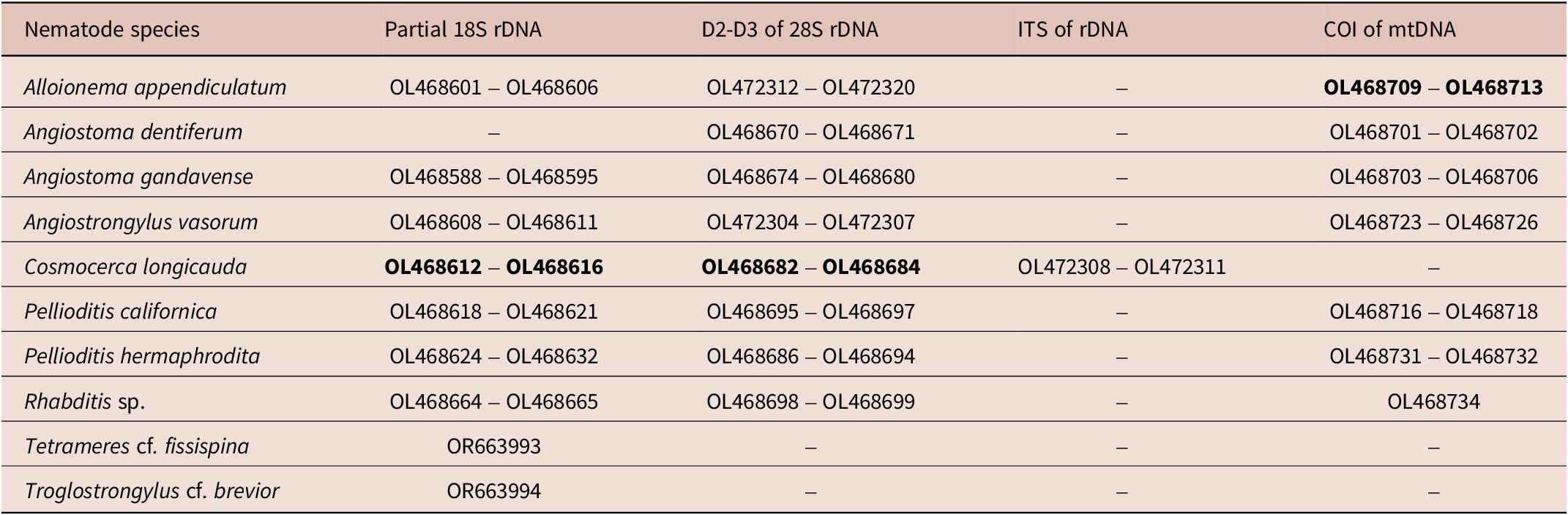
In total, 40 partial 18S, 38 D2-D3 of 28S and 4 ITS of rDNA sequences, and 31 COI of mitochondrial DNA sequences were generated from ten nematode species of which the COI sequences for A. appendiculatum and the 18S and 28S sequences for C. longicauda were generated for the first time (Table 3).
Table 3. GenBank accession numbers of submitted sequences of the slug- and snail-associated nematode species found in this study. Accession numbers in bold are for sequences generated for the first time
Four ex vivo cultures of P. californica and six ex vivo cultures of P. hermaphrodita were established from single hermaphrodites. Ten to 15 days after initiation of the cultures, the first generation of juveniles was observed that developed into gravid hermaphrodites in 24 – 48 h. The cultures were maintained for one month to check for the emergence of males; however, no males were observed in any of the ten cultures.
Pellioditis californica Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley, Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016

Figure 2. Light microscopy (LM) microphotographs and scanning electron microscopy (SEM) images of Pellioditis californica hermaphrodites. (A) Face view; (B – C) anterior end showing lip region; (D) total body; (E – F) anterior body showing up to pharynx end; (G – H) tail region.
Morphological description (Figure 2; Table 3)
Hermaphrodite: (Based on 28 specimens cultured ex vivo on lung tissue of their host.) Body straight or slightly curved in the middle when heat-killed, robust, elongate, gradually tapering to blunt anterior end. Annules fine; lateral field with six incisures. Lip region 16.9 (15 – 19) μm wide, flattened anteriorly, continuous with body, six lips grouped in pairs. One labial papilla emerging from each lip with clear apical dendrite of inner labial papilla. One outer cephalic papilla prominent on each subventral lip and two less prominent on each dorsal lip. Stoma triangular in cross-section, 19.2 (16 – 22) μm long, cheilostom, gymnostom, and stegostom prominent. Stegostom ends in two subventral metarhabdions. Pharyngeal collar reaching up to level of gymnostom. Corpus cylindrical, 119 (101 – 130) μm long, 2.5 times longer than isthmus, gradually expanding into non-valvular metacorpus, narrowing into isthmus, and ending in a bulbous, valvular basal bulb. Nerve ring surrounding anterior of isthmus, at 68.9% of pharynx length. Deirids observed. Secretory-excretory pore opening posteriorly, near base of basal bulb or cardia level. Cardia conoid. Reproductive system didelphic–amphidelphic, anterior and posterior ovaries reflexed, occupying 23.6% and 22.9% of body length, respectively. Oviduct short, filled with sperm in young gravid hermaphrodites; gonads of mature hermaphrodites filled with elliptical eggs (59 x 38 μm), juveniles observed hatching inside hermaphrodites. Vulva transverse slit; vulval lips slightly protruding, located at 50% of body length. Intestine ends in balloon-shaped rectum, 29.8 (23.6 – 37.9) μm long, somewhat shorter than anal body diameter, with four associated cell bodies and sphincter. Anus transverse slit. Anal body diameter 37.7 (27 – 51) μm, about 30% of tail length. Phasmids prominent, located at 37% of tail length. Tail elongate conoid, wider part funnel-shaped.
Male: No males were found in the ex vivo cultures.
Pellioditis hermaphrodita (Schneider, 1859) Andrássy 1983

Figure 3. Light microscopy (LM) microphotographs and scanning electron microscopy (SEM) images of Pellioditis hermaphrodita hermaphrodites. (A) Total bodies; (B) anterior body showing up to pharynx end; (C – D) face view; (E – F) anterior end showing lip region; (G) lateral field; (H) eggs; (I – K) tail region.
Morphological description (Figure 3; Table 3)
Hermaphrodite: (Based on 28 specimens cultured ex vivo on lung tissue of their host). Body straight or slightly curved when heat-killed, robust, elongate, gradually tapering to blunt anterior end. Annules fine. Lateral field with six incisures. Lip region 15.9 (14 – 19) μm wide, flattened anteriorly, continuous with body; six lips grouped in pairs. One labial papilla emerging from each lip with clear apical dendrite of inner labial papilla. One outer cephalic papilla prominent on each subventral lip and two less prominent on each dorsal lip. Stoma triangular in cross-section, 17.6 (16 – 20) μm long; cheilostom, gymnostom, and stegostom prominent. Stegostom ends in two subventral, isomorphic metarhabdions. Pharyngeal collar reaching up to level of gymnostom. Corpus cylindrical, 105 (93 – 116) μm long, 2.3 times longer than isthmus, gradually expanding into non-valvular metacorpus, narrowing into isthmus, and ending in pyriform, valvular basal bulb. Nerve ring surrounding anterior of isthmus at 68.4% of pharynx length. Deirids prominent. Secretory–excretory pore opening at level of the middle or near base of basal bulb. Cardia conoid. Reproductive system didelphic–amphidelphic, anterior and posterior ovaries reflexed, occupying 25.2% and 23.7% of body length, respectively. Oviduct short, filled with sperm in young gravid hermaphrodites; gonads of mature hermaphrodites filled with elliptical eggs (48 x 35 μm); juveniles observed hatching inside hermaphrodites. Vulva transverse slit; vulval lips slightly protruding, located at 50% of body length. Intestine ends in balloon-shaped rectum, 28.7 (24 – 37) μm long, almost as long as anal body diameter, with four associated cell bodies and sphincter. Anus transverse slit. Anal body diameter 30.5 (24 – 37) μm, about one-third of tail length. Phasmids prominent, located at 37% of tail length. Tail elongate conoid, tapering to slightly filiform end.
Male. No males were found in the ex vivo cultures.
Molecular characterisation and phylogenetic relationships
Four identical 18S (OL468618 – OL468621; up to 847 bp), 3 identical D2-D3 (OL468695 – OL468697; up to 888 bp), and three COI (OL468716, 681bp; OL468717 – OL468718, up to 423 bp; upon alignment of both regions gave a contig of 903 bp) sequences were generated for P. californica. The 18S sequences were found to be identical to sequences of P. californica from the USA (KM510210), Canada (MT135094), and New Zealand (MT179851) (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014; Brophy et al. Reference Brophy, Howe, Denver and Luong2020; Howe et al. Reference Howe, Ha, Colton, De Ley, Rae, Ross, Wilson, Nermuť, Zhao, Mc Donnell and Denver2020). The D2-D3 sequences were also identical to sequence of a P. californica isolated from the USA (KM510199) (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014). The COI sequence (OL468716) was 98% similar (11-bp difference) to the only available COI sequence (KM555043) of C. californica (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014, Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016).
Nine identical 18S (OL468624 – OL468632; up to 865 bp), nine identical D2-D3 (OL468686 – OL468694; up to 873 bp), and two COI sequences (OL468731, 627 bp; and OL468732, 414 bp; upon alignment gave a contig of 900 bp) were generated for P. hermaphrodita. The 18S sequences were found identical to sequences of previously isolated P. hermaphrodita from Belgium (MK214808), New Zealand (JQ965811), and Norway (KC883637), and 99.9% (1-bp difference) similar to isolate from the USA (KM510208) (Wilson et al. Reference Wilson, Burch, Tourna, Aalders and Barker2012; Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014; Ross et al. Reference Ross, Ivanova, Hatteland, Brurberg and Haukeland2016; Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019). The D2-D3 sequences were also identical to sequences of previously isolated P. hermaphrodita from Belgium (MK214812) and the USA (KM510194) (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014; Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019). Finally, the COI sequence (OL468731) was 96.5% similar (12-bp difference) to P. hermaphrodita isolate (KM555041) from the USA (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014), and 97% similar (11-bp difference) to that (MK205359) collected from Belgium (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019).
In the inferred phylogenetic tree based on the 18S alignment, our P. californica sequences formed an unresolved clade together with other sequences of P. californica, P. papillosa, and unidentified Pellioditis spp. (Posterior probabilities, PP=1) (Figure 4). On the other hand, our P. hermaphrodita sequences formed a clade together with other sequences of the same species (PP=0.82) and appeared as sister to P. tawfiki (PP=1). The 18S sequences of the other mollusc parasitic nematodes generated in this study were also included in the tree (Figure 4), and sequences of the genera Pellioditis and Angiostoma appeared together in an unresolved clade (PP=1) that is sister to Agfa (PP=1). The observed close relationship between these two genera were similarly reported by Tandingan De Ley et al. (Reference Tandingan De Ley, Kiontke, Bert, Sudhaus and Fitch2023). A further check in the resolution of this clade was done by constructing D2-D3 tree using an alignment of only sequences of Pellioditis and Angiostoma species (Figure 5). In this tree, P. californica appeared as sister to P. papillosa (PP=1), and P. hermaphrodita appeared as a clade with a sister relationship to A. limacis with a weak support (PP=0.52).

Figure 4. Phylogenetic tree based on partial 18S rDNA sequences of snail and slug parasitic nematodes as inferred by Bayesian Inference (BI) using the GTR + I + G nucleotide substitution model. The branch support is indicated by posterior probabilities at each node. Sequences highlighted in green were generated in this study.
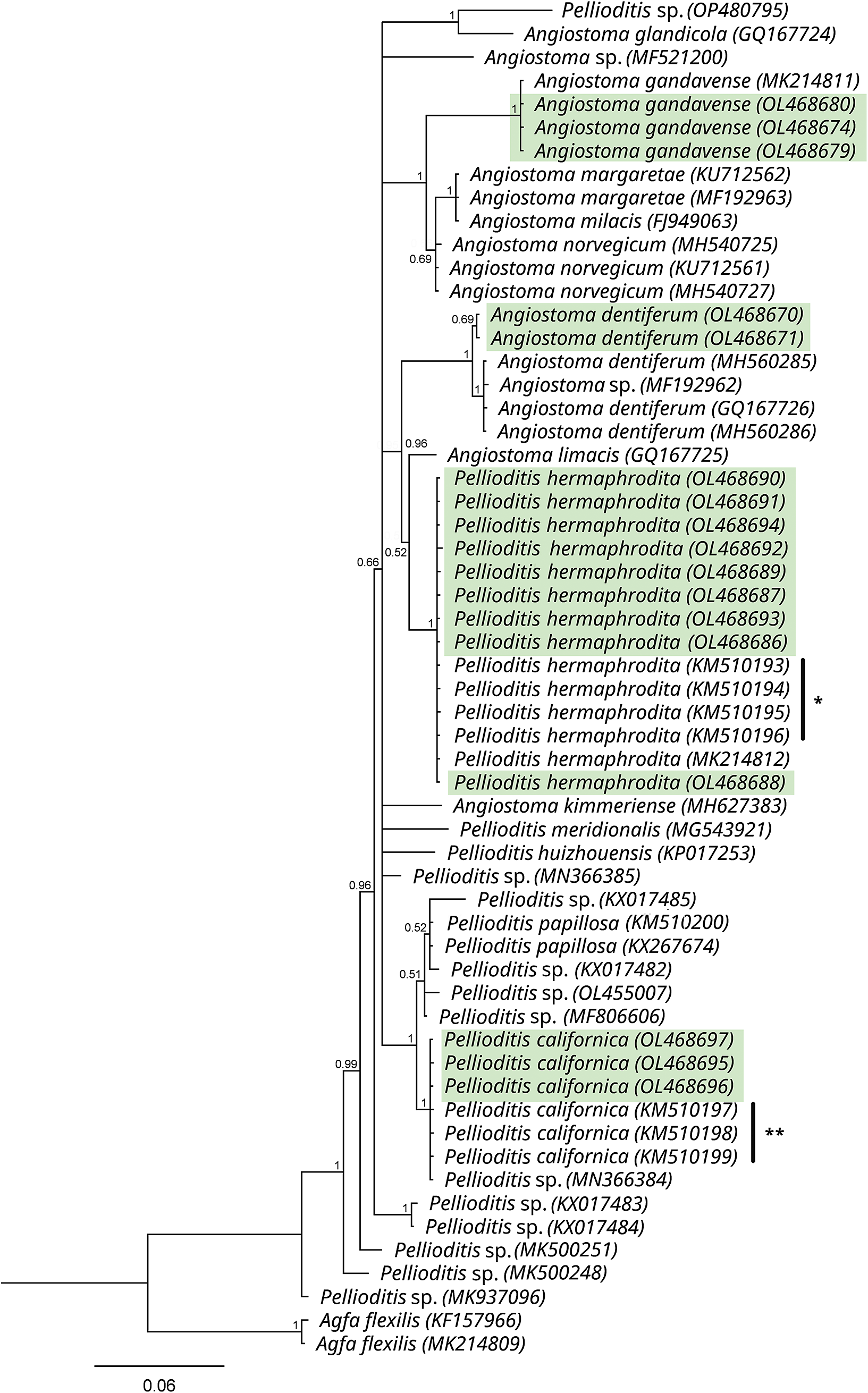
Figure 5. Phylogenetic tree based on the D2-D3 of 28S rDNA sequences of Angiostoma spp. and Pellioditis spp. as inferred by Bayesian Inference (BI) using the GTR + I + G nucleotide substitution model. The branch support is indicated by posterior probabilities at each node. Sequences highlighted in green were generated in this study. (*Sequences of P. hermaphrodita; **Sequences of P. californica from the USA published in Tandingan De Ley et al., Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014).
Remarks
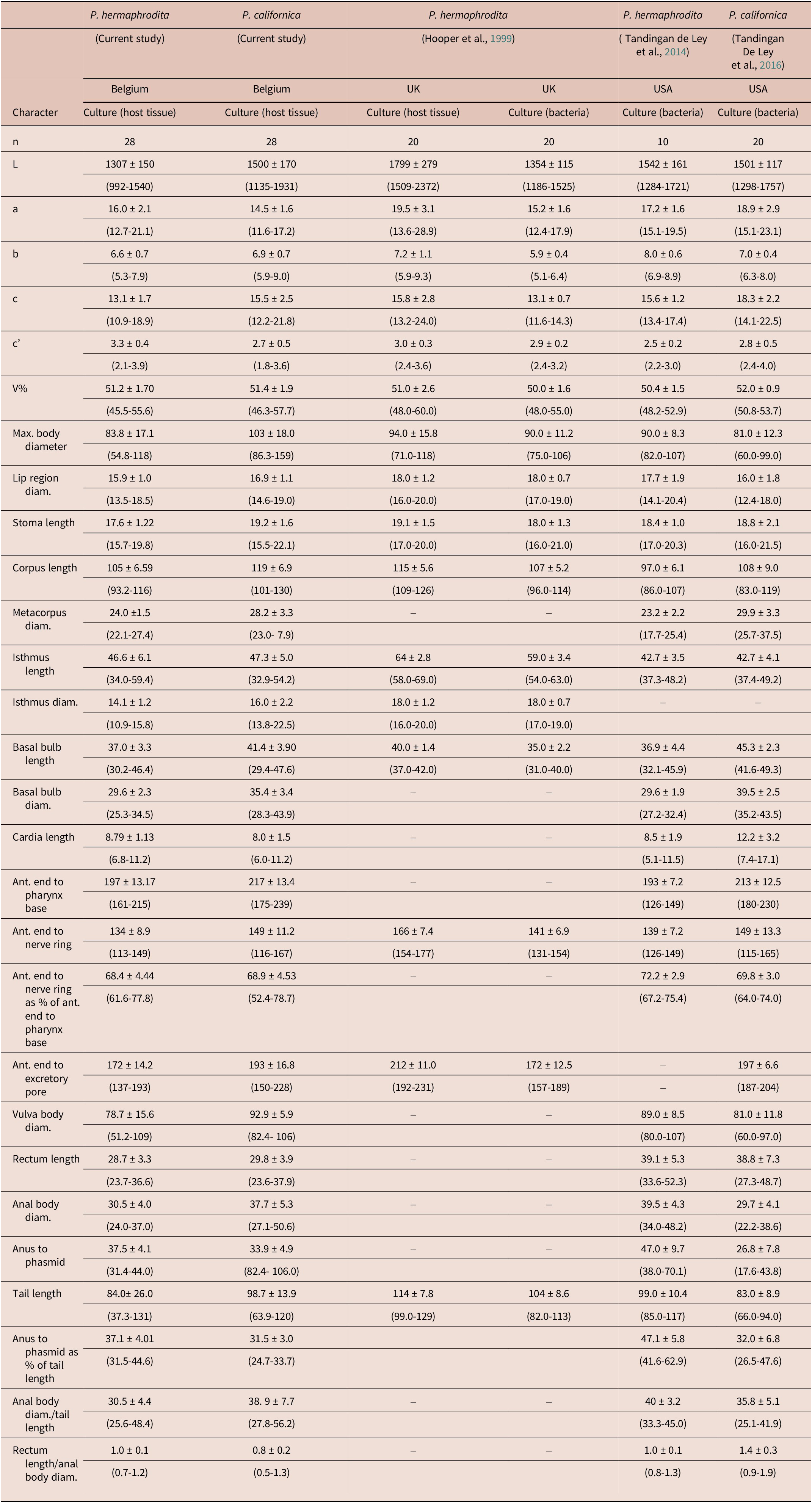
The hermaphrodite morphology and morphometrics of P. californica largely agreed with the original description of the species (Tandingan de Ley et al. Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016). Similarly, our P. hermaphrodita population agreed with the original description by Mengert (Reference Mengert1953) and the morphometrics data also fall within the range of the measurements from cultured nematodes in previous descriptions of the species (Hooper et al. Reference Hooper, Wilson, Rowe and Glen1999; Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014). However, the general morphometric data of both of our populations showed some variations such as in the body length and width, and tail length (see Table 4 for details). This was because of the inclusion of both young and matured hermaphrodites in our measurements. Nevertheless, the most important morphological differences observed between the two Pellioditis species was the tail shape (i.e., conical that appears almost like a funnel for P. californica vs more elongate conoid for P. hermaphrodita). However, in addition to tail shape, Tandingan de Ley et al. (Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014) considered tail length as also one of the main distinguishing features between the two species; however as noted in the current study, measurements can be influenced by maturity level of the nematodes. In addition, they can also be affected by the diet on which nematodes are cultured, for instance, nematodes grown in slugs tend to be larger than those grown on bacterial agar plates (Hooper et al. Reference Hooper, Wilson, Rowe and Glen1999). Therefore, it appears that tail shape rather than length is more reliable in separating the two species. However, molecular confirmation of Pellioditis spp. is generally required because of the high morphological similarities and overlapping morphometrics amongst the species (Tandingan de Ley et al. Reference Tandingan De Ley, McDonnell, Lopez, Paine and De Ley2014, Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016).
Table 4. Comparison of the morphometrics of hermaphrodites of the Pellioditis hermaphrodita and P. californica populations from Belgium with the morphometrics of P. hermaphrodita and P. californica populations from the UK and the USA. Measurements were made from fixed specimens and are given in μm and in the format mean ± standard deviation (range)
Discussion
The diversity of the gastropods and their associated nematodes in Belgium is remarkable (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019; current study). In the current work, a total of nine slug and snail species belonging to five families and 11 mollusc-associated nematode species belonging to eight families were identified. Of the seven slug species collected in Belgium by Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019), only one species (i.e., L. maximus) was recollected in this study, whereas the remaining species (i.e., A. ater, A. flagellus, A. fasciatus, A. lusitanicus, L. flavus, and L. valentiana) were not found. On the other hand, A. vulgaris, the most commonly detected slug species in this study, was not found in the former study. Similarly, of the seven molluscs nematode species detected by Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019), three species (i.e., A. flexilis, A. limacis, and A. norvegicum) were not redetected in this study; however, six other nematode species not detected by the previous study were found here. In the survey of Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019), the slugs were collected from household gardens and a forest, whereas in this study the molluscs were collected mainly from forests, parks, botanical gardens, and nature reserves. This difference in habitats may explain the difference in the biodiversity of the collected gastropods and their associated nematode species. It underlines the importance of collecting samples from diverse habitats to obtain a better picture of the biodiversity of slugs and snails and associated nematode species in a region.
Two of the nematode species found in this study (i.e., C. longicauda and P. californica) were recorded for the first time in Belgium, whereas five other species (i.e., A. vasorum, A. appendiculatum, A. dentiferum, A. gandavense, and P. hermaphrodita) were previously reported in the country (Jolly et al. Reference Jolly, Poncelet, Lempereur, Caron, Bayrou, Cassart, Grimm and Losson2014; Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019;). Angiostoma gandavense, described from East Flanders of Belgium (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019) was herein revealed to be more widespread in the country, as evidenced by its presence in Liège of Southeastern Belgium. In this study, on average, about one out of five slugs and snails examined (20%) was infected with at least one nematode species. This prevalence of nematodes is comparable to that of the survey conducted in Norway which found 18.7% of 611 slugs collected as infected by nematodes (Ross et al.,2016), and to that in the former survey in Belgium (i.e., infection of 22.8% of 239 slugs) (Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019). In contrast, a survey of native and introduced species of terrestrial slugs conducted in the Western Cape Province, South Africa, found only 6% of 521 slugs collected as infected by nematodes (Ross et al. Reference Ross, Ivanova, Sirgel, Malan and Wilson2012). The higher prevalence of nematodes associated with slugs and snails in Belgium suggests that gastropod fauna in Belgium is more susceptible to infection by nematodes. Also, some similarities and differences in the nematode infection rate of a few well-known slug species were found when compared with existing reports; for instance, in Canada, Brophy et al. (Reference Brophy, Howe, Denver and Luong2020) reported a nematode infection rate of 3.2% of 2,158 examined Deroceras reticulatum specimens and 24.6% of 65 examined A. valentianus, whereas our study found the infection rate of 2.2% of the 91 examined D. reticulatum specimens and 14.3% of the 28 examined A. valentianus specimens, respectively.
Pellioditis was the most commonly found nematode genus in our study (i.e., it was found at nine of the 13 localities sampled and in six out of the nine slug and snail species collected). Based on morphometrics and morphology, and molecular characterisations, the presence of P. californica and P. hermaphrodita could be confirmed. Following the first description of P. californica from the USA (Tandingan De Ley et al. Reference Tandingan De Ley, Holovachov, Mc Donnell, Bert, Paine and De Ley2016), this species was subsequently reported in Europe (Carnaghi et al. Reference Carnaghi, Rae, De Ley, Johnston, Kindermann, Mc Donnell, O’Hanlon, Reich, Sheahan, Williams and Gormally2017) and, more recently, in Germany (Keyte et al. Reference Keyte, Grannell, Sheehy, Shepherd and Rae2022). It was also recovered in other countries including Canada (Brophy et al. Reference Brophy, Howe, Denver and Luong2020) and New Zealand (Wilson et al. Reference Wilson, Wilson, Aalders and Tourna2016). The current study reports P. californica for the first time in Belgium. On the other hand, P. hermaphrodita was recovered for the second time in Belgium after it was found by Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019), but the identification of the species was based only on molecular data. This species has a cosmopolitan distribution and has been recovered from gastropod molluscs worldwide including Germany (Mengert Reference Mengert1953), France (Maupas Reference Maupas1900; Coupland Reference Coupland1995), the UK (Wilson et al. Reference Wilson, Glen and George1993), Iran (Karimi et al. Reference Karimi, Kharazi-Pakdel and Robert2003), Czech Republic (Nermuť et al. Reference Nermuť, Půža and Mráček2010), Egypt (Genena et al. Reference Genena, Mostafa, Fouly and Yousef2011), New Zealand (Wilson et al. Reference Wilson, Burch, Tourna, Aalders and Barker2012), South Africa (Ross et al. Reference Ross, Ivanova, Sirgel, Malan and Wilson2012), the USA (Tandigan De Ley et al. 2014), China (Huang et al. Reference Huang, Ye, Ren and Zhao2015), Norway (Ross et al. Reference Ross, Ivanova, Hatteland, Brurberg and Haukeland2016), and Japan (Waki Reference Waki2017). It is most well-known for its biocontrol efficacy against molluscan pest (Wilson et al. Reference Wilson, Glen and George1993; Rae et al. Reference Rae, Verdun, Grewal, Robertson and Wilson2007; Mc Donnell et al. Reference Mc Donnell, Colton, Howe and Denver2020; Tandingan De Ley et al. Reference Tandingan De Ley, Schurkman, Wilen and Dillman2020). A comprehensive morphological and molecular characterisations of P. californica and P. hermaphrodita are presented from Belgium for the first time in this paper.
As also reported in previous studies, some nematodes such as Agfa spp. and Angiostoma spp. can complete their life cycles inside mollusc hosts (Ivanova and Wilson, Reference Ivanova and Wilson2009, Ross et al. Reference Ross, Ivanova, Spiridonov, Waeyenberge, Moens, Nicol and Wilson2010, Singh et al. Reference Singh, Couvreur, Decraemer and Bert2019) indicating their obligate parasitism, the current study also observed adults and juveniles of Angiostoma spp. within the same infected hosts. Another interesting observation, which was also similarly made by Singh et al. (Reference Singh, Couvreur, Decraemer and Bert2019), was the co-infections of A. dentiferum and P. hermaphrodita within the same host where the former occupies the intestine and the latter occupies below the mantle, oftentimes in the pallial cavity of the host. Although both the nematode significantly differ in their morphology (e.g., stoma shape and size) and mode of parasitism (obligate vs facultative), phylogenetic inference showed that Angiostoma and Pellioditis are closely related (see phylogenetic analysis above; Tandigan De Ley et al. Reference Tandingan De Ley, Kiontke, Bert, Sudhaus and Fitch2023). The significance of such co-infection of nematodes with different modes of parasitism should be further investigated to enhance our understanding of mollusc parasitism by the nematodes, and also to determine whether Angiostoma has potential as biocontrol agents. Further interesting observation made in this study was the recovery of J4 (4th-stage juveniles) of A. appendiculatum from the digestive tubules of the digestive gland in one slug specimen and from below the mantle cavity in two slug specimens. This nematode species has an alternate parasitic-saprophytic life cycle – i.e. infective juveniles invade the foot muscle to develop into J4 and thereafter leave their host to continue their life cycle in the soil as free-living nematodes (Nermuť et al. Reference Nermuť, Půža and Mráček2019). To our knowledge, this is the first record of the recovery of J4 of A. appendiculatum from host digestive tubules other than the foot muscle. Juveniles inhabiting the pallial cavity (a double fold of the mantle which encloses a water space) vs the foot was one of the characters used to differentiate Neoalloionema tricaudatum from A. appendiculatum (Ivanova et al. Reference Ivanova, Van Luc and Spiridonov2015). However, our above observations suggest that the difference in infection site in the host may not be a reliable character to differentiate these two nematode species.
Remarkably, this study also revealed the presence of an important vertebrate nematode parasite in the mollusc specimens (i.e., juveniles [J1 – J3] of A. vasorum were found in the foot muscle of A. vulgaris). Angiostrongylus vasorum, also known as French heartworm, is a lungworm of foxes and, inter alia, of domestic dogs that utilizes terrestrial gastropods as intermediate hosts. Recently, its spread in North America and Europe has increased the veterinary relevance of this nematode species (Morgan et al. Reference Morgan, Modry, Paredes-Esquivel, Foronda and Traversa2021). In Belgium, the first fatal case of canine angiostrongylosis caused by A. vasorum was reported in 2013 (Jolly et al. Reference Jolly, Poncelet, Lempereur, Caron, Bayrou, Cassart, Grimm and Losson2014). A subsequent sero-epidemiological study demonstrated a high seroprevalence of A. vasorum in southern Belgium (Lempereur et al. Reference Lempereur, Martinelle, Marechal, Bayrou, Dalemans, Schnyder and Losson2016). The recovery of A. vasorum in the current study revealed that this important canine parasite is also present in the northern part of the country.
Financial support
This research was supported by VLIR_OUS ICP scholarship.
Competing interest
None.











