Vitamin D (or 25-hydroxyvitamin D, 25(OH)D) is a fat-soluble prohormone obtained primarily through solar UVB radiation exposure( 1 ). A well-documented lag time of 6–8 weeks exists between solar UVB exposure (or lack thereof) and resulting serum 25(OH)D concentrations( Reference Lucas, Bolland and Grey 2 ). Season and latitude are two main environmental determinants of 25(OH)D status, and from October to March at locations above 40°N, solar UVB exposure is insufficient to permit cutaneous vitamin D synthesis due to the large solar radiation angle( Reference Holick 3 ). The term ‘vitamin D winter’ has been coined to describe this period of time( Reference Engelsen, Brustad and Aksnes 4 ).
Increasing age, BMI and skin pigmentation are inversely related to serum 25(OH)D concentration( Reference Chen, Chimeh and Lu 5 , Reference Gozdzik, Barta and Wu 6 ). Cloud coverage, air pollution, clothing coverage and sunscreen use decrease cutaneous vitamin D synthesis, as they interfere with skin UVB absorption( Reference Holick 3 – Reference Chen, Chimeh and Lu 5 ). Conversely, UVB radiation from sunny vacations and tanning bed use has been linked to increased 25(OH)D concentrations( Reference Burgaz, Akesson and Oster 7 , Reference Tangpricha, Turner and Spina 8 ). Natural and fortified foods and supplements are other sources of vitamin D( 1 , Reference Burgaz, Akesson and Oster 7 ). Acute vitamin D toxicity can occur if serum concentration approaches or exceeds 375 nmol/l( Reference Jones 9 ), although some experts will cite 250 nmol/l. Toxicity from dietary consumption is highly unlikely in the general population( 10 ).
The US Institute of Medicine (IOM) has recently updated the definition of vitamin D deficiency (risk for rickets and osteomalacia) as serum 25(OH)D concentration <30 nmol/l (12 ng/ml) and the definition of inadequate vitamin D for bone health (risk of osteoporosis) as 30 to <50 nmol/l (12 to <20 ng/ml)( 10 ). Serum 25(OH)D concentrations below 50 nmol/l have been associated with a 30–50 % increase in incidence and mortality of colon, prostate and breast cancers( Reference Holick 11 ), and concentrations above 100 nmol/l have been associated with a 62 % decrease in risk of multiple sclerosis( Reference Munger, Levin and Hollis 12 ). Solar UV exposure in early life has been associated with a 44 % reduction in breast cancer risk in a prospective cohort of Swedish women( Reference Yang, Veierød and Löf 13 ) and with a 29 % risk reduction in a case–control study of Canadian women( Reference Anderson, Cotterchio and Kirsh 14 ). However, the IOM review found that current evidence was insufficient to conclude a causal role for vitamin D in non-bone-related health conditions( 10 ).
Epidemiological studies of vitamin D status in relation to disease risk have traditionally employed 25(OH)D as a biomarker of available vitamin D, while the biologically active form is its metabolite, 1,25-dihydroxyvitamin D or 1,25(OH)2D( Reference Jacobs, Martinez and Jurutka 15 ). 25(OH)D has a circulating half-life of 15 d and is thus is a better marker of vitamin D repletion than 1,25(OH)2D, which has a short circulating half-life of 15 h and is tightly regulated in blood serum by parathyroid hormone, Ca, fibroblast growth factor-23 and phosphate( 1 ). However, in terms of non-bone-health-related conditions, uncertainty remains as to whether or not 25(OH)D status represents vitamin-D-related biological activity affecting disease risk or presence of other disease risk factors associated with 25(OH)D status( Reference Jacobs, Martinez and Jurutka 15 ).
A 2010 Ontario study found that almost 40 % of working-age Ontario women did not meet the Dietary Reference Intake for vitamin D of 5 μg/d (200 IU/d)( Reference Anderson, Cotterchio and Boucher 16 ), a percentage now likely to be even higher since the IOM has raised the daily requirement to a recommended dietary intake of 15 μg/d (600 IU/d) for females aged 1–70 years( 10 ). Since the IOM estimates that mean daily vitamin D intake among Canadian women aged 19–50 years ranges between 4·7 and 5·2 μg/d (188 and 208 IU/d)( 10 ), vitamin D deficiency is a concern in this population and determinants of serum 25(OH)D concentration should be explored to help identify strategies for prevention. The objectives of the present study were to describe seasonal variation in serum 25(OH)D concentrations, prevalence of vitamin D inadequacy and deficiency, and the UV and dietary contributions to the prediction of serum 25(OH)D concentrations in a population of premenopausal nurses in Kingston, Ontario, Canada (44°N).
Experimental methods
A cross-sectional study was conducted among premenopausal nurses from Kingston General Hospital who volunteered to participate. Recruitment began in April 2008 through posters, pamphlets and information sessions. Participants were full-time nurses working the shift schedule of two 12 h days followed by two 12 h nights, followed by five days off. Nurses self-excluded if they had used melatonin supplements or had been pregnant or lactating within the past 6 months. While ninety-four nurses provided informed consent for all aspects of data collection, eleven were excluded due to pregnancy, change to part-time status, failure to provide a blood sample or study withdrawal. Thus, eighty-three (88 %) nurses were included in the present analysis. Nurses participated in data collection for two seasonal time periods (winter/spring and summer/autumn) in either 2008 or 2009 to give two data collection periods for each nurse. All nurses completed data collection, with sixty-one participating in two seasons and twenty-two participating in only one season. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Queen's University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. Written informed consent was obtained from all subjects/patients.
Weight and height were measured by a trained nurse for all but two participants who reported by telephone. Participants completed a questionnaire and provided an 8 h fasting blood sample upon waking prior to a day shift. Blood samples were collected in 3·5 ml serum separator tubes, and isolated serum was separated into 100 μl aliquots and stored in a dark −80°C freezer. Total serum concentrations (nmol/l) of the biomarker 25(OH)D (representing both serum 25(OH)D2 and 25(OH)D3) were quantified using the DiaSorin 25(OH)D RIA, with all samples run in duplicate within each assay. Two counts per minute (CPM) values were thus calculated for each sample. For samples taken in the winter/spring, the intra-assay CV for CPM values was 0·99 (P < 0·0001) and for samples taken in the summer/autumn, this value was 0·98 (P < 0·0001). Within-assay quality control was ensured through calibration with six calibrator samples included with the RIA, where % B/B0 was plotted v. log concentration of the sample; % B/B0 is defined as [(CPM of calibrator or unknown sample – CPM of non-specific binding buffer)/(CPM of 0 calibrator – CPM of non-specific binding buffer)] × 100 %.
The lifestyle section of the study questionnaire included type and frequency of vitamin D supplement use, tanning bed use, time spent outdoors from 11.00 to 15.00 hours in summer and early autumn, clothing coverage, sunscreen use and vacations to a sunny/warm destination taken within the last 6 months. The dietary section of the questionnaire focused on frequency of consumption of natural and fortified food sources of vitamin D in an average week in the past 6 months( Reference Bolek-Berquist, Elliott and Gangnon 17 ). Both sections were adapted from the Ausimmune Study and used with permission from Dr Robyn Lucas, Canberra, Australia( Reference Lucas, Ponsonby and McMichael 18 ). Skin type was self-classified using Fitzpatrick's skin pigmentation scale( Reference Fitzpatrick 19 ).
Mean serum 25(OH)D concentrations were calculated to describe monthly variation in vitamin D status, and paired t tests were performed to assess seasonal variation. General linear multivariable regression modelling was conducted to assess associations between potential determinants and serum 25(OH)D concentration following the winter/spring and summer/autumn seasons separately, while controlling for age and BMI. Generalized linear mixed models were used to allow for seasonal variation in solar UVB radiation by accounting for repeated observations over two seasons for the sixty-one participants with data for two seasons( Reference Littell, Stroup and Freund 20 ). All models were assessed for potential confounders( Reference Rothman and Greenland 21 ). Statistical analyses were performed using the statistical software package SAS version 9·2.
Results
Descriptive results
Participants’ mean age was 36 (sd 8·3) years, with mean BMI of just over 27 kg/m2 (Table 1). Almost all nurses self-identified as white, except five who were Asian, Latin or mixed. As seen in Table 2, lifestyle behaviours did not differ between seasons, although sunny vacations taken within the past 6 months were reported more frequently in the winter/spring (42 %) than in the summer/autumn (15 %).
Table 1 Personal characteristics of the study population: female, premenopausal, shift-working nurses (n 83), Kingston, Ontario, Canada, April 2008 to July 2009

*Only one nurse was underweight, so the underweight category was combined with the normal weight category.
Table 2 Seasonal characteristics and behaviours of the study population: female, premenopausal, shift-working nurses (n 83), Kingston, Ontario, Canada, April 2008 to July 2009
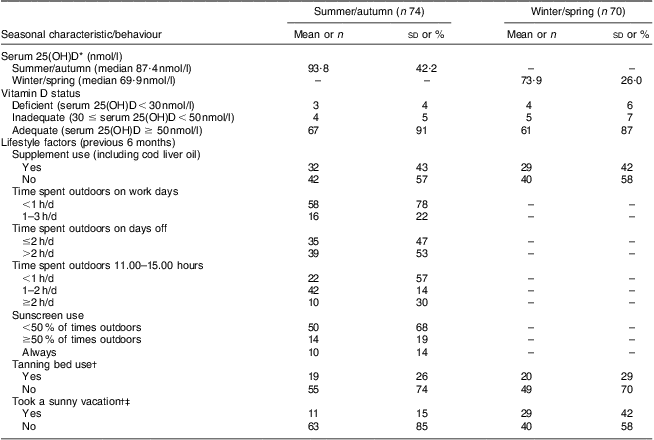
25(OH)D, 25-hydroxyvitamin D.
*Serum 25(OH)D data presented as mean and standard deviation; all other data presented as number and percentage.
†Frequency of tanning bed use ranged from once per week to a few times in 6 months.
‡Sunny vacation durations ranged from 6 to 18 d in length during the summer/autumn and from 6 to 28 d in length during the winter/spring.
Serum 25-hydroxyvitamin D concentration
Median serum 25(OH)D concentration was higher during summer/autumn (87·4 nmol/l) than during winter/spring (69·9 nmol/l). Following summer/autumn months, 4 % of women were vitamin D deficient and another 5 % had inadequate concentrations for bone health. Following winter/spring months, these percentages were 6 % and 7 %, respectively. Summer/autumn and winter/spring serum 25(OH)D concentrations were moderately positively correlated, with a Spearman's rank correlation coefficient of 0·69.
Median serum 25(OH)D concentrations among nurses by calendar month are displayed in Fig. 1. Median concentrations ranged from 65 nmol/l (May) to 117 nmol/l (August). Greater variation in individual serum 25(OH)D concentrations was observed following summer/autumn months (range 16–250 nmol/l) than following winter/spring months (17–135 nmol/l). No toxic 25(OH)D concentration was observed. Paired t tests detected a difference in mean serum 25(OH)D concentrations between the two seasonal data collection periods (P < 0·0001).
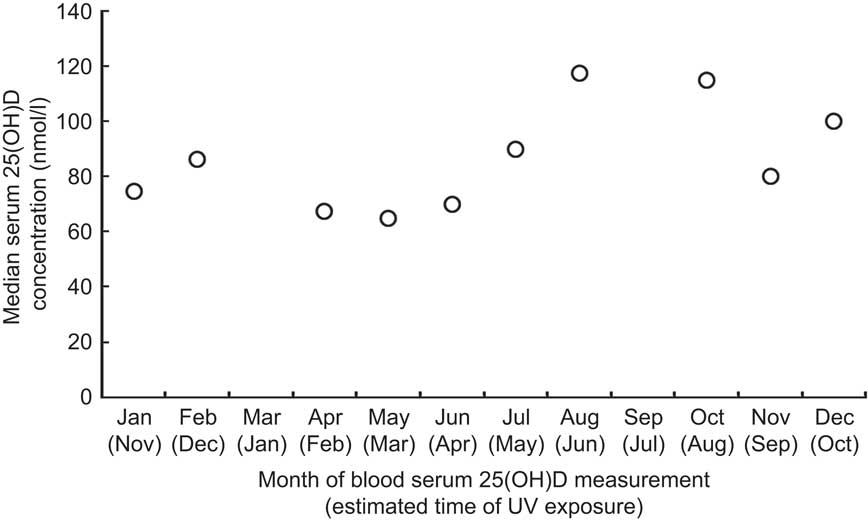
Fig. 1 Median serum 25-hydroxyvitamin D (25(OH)D) concentration by month among the study population: female, premenopausal, shift-working nurses (n 83), Kingston, Ontario, Canada, April 2008 to July 2009. No data were collected in March or September
Predictors of serum 25-hydroxyvitamin D concentration
Table 3 shows seasonal predictors of serum 25(OH)D concentrations. When seasons were analysed separately, tanning bed use during summer/autumn and winter/spring was a significant predictor of serum 25(OH)D (P < 0·0001 and P = 0·03, respectively), while vitamin D supplement use in summer/autumn and time spent outdoors between 11.00 and 15.00 hours in summer/autumn were of borderline significance (P = 0·05 and P = 0·06, respectively). Dietary factors, skin pigmentation, sunscreen use and having taken a vacation to a sunny/warm destination in the past 6 months were not associated with serum 25(OH)D concentration in either season, and vitamin D supplement use was not associated with serum 25(OH)D concentration in the winter/spring. These variables were subsequently not included in final models. When seasons were analysed together, tanning bed use in the past 6 months was associated with an increase in serum 25(OH)D concentration of 23·24 nmol/l on average (95 % CI 8·78, 37·69 nmol/l, P = 0·002).
Table 3 Summary of associations between potential seasonal predictors and serum 25(OH)D variation (nmol/l) among the study population: female, premenopausal, shift-working nurses (n 83), Kingston, Ontario, Canada, April 2008 to July 2009

25(OH)D, 25-hydroxyvitamin D; GLM, general linear multivariable.
*One participant excluded due to missing BMI value.
†Two participants excluded due to missing BMI and tanning bed use values.
‡P values adjusted for age, BMI, tanning bed use in the past 6 months, supplement use, time spent outdoors 11.00–15.00 hours and year of data collection.
§P values adjusted for age, BMI, supplement use and year of data collection.
∥P values adjusted for age, BMI, year of data collection and season.
¶P for trend.
Discussion
In the present cross-sectional study of female nurses in Ontario, Canada, we found that serum 25(OH)D concentrations tended towards the low end of the adequate range for bone health (≥50 nmol/l). After summer/autumn, 9 % of women had deficient or inadequate serum 25(OH)D and after winter/spring this proportion was 13 %. According to the 2011 IOM vitamin D guidelines for bone health, these women are at increased risk for osteoporosis and/or osteomalacia. Tanning bed use was the strongest modifiable predictor that we identified to increase serum 25(OH)D concentration, followed by use of vitamin D supplements.
Vitamin D supplementation was a significant determinant of serum 25(OH)D concentrations in the summer/autumn season. One other study also found this result, attributed in that study to confounding by time spent outdoors during the summer( Reference Vieth, Cole and Hawker 22 ). In our analysis we did control for time spent outdoors, and vitamin D supplementation remained a statistically significant predictor of serum 25(OH)D concentration. Our results are consistent with previous reports demonstrating that supplement use improves serum 25(OH)D concentration and prevents deficiency( Reference Burgaz, Akesson and Oster 7 , Reference Nelson, Blum and Hollis 23 ). Supplement use is a safe means to improve vitamin D status compared with potential harm from solar or artificial UV exposure: the IOM reports an upper tolerable limit of 100 μg/d (4000 IU/d), a relatively high amount( 10 ).
While previous studies have identified foods containing vitamin D as predictors of serum 25(OH)D concentration following the winter( Reference Gozdzik, Barta and Wu 6 – Reference Tangpricha, Turner and Spina 8 ), particularly milk( Reference Greenfield, Park and Farahani 24 , Reference Sahota, Barnett and Lesosky 25 ), we did not find any association with dietary sources of vitamin D. Because the vitamin D content of most foods is low relative to the amount produced in skin( Reference Chen, Chimeh and Lu 5 ), we may not have been able to detect the relatively small contribution of diet. Skin type, another factor known to be associated with vitamin D status, was not associated with serum 25(OH)D concentration in our study likely because of the lack of variation in this factor as almost all women in our study were white.
Recent data from the 2007–2009 Canadian Health Measures Survey (CHMS) also using the current IOM guidelines report that prevalence of deficient or inadequate vitamin D status is 20 % among women aged 20–39 years across Canada following summer and 31 % following winter( Reference Whiting, Langlois and Vatanparast 26 ). Other studies conducted at similar latitudes have noted as much as 73 % of their study population to have inadequate vitamin D status( Reference Gozdzik, Barta and Wu 6 , Reference Kull, Kallikorm and Tamm 27 ). We may have observed a comparatively low proportion with deficient and inadequate vitamin D status because younger age, white ethnicity, higher BMI and tanning bed use were prevalent in our study population and are associated with protection against deficient and inadequate vitamin D status( Reference Chen, Chimeh and Lu 5 – Reference Tangpricha, Turner and Spina 8 ). Despite these protective characteristics, deficient and inadequate vitamin D status was not rare in our study following the winter/spring months. Together, results from our study and others support the need to find ways to increase vitamin D status especially during winter months.
While just less than 30 % of study participants used a tanning bed in the 6 months prior to answering the questionnaire, this habit was a strong predictor of serum 25(OH)D concentration. The proportion of women reporting tanning bed use in our study is higher than the national average for Canada, where 15 % of women aged 25–44 years reported tanning bed use in the 2006 National Sun Survey( 28 ). Tanning bed use is most common among white ethnic groups and among higher income groups( Reference Hartman, Guy and Holman 29 , Reference Woo and Eide 30 ) as represented by the working nurses in our study, which may be why tanning bed use was more common in our study than among the general Canadian population of the same age and gender. Although the tanning industry claims that tanning bed use helps prevent vitamin D deficiency( Reference Autier, Dore and Breitbart 31 ), the health hazards from exposure to tanning beds are well documented( Reference Lim, James and Rigel 32 ). Tanning beds emit variable amounts of UVA and UVB radiation in doses often higher than that emitted by the sun, resulting in increased risk for UV overexposure( Reference Lim, James and Rigel 32 ). The International Agency for Research on Cancer has classified tanning beds as a cause of skin cancer( Reference El Ghissassi, Baan and Straif 33 ), supported by extensive literature showing that their use causes higher risks for melanoma and other skin cancers( 34 , Reference Gallagher, Spinelli and Lee 35 ). Tanning bed use is not advocated as a safe form of obtaining UV exposure for vitamin D synthesis and should not be used to obtain vitamin D among otherwise healthy populations( 34 ).
Legislation in many countries is being implemented to prevent harmful exposure from tanning beds specifically among young women and teens, with Brazil becoming the first country to ban commercial tanning bed use altogether( 36 ). Within Canada, legislation banning tanning beds among minors and enforcing other safety regulations exists in New Brunswick and in Victoria, British Columbia; was introduced into Ontario Parliament on 7 March 2013; and is anticipated in other provinces( Reference Matthews 37 , 38 ). Young white women are the population subgroup with the highest rate of tanning bed use( Reference Hartman, Guy and Holman 29 , Reference Woo and Eide 30 ), and in Ontario and nationally this group is most unlikely to meet the daily dietary required intake for vitamin D( 10 , Reference Anderson, Cotterchio and Boucher 16 , Reference Nelson, Blum and Hollis 23 ). As tanning bed use is increasingly curtailed, our study supports the need for increasing emphasis on vitamin D supplementation, especially in the winter months.
The present research has several strengths. Vitamin D status was measured using the objective serum biomarker, 25(OH)D. Although the utility of 25(OH)D in representing risk for non-bone-related health conditions is uncertain, this biomarker is the best biomarker of vitamin D status( 1 ). Although we did not assess between-assay variation, the within-assay correlations between samples run in duplicate were extremely high and all assays were performed by a single technician. Our study population of premenopausal women is of particular public health importance with respect to vitamin D, as young white women are the population subgroup most at risk in North America for low vitamin D status and engaging in tanning behaviours( 10 , Reference Hartman, Guy and Holman 29 , Reference Woo and Eide 30 ). Studies similar to ours have been conducted in other global locations and latitudes( Reference Burgaz, Akesson and Oster 7 , Reference Tangpricha, Turner and Spina 8 , Reference Vieth, Cole and Hawker 22 , Reference Sahota, Barnett and Lesosky 25 , Reference Brot, Vestergaard and Kolthoff 39 , Reference Nesby-O'Dell, Scanlon and Cogswell 40 ), although few studies have been conducted in Canada and these were prior to the recent IOM report( Reference Gozdzik, Barta and Wu 6 , Reference Greenfield, Park and Farahani 24 , Reference Sahota, Barnett and Lesosky 25 , Reference Rucker, Allan and Fick 41 ). The CHMS analysis was performed according to the 2011 IOM guidelines, although our study additionally examined monthly vitamin D variation and tanning bed use. Our descriptive assessment of monthly 25(OH)D concentrations allowed enhanced precision in measurement of annual variation in vitamin D status and supports the concept of a ‘vitamin D winter’( Reference Engelsen, Brustad and Aksnes 4 ). The examination of tanning bed use as a predictor of vitamin D status according to the 2011 IOM guidelines has not yet been done in Canada outside the present study.
The study is not without limitations. Our small sample size and relatively homogeneous study population of white, working-age, female nurses may have reduced our ability to detect predictors of serum 25(OH)D concentration that have small variability in our population. Regarding generalizability, results can be confidently extrapolated to white, working-age women at approximately the same latitude (44°N). Sample size limitations reduced the precision of estimates in our multivariable single season models, which may explain why the effect for tanning bed use was smaller in the winter/spring relative to summer/autumn and why supplementation had a non-statistically significant effect in the winter/spring. Some degree of recall error non-differential by vitamin D status may have occurred with our self-report questionnaire, causing our results to underestimate the effects of tanning bed use, supplement use and other factors on vitamin D status. However, a self-report questionnaire of past sun exposure with questions similar to ours has been shown to be valid when compared with objective measures of UV skin damage and serum 25(OH)D and test–retest κ statistics ranging from 0·43 to 0·74 for self-reported sun exposure( Reference van der Mei, Blizzard and Ponsonby 42 ). Misclassification of skin type was possible, as validity of the Fitzpatrick classification system has been questioned due to its dependence on self-reported data( Reference Fitzpatrick 19 , Reference Rampen, Fleuren and Boo 43 ), although this scale is used internationally. Diet was assessed descriptively, rather than through objective measures( Reference Millen and Bodnar 44 ).
Conclusions
Although we found that most nurses had adequate serum vitamin D status for bone health, 13 % were at risk for osteoporosis and/or osteomalacia following winter months. Women who use tanning beds are likely to be at increased risk for premature skin ageing and skin cancers; these health risks outweigh any perceived benefits of tanning bed use. Public health strategies promoting vitamin D supplementation and consumption of fortified foods (e.g. fortified milk) are essential to protect against osteopenia and osteomalacia among this population subgroup. While the biological activity of vitamin D in relation to non-bony disease risk becomes better understood through research( Reference Jacobs, Martinez and Jurutka 15 ), we must take action now to improve the vitamin D status of young women while enforcing safe sun practices and recommendations against tanning bed use.
Acknowledgements
Sources of funding: Financial support for this research was provided by the Workplace Safety and Insurance Board (Ontario, Canada). S.C.W. was supported by the Terry Fox Foundation/Canadian Institutes of Health Research Transdisciplinary Training Program in Cancer Research at Queen's University. J.T. was supported by a Mid-Career Investigator Award for the Ontario Women's Health Council/Canadian Institutes of Health Research. Conflict of interest: The authors have no conflicting interests. Authors’ contributions: S.C.W. performed the RIA for serum 25(OH)D and the statistical analysis and contributed to interpretation of the results and drafting of the manuscript. G.J. oversaw the RIA, contributed to interpretation of the results and provided revisions of the manuscript. L.C.K. performed the statistical analysis and contributed to interpretation of the results and drafting of the manuscript. A.G. contributed to the study design and provided revisions of the manuscript. Q.M. performed the statistical analysis and contributed to interpretation of the results. J.T. contributed to the study design, coordination and participant recruitment. K.J.A. conceived of the study, contributed to its design and coordination, directed the analysis and provided revisions of the manuscript. All authors have seen and approved the contents of this manuscript. Acknowledgements: The authors thank the nurses who participated in the study; the co-investigators of the larger study, Drs Harriet Richardson, Ian Janssen and Charles Graham; the study staff, Kathy Bowes, Deborah Emerton, Karen Lollar, Krista Smith, Shannyn McDonald-Goodfellow and Mark McPherson; Valarie Byford and Martin Kaufmann for their support with laboratory analyses; and Dr Robyn Lucas for providing permission to adapt sections of the Ausimmune Study questionnaire.






