Introduction
The assessment of a species’ extinction risk is the first step towards its conservation. Species for which ecological and population data are lacking are categorized on the IUCN Red List as Data Deficient (IUCN, 2012), but it is preferable that such species are assessed fully as they could potentially be threatened. One such species is Marca's marmoset Mico marcai, endemic to southern Amazonia, an area heavily impacted by the advancing Brazilian agricultural frontier. In the Brazilian National Threat Assessment of Primates (ICMBio, 2018) M. marcai was the only marmoset categorized as Data Deficient, and it has the same categorization on the IUCN Red List (Rylands & Silva Jr, Reference Rylands and Silva2008). This primate was first observed and collected by the Roosevelt–Rondon Expedition in 1914 but remained overlooked in the National Museum of Rio de Janeiro mammal collection for 79 years until Alperin (Reference Alperin1993), in a revision of all marmosets of the argentata group, described it as a new taxon, Callithrix argentata marcai. This taxon was later elevated to full species and included in the genus Mico (Rylands et al., Reference Rylands, Schneider, Langguth, Mittermeier, Groves and Rodríguez-Luna2000).
The museum tag of the type specimen of M. marcai indicates it was collected at the confluence of the Roosevelt and Aripuanã Rivers (Alperin, Reference Alperin2002; Fig. 1). In 2000 van Roosmalen et al. (Reference Van Roosmalen, Van Roosmalen, Mittermeier and Rylands2000) described a new species of marmoset, M. manicorensis; its type locality is the confluence of the Manicoré and Madeira Rivers (Fig. 1). However, the hypothesized distribution of M. manicorensis encompasses the Manicoré–Aripuanã interfluve, including the type locality of M. marcai. Based on examination of the few available specimens, Garbino (Reference Garbino2014) proposed that the van Roosmalen et al. (Reference Van Roosmalen, Van Roosmalen, Mittermeier and Rylands2000) manicorensis was a junior synonym of marcai. Silva et al. (Reference Silva, Nunes and Bastos2013) presented the first information on the species occurrence in the wild, near its type locality, and discussed potential threats to the species.

Fig. 1 Locations of the 10 transects used to survey for Mico marcai during January–February 2015, sightings from 2012–2014, earlier records from other observers (van Roosmalen et al., Reference Van Roosmalen, Van Roosmalen, Mittermeier and Rylands2000; Rohe, Reference Röhe, Py-Daniel, Deus, Henriques, Pimpão and Ribeiro2007; Garbino, Reference Garbino2014) and the type locality (Alperin, Reference Alperin2002), in the Aripuanã–Marmelos interfluve of the Brazilian Amazon. Juma SDR, Açaí Grande Juma Development Reserve.
Here we present the first investigation of the distribution and potential population size of M. marcai, using both field surveys and existing records, and assess the species’ conservation status. In addition, using spatial predictive modelling, we predict the potential effect of two alternative land-use scenarios on the species’ habitat. We believe this is the first study to categorize the conservation status of a Data Deficient Amazonian primate using uring a combination of field surveys and remote sensing data.
Study area
This study was carried out in the Marmelos–Aripuanã interfluve, two right bank tributaries of the Madeira River in Brazil (Fig. 1). The climate is tropical, with a short dry season in July–September, a mean annual temperature of 28 °C and a mean annual precipitation of 2,500–3,000 mm/year (Hayakawa & Rossetti, Reference Hayakawa and Rossetti2015). The vegetation comprises mostly upland forest, and seasonally flooded forests (open and dense lowland rainforest), and patches of pioneer and savannah-like vegetation (Supplementary Fig. 1; Anderson, Reference Anderson1981). This region lies within the arc of deforestation region of Amazonia, which is under severe threat from the rapidly expanding Brazilian agricultural frontier, urban encroachment, logging and infrastructure projects (Nepstad et al., Reference Nepstad, Carvalho, Cristina, Alencar, Paulo and Bishop2001; Vieira et al., Reference Vieira, Toledo, Silva and Higuchi2008). The study area lies in the municipality of Manicoré that, with Apuí municipality, is the main area for livestock production in Amazonas State, forming the arc of cattle ranching (Carrero et al., Reference Carrero, Albuja, Frizo, Hoffman, Alves and Bezerra2015). The area is considered a deforestation hotspot because of the presence of the federal road BR-230, also known as the Trans-Amazonian Highway (Fearnside et al., Reference Fearnside, de Graça, Keizer, Maldonado, Barbosa and Nogueira2009; Carrero & Fearnside, Reference Carrero and Fearnside2011).
Methods
Surveys
During 2012–2014 we carried out six expeditions to the Marmelos–Aripuanã interfluve to survey for marmosets and other primates, totalling 63 days of fieldwork. Our surveys included the confluence of the Roosevelt and Aripuanã rivers, the upper and lower Manicoré River, and the mid Aripuanã River (Fig. 1). Surveys were conducted using existing trails and roads, and with small boats. We recorded the location of all sightings, with a GPS, and, using these locality records and data from the literature (Ferrari, Reference Ferrari1993; van Roosmalen et al., Reference Van Roosmalen, Van Roosmalen, Mittermeier and Rylands2000; Alperin, Reference Alperin2002; Röhe, Reference Röhe, Py-Daniel, Deus, Henriques, Pimpão and Ribeiro2007; Garbino, Reference Garbino2014), we defined the species’ extent of occurrence (EOO, sensu IUCN, 2012). We followed the IUCN (2012) guidelines to calculate the EOO as the minimum convex polygon that contains all of the species’ records (IUCN, 2012). Assuming rivers are effective barriers to primate dispersal (Ayres & Clutton-Brock, Reference Ayres, de Malghaes Lima, Martins, Barreiros, Robinson and Redford1992), we adjusted this EOO accordingly to measure the total area potentially occupied by the species (i.e. its geographical range).
To estimate the species’ density and abundance we surveyed two sites during January–February 2015. Survey transects were near the species’ type locality and along the lower Manicoré River. In total, we opened six transects in the former site and four in the latter, averaging 3.07 ± SD 0.63 km in length (Fig. 1). The positioning of transects was by randomization, using ArcGIS 10.2.2 (ESRI, Redlands, USA), of each trail starting point and direction from the main access points (roads or rivers). Nine transects were opened perpendicularly to these access points so that any gradient of environment and primate density from the start of the trail to the interior of the forest was accounted for. Transects were at least 2 km apart to avoid spatial dependence. We followed standardized field protocols for data collection, using distance sampling (Buckland et al., Reference Buckland, Anderson, Burnham and Laake1993) to estimate marmoset densities. Two observers walked transects at a mean speed of 1.5 km/h, recording the number of individuals sighted and the perpendicular distance from the transect to the centre of the group. Transects were surveyed during 7.00–11.00 in one direction and 14.00–17.00 in the reverse direction. Each transect was surveyed at least three times, with a 2-day break between surveys to reduce the impact of the observers’ presence on the detection rate. We estimated the density of marmosets using Distance 7.1 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). This analysis fits detection functions to provide the probability of detecting groups and estimate the number of individuals potentially missed by the observers. The encounter rate (groups/km) and the mean number of individuals per group were used to estimate density.
We first used a χ2 test to determine the appropriate truncations and perpendicular distances intervals for adjusting the detection functions, at P > 0.6. We compared the adjustments of the detection functions using the Akaike Information Criterion (AIC). The model with the smallest AIC value was considered the best-fit for the data. If more than one function was considered a good fit (i.e. the difference in their AIC values, ∆AIC, was < 2), we selected the model for which the density estimate had the lowest coefficient of variation. We then used the density value to estimate the abundance of M. marcai as A = D * a, where A is abundance, D is density and a is the species’ geographical range.
Evaluation of conservation status
As recommended by IUCN (2012), we multiplied the lower confidence interval of the species’ density by the predicted geographical area to obtain a conservative, naive estimate of population size. We calculated forest lost up to 2017 within the estimated geographical range of the species and used predictive deforestation models to assess how much of the species range will be lost by the end of 2035 (in 18 years). The IUCN criterion A assesses extinction risk over three generations, and 18 years is c. three generations for Mico leucippe (Mittermeier & Rylands, Reference Mittermeier and Rylands2008; Nishijima et al., Reference Nishijima, Saitoh, Tanaka, Ohsato-Suzuki, Ohno and Kitajima2012; data on generation time or lifespan for M. marcai are unavailable).
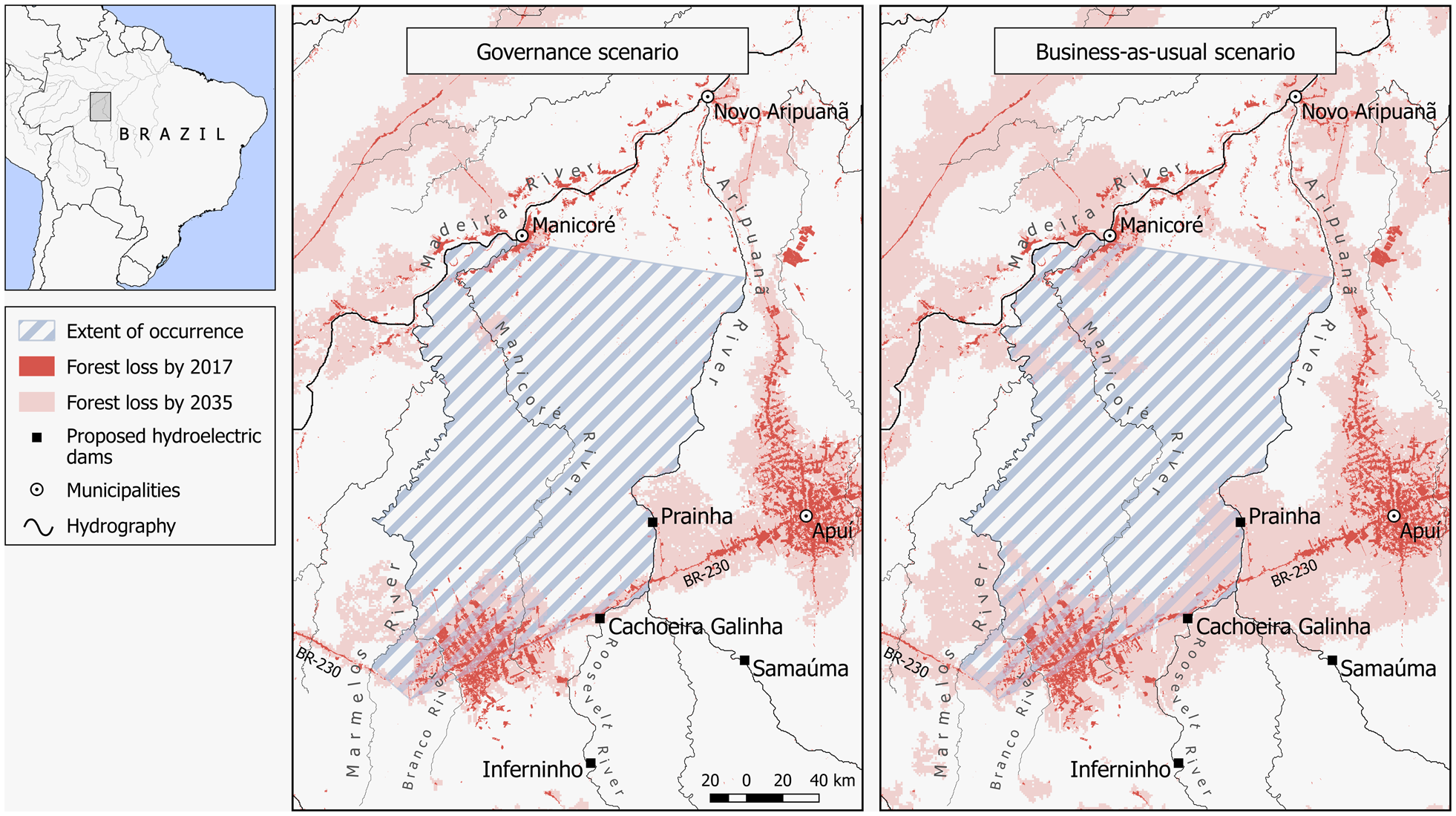
Data on the annual rate of deforestation during 2000–2017 were obtained from PRODES (2018). For predicted forest loss, we considered two scenarios (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006): (1) governance, which assumes current deforestation trends but with a 50% cap in forest loss as a result of current laws that prohibit farmers from clearing > 50% of forest on their properties, and that existing and proposed protected areas are effectively managed, and (2) business-as-usual, which considers current deforestation trends across the Amazon basin plus the effect of infrastructure development and low effectiveness in the management of protected areas.
We calculated the amount of forest loss in each scenario and the percentage that lies within the geographical range of M. marcai, to estimate the forest loss to be expected by 2035. We then used the IUCN Red List criteria (IUCN, 2102) to evaluate the risk of extinction.
Results
Geographical range
During 2012–2014 we observed M. marcai groups in 13 localities: (1) along the left bank of the Aripuanã and Roosevelt Rivers, (2) on both banks of the Manicoré River, and (3) on both banks of the Branco River, a small tributary of the Marmelos River (Fig. 1; Table 1). Based on these observations and data from the literature, we calculated the adjusted EOO to be 31,073 km2, limited to the east by the Aripuanã River, to the west by the Marmelos River, to the north by the Madeira River and to the south by the open savannah vegetation of the Campos Amazônicos National Park, an area believed to be a distribution limit for other marmoset species (Ferrari, Reference Ferrari1993; Garbino, Reference Garbino2014; Fig. 1).
Table 1 Occurrence records (with decimal latitude and longitude) of Mico marcai from our field surveys in the Ariupanã–Marmelos interfluve (Fig. 1) and published data.

Density and abundance
In total we walked 271.6 km on the 10 transects. We observed groups of M. marcai on 30 occasions, giving an encounter rate of 0.11 individuals/km (CV 21.80). The best distribution of perpendicular distances was obtained with five intervals of 10 m each (χ2 = 0.52, df = 4, P = 0.91; Fig. 2). The Uniform function with one cosine adjustment term provided the best fit (AIC 82.22). The number of individuals detected per group increased with perpendicular distance (r = −0.22; P = 0.13) and we therefore estimated mean group size using linear regression, giving a value of 4.09 individuals/group (CI 3.23–5.16, CV 11.41, range 1–11). Density was estimated to be 8.31 individuals/km2 (CI 4.85–14.22, CV 25.94) and group density 2.03 per km2 (CI 1.23–3.36; CV 23.29). The naive estimate of abundance within the species’ range was 258,218 individuals (CI 150,705–441,860).
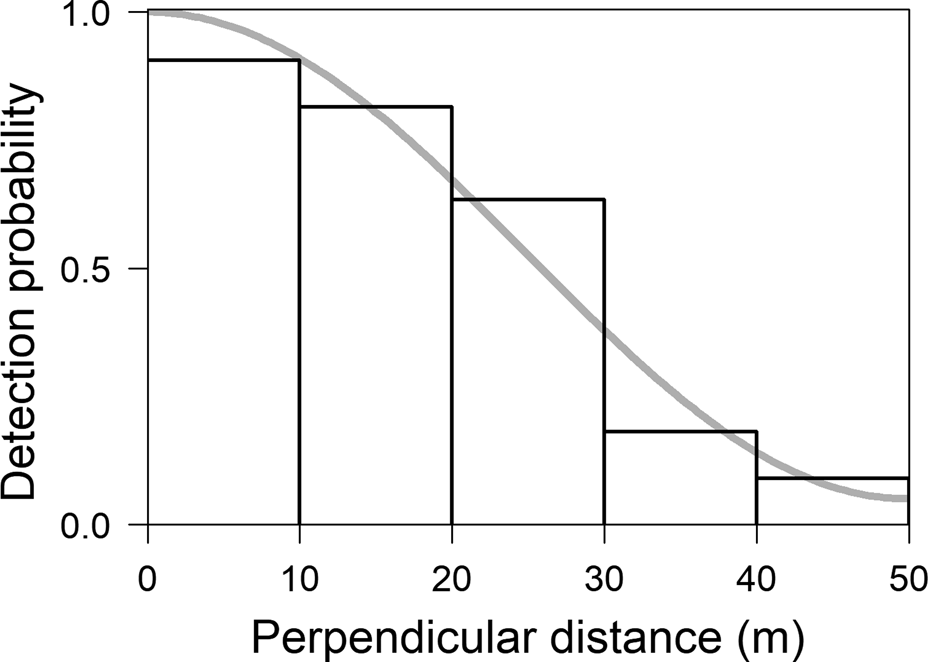
Fig. 2 Distribution of perpendicular distances of observations of M. marcai on transects in the Marmelos–Aripuanã interfluve (Fig. 1). The trend line indicates the best detection function fitted to the distance classes.
Conservation status
Data from PRODES (2018) indicated a loss of 1,266.23 km2 of forest cover up to 2017 (4% of the species’ estimated geographical range), and spatial predictive models (Soares-Filho et al., Reference Soares-Filho, Nepstad, Curran, Cerqueira, Garcia and Ramos2006) indicate that 5,800 km2 (19%) of forest will be lost in the next 18 years under the governance scenario, and 10,396 km2 (33%) under the business-as-usual scenario (Fig. 3). Using our conservative estimate of population size (150,705 individuals), these levels of forest loss extrapolate to a loss of 13,430 M. marcai under the governance and 49,733 under the business-as-usual scenarios by 2035. This indicates that, according to the business-as-usual scenario, the species should be categorized as Vulnerable based on criteria A3cd (an estimated 30% population reduction projected over the next 18 years, c. three generations), as a result of a predicted decline in EOO.
Discussion
IUCN Red List guidelines (IUCN, 2012) recommend that species should be assessed using all available evidence to avoid, if possible, placing a species in the Data Deficient category (IUCN 2012). This category does not mean a species is without threats, but rather that it is a priority for research, and there are examples of Data Deficient species being categorized as threatened once relevant data became available (Bland et al., Reference Bland, Collen, Orme and Bielby2015). This is also the case for M. marcai, which we recommend should be categorized as Vulnerable.
Our analysis indicates that M. marcai has an estimated minimum population of 150,705 individuals in a geographical range of 31,073 km2. Variation in group size may bias density estimates but the coefficient of variation of the group size for Mico marcai is 11.41% with a confidence interval of 3–5, which is lower than estimated group sizes of other Neotropical primate species (i.e. those in the genera Saimiri and Sapajus; Peres Reference Peres1993). Furthermore, despite employing distance sampling in only two regions within the species’ EOO, we surveyed the predominant forest type (dense lowland rainforest) within the species’ range rather than the few patches of open lowland rainforest and pioneer vegetation.
Despite a relatively large estimated population, the high rate of deforestation in this region, caused by the ever-expanding Brazilian agriculture frontier and infrastructure development (roads and hydroelectric power plants), poses a grave threat to the survival of M. marcai and other marmoset species. A projected loss of 33% of the species total range by 2035 under a business-as-usual scenario is a bleak outlook.
Although part of the current geographical range of M. marcai is theoretically conserved by Indigenous Lands and protected areas, these units are under pressure from the current trend of protected area downgrading, downsizing, and degazettement in the Brazilian Amazon (Bernard et al., Reference Bernard, Penna and Araújo2014; Ferreira et al., Reference Ferreira, Aragao, Barlow, Barreto, Berenguer and Bustamante2014; Pack et al., Reference Pack, Ferreira, Krithivasan, Murrow, Bernard and Mascia2016). Three main factors drive this, decreasing the effectiveness of protected areas within M. marcai’s range: (1) political instability and changes in governmental policies on land use and conservation in the Amazon, (2) planned hydroelectric dams, especially on the southern tributaries of the Amazon River, and (3) increases in human settlements surrounding the Indigenous Lands and protected areas. Four hydroelectric dams will be constructed within M. marcai’s range, flooding an area of 1,118.42 km2 (ANEEL, 2012). The planned dams and reservoirs of Prainha and Samaúma on the Aripuanã River, and the reservoirs Inferninho and Cachoeira Galinha on the Roosevelt River, will reduce the area of occurrence of M. marcai and two other marmosets: the sympatric Callibella humilis and the marmoset found along the right bank of Aripuanã River, Mico chrysoleucos (Silva et al., Reference Silva, Costa-Araújo, Boubli, Santana, Franco and Bertuol2018a,Reference Silva, Endo, de Sousa e Silva Jünior, Marcelo and Santosb). In addition, the Trans-Amazonian Highway, which has negatively affected the conservation of southern Amazonia (Kirby et al., Reference Kirby, Laurance, Albernaz, Schroth, Fearnside and Bergen2006; Carrero & Fearnside, Reference Kirby, Laurance, Albernaz, Schroth, Fearnside and Bergen2011), bisects the range of M. marcai, and the municipalities of Apuí and Manicoré lie in the arc of cattle ranching.
In 2013 the Brazilian government, through the Chico Mendes Institute for Biodiversity Conservation, assessed the conservation status of Brazilian primates (ICMBio, 2018), in which nine Mico species were categorized as Least Concern, two as Near Threatened, and one as Vulnerable, with only M. marcai categorized as Data Deficient. However, the threats to Amazonian marmosets have been documented in only a few studies (Gonçalves et al., Reference Gonçalves, Ferrari, Silva, Coutinho, Menezes, Schneider and Marsh2003; Ochoa-Quintero et al., Reference Ochoa-Quintero, Chang, Gardner, Rezende Messias, Sutherland and Delben2017) and the range of many of these species has been estimated from relatively few records (Ferrari, Reference Ferrari1993; Silva Jr & Noronha, Reference Silva and Noronha1995; van Roosmalen et al., Reference Van Roosmalen, Van Roosmalen, Mittermeier and Rylands2000; Noronha et al. Reference Noronha, Spironello and Ferreira2007; Fialho, Reference Fialho2010; Garbino, Reference Garbino2011). Most Mico species inhabit the arc of deforestation, where forest loss and other threats are similar to or higher than those estimated here for M. marcai. For example, Ochoa-Quintero et al. (Reference Ochoa-Quintero, Chang, Gardner, Rezende Messias, Sutherland and Delben2017) predicted a decline of > 50% of the potential distribution of M. rondoni by 2030 as a result of forest loss, which meets the IUCN criteria for Endangered (A3). As a follow-up of our fieldwork and data analysis, we have passed our results and recommendations to the Red List Authority. We advocate that the conservation status of all Amazonian marmosets should be re-examined following the methods we have used for M. marcai.
Acknowledgements
Data collection and analysis were supported by International Association for Conscientiology Expansion, Mamirauá Institute for Sustainable Development, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 200502/2015-8), Conservation Leadership Programme, Conservation International, Primate Conservation Inc., International Primatological Society, and Idea Wild. We thank the Gordon and Betty Moore Foundation (Grant Agreement for Instituto de Desenvolvimento Sustentável Mamirauá, #5344), Isaac Theobald and Aldeísa for logistical support, and Catitu and José’s family for support in the field.
Author contributions
Study design: FES, HEB, JRG, LPL, RCA, IJL, CLBF, AST and MIS; data collection: FES, JRG, LPL, RCA, IJL, ATS and MIS; data analysis: FES, HEB, JRG, CLBF and JPB; all authors contributed to interpretation of results and writing.
Conflicts of interest
None.
Ethical standards
This research adhered to Brazilian law governing primate research and the principles of he American Society of Primatologists for the ethical treatment of primates. Research permits were granted by ICMBio/SISBio (number 44707-2).