INTRODUCTION
Verocytotoxin-producing Escherichia coli (VTEC) infections contribute to severe infectious gastrointestinal illness in the UK, resulting in 15–20 laboratory-confirmed cases per 1 000 000 population annually in England between 2005 and 2011[1]. Almost 50% of cases are in children aged <16 years, and rates of infection are highest in children aged <5 years. Complications include haemolytic uraemic syndrome (HUS), which occurs in about 6% of all cases (and in 15% of children aged <5 years), of whom about 5% die [Reference Gould2]. The infectious dose of VTEC is low [Reference Tuttle3], and outbreaks can occur following exposure to a common source such as contaminated food or through person-to-person spread due to faecal–oral ingestion [Reference Pennington4]. VTEC infections also have economic impact due to medical costs and lost productivity [Reference Roberts, Upton and Azene5].
The Health Protection Agency (HPA) guidelines request that VTEC cases be notified to the local health protection unit (HPU) within 24 h of clinical suspicion or laboratory isolation [6]. The HPU records details of cases and their public health management in a web-based information system called HPZone [7]. Information collected via a standard questionnaire is also entered into the VTEC national enhanced surveillance system [8]. Public health control measures include excluding children aged ⩽5 years with presumptive VTEC infection from the childcare facility (nursery or school) until two consecutive faecal specimens are culture negative. These should be collected after resolution of symptoms and at least 24 h apart. Exclusion is implemented to avoid transmission within the childcare facility. Working parents caring for excluded children experience disruption with potential stress and financial loss.
A few studies estimated the duration of the infectious (shedding) period, defined as the interval from date of onset of diarrhoea to the specimen date of the first of two consecutive negative stool cultures to be <3 weeks, based on small numbers [Reference Wells9–Reference Wahl11]. Others have reported longer durations [Reference Miliwebsky12, Reference Shah13]. In a retrospective study performed in England and Wales, 10% of VTEC isolates between 1995 and 1998 were part of an outbreak, and 6/67 outbreaks occurred within a nursery or school [Reference Willshaw14]. To our knowledge, neither the duration of shedding in children aged ⩽5 years, nor rates of transmission within childcare facilities have been recently estimated in England.
The study objectives are therefore to assess the duration of shedding and estimate the risk of transmission from infectious young children in childcare facilities in England to help inform any revision of national guidance.
METHODS
Study design
We conducted a descriptive study of laboratory-confirmed VTEC cases aged ⩽5 years, residing in England and attending childcare facilities including schools and nurseries, with onset between 1 January 2010 and 7 July 2011, and for whom data was available on HPZone.
Case definition
Microbiological definitions
A confirmed case was defined as an individual with VTEC isolation confirmed by PCR identification of verocytotoxin-encoding genes by the reference laboratory (Laboratory of Gastrointestinal Pathogens at HPA Colindale, London) [Reference Jenkins15]. Absence of VTEC in clearance specimens was assessed by local laboratories for E. coli O157 [16] and by the reference laboratory for non-O157 VTEC.
Epidemiological definitions (based on HPA guidance) [ 8 ]:
-
• A primary case was defined as a case believed to have introduced the disease into the childcare facility, indicated by the absence of any confirmed cases in the facility prior to the onset of symptoms in the case.
-
• A case was defined as co-primary if he/she had a date of symptom onset (or of specimen collection if asymptomatic) within one incubation period (4 days) of the primary case.
-
• A case was defined as secondary if he/she had a date of symptom onset, or specimen collection if asymptomatic, more than 4 days after the primary case. Their main risk factor was believed to be ‘exposure to a primary case’.
Data collection
We obtained data from the national enhanced VTEC surveillance system, including case demographics, clinical details, laboratory results (serogroup and phage type), and names and addresses of childcare facilities for all cases. Further information was collected from the public health management system (HPZone), including the date of the first of two consecutive negative specimens, the date the child was cleared to return to their childcare facility, whether difficulties were experienced in implementing exclusion, whether the child attended the facility while infectious and if so, the number of days attended and whether the HPU alerted the childcare facility to be vigilant for other cases. A case was considered infectious at the facility if HPZone records indicated he/she was definitely or probably shedding (culture positive for VTEC), or symptomatic with diarrhoea while at the facility.
Data analysis
The duration of shedding was calculated as the interval from date of onset of illness to the date of the first of two consecutive negative stool specimens. The duration of exclusion was calculated as the interval from date of onset to the date when the child was cleared to return to the childcare setting. The date of onset was used as a proxy for the date of actual exclusion because of the greater likelihood of accuracy of recording of the onset date, and the likelihood that parents would have removed symptomatic children from childcare facilities during the acute illness. Where one or more of these dates was unknown, the case was excluded from these analyses. The median duration and interquartile (IQR) range for shedding duration were estimated in days. Simple linear regression was performed to ascertain the effect of age on log-transformed duration of shedding. Wilcoxon and Kruskal–Wallis tests were performed to compare the median duration of carriage by gender and phage type, respectively. We also calculated the proportion of cases where difficulties in implementing exclusion were documented.
We describe the transmission of VTEC within childcare facilities according to three indicators:
-
(1) The proportion of infectious children attending childcare facilities in the study population and the duration of attendance while infectious. We also ascertained whether the HPU had alerted the facility.
-
(2) The proportion of facilities with at least one secondary case in the total number of facilities attended by at least one infectious primary or co-primary VTEC case. In addition, we described each incident involving at least one secondary case in terms of the number, age and type of cases (symptomatic or asymptomatic; primary, co-primary or secondary), and the possible transmission mechanism (at home, at the facility, at another setting).
-
(3) The proportion of secondary cases in children exposed to primary or co-primary cases within childcare facilities with at least one infectious case. As the exact number of exposed children was unknown we simulated it according to a Poisson distribution, assuming an average number ranging from 10 to 30 children in a group, with all children considered to be equally in contact with one another.
Transmission was assumed to have occurred at home if the VTEC cases shared the same home address, at the childcare facility if the VTEC cases attended the same facility but lived at different addresses, or at any other setting if documented on HPZone.
RESULTS
The national enhanced VTEC surveillance system included 349 confirmed VTEC cases aged ⩽5 years, of which 234 (67%) attended childcare facilities. Our study population comprised 225 (96·2%) of these children attending 201 childcare facilities, on whom information was available on HPZone.
Description of all VTEC cases in the study population
The median age was 3 (IQR 2–4) years and 52·4% (n = 118) of the children were female. Of the 184 (81·8%) cases with information available on ethnicity, 156 (84·8%) were white, 13 (7·1%) were Asian or Asian British, seven (3·8%) Black or Black British, and eight (4·3%) mixed or other.
The most common symptoms reported included: diarrhoea (97·3%, 181/186 cases with known information), and/or bloody diarrhoea (59%, 98/166 cases). Twenty-one (9·3%) cases were asymptomatic, 220 (98·7%) cases were serogroup E. coli O157, three were E. coli O26 and two did not have serogroup recorded. The three most common phage types were PT21/28 (37%), PT8 (30%), and PT2 (10%).
Duration of shedding in children aged ⩽5 years
The median duration of shedding was 31 days (IQR 17–41) based on the 151 cases for whom this information was available (Table 1, Fig. 1). Of these, 73 (48%, 95% CI 40–56) were shedding for up to 30 days, 66 (44%, 95% CI 36–52) were shedding between 31 and 60 days and 12 (8%, 95% CI 4–12) continued to shed for more than 60 days (Fig. 2).
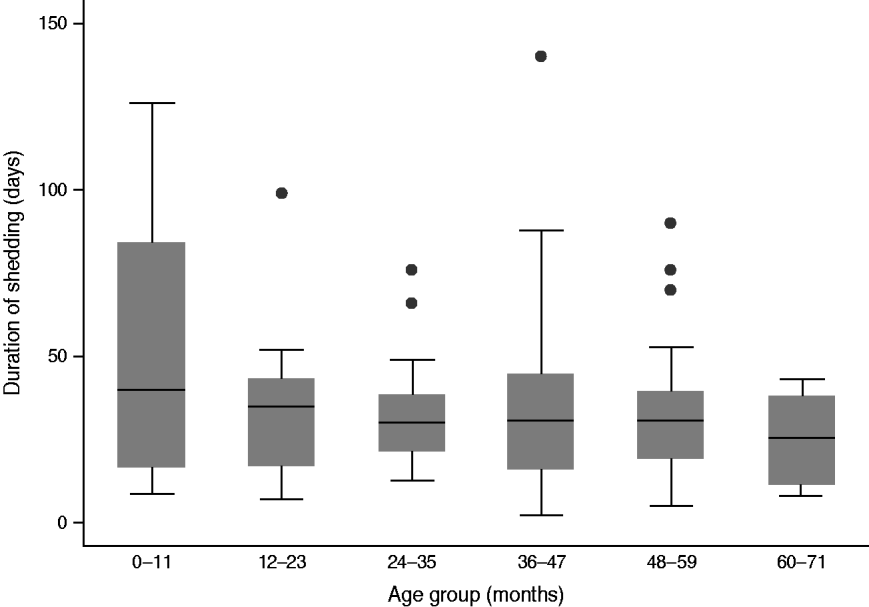
Fig. 1. Duration of shedding of Verocytotoxin-producing Escherichia coli in days by age group of child, England, 2010–2011 (n = 151). [Grey bars: interquartile range (IQR); horizontal line within bar: median; whiskers: 1·5 IQR beyond 25th and 75th percentiles; outliers: >1·5 IQR beyond 25th and 75th percentiles].
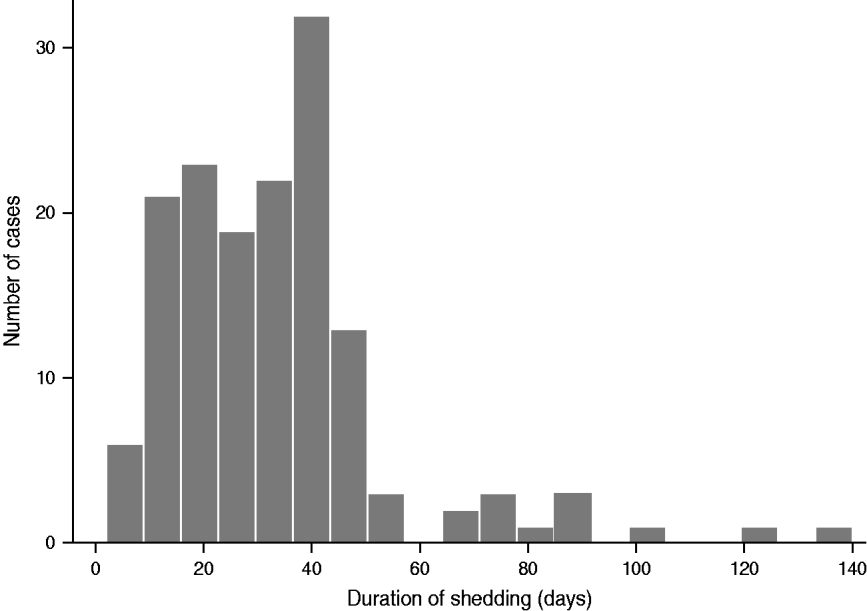
Fig. 2. Number of cases aged ⩽5 years, shedding Verocytotoxin-producing Escherichia coli by duration in days, England, 2010–2011 (n = 151).
Table 1. Median duration and range of shedding of Verocytotoxin-producing Escherichia coli in days by age and gender in children attending childcare settings, England, 2010–2011
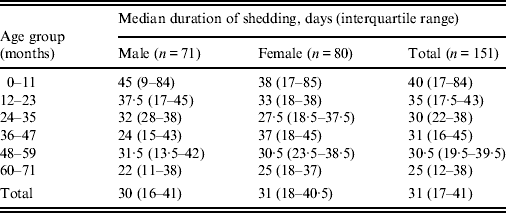
Younger children shed for longer; there was a 7% drop in duration of shedding per year of age (t test for slope of linear regression: P = 0·04, 95% CI 1–14). There was no significant difference in median duration of shedding by gender (Wilcoxon P = 0·7) or phage type (Kruskal–Wallis P = 0·27). The median duration of exclusion was 39·5 days (IQR 28–52, n = 162), and was at least 2 weeks longer than the duration of shedding in 34/150 cases (23%, 95% CI 16–30) where both duration of shedding and exclusion were known.
Difficulties in implementing exclusion were documented for 61 (30%) of 204 excluded cases. In these 61 cases, parental anxiety and/or communication issues were most frequently reported (n = 39, 64%), followed by disruption to family and social isolation (n = 28, 46%), issues with sampling including delays and loss of samples (n = 20, 33%), financial issues (n = 17, 28%) and childcare issues (n = 9, 15%).
VTEC transmission in children in childcare facilities
Of the 172 VTEC cases with available information, 51% (95% CI 44–59, n = 88) were infectious at the facility. The mean age of those infectious at facilities was no different from those not infectious at facilities (t test, P>0·05). The number of days the case attended their childcare facility while infectious was known for 63 (72%) cases. Of these, the median number of days attended was 2 (IQR 1–3·5), and 44 (70%, 95% CI 59–81) spent ⩽2 days at their facility. The HPU alerted the childcare facility to the risk of transmission via letter or phone call for 74% (95% CI 67–80) of the cases (135/183 with available information). An alert was raised for 93% of the children known to have been infectious at the facility vs. 59% if not infectious at the facility (P<0·0001, χ 2).
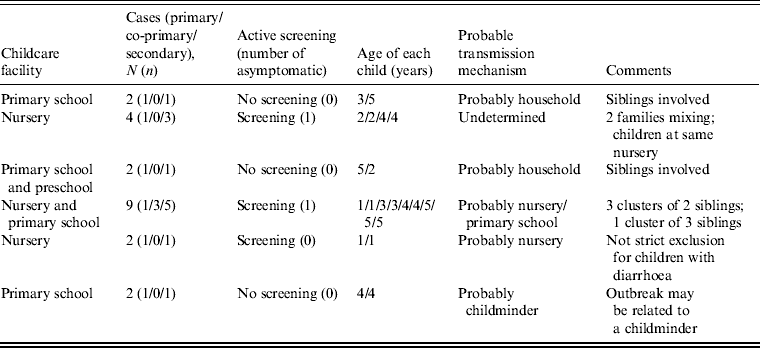
We had information about case attendance while infectious for 155 (77%) facilities out of 201. Of those 155, 83 (53·5%) facilities had been attended by at least one primary or co-primary infectious case. Secondary cases were identified at six of these 83 facilities involving a total of 21 cases with the following distribution: six primary, three co-primary, and 12 secondary cases including two asymptomatic (Table 2). The proportion of facilities with identified transmission incidents for all facilities with at least one infectious case was estimated at 7·2% (95% CI 3–16). The ratio of secondary to primary/co-primary cases was estimated as 1·3. Among the six incidents, three were considered most likely to be caused by person-to-person transmission within the household or at a childminder's; two by transmission at the childcare facility and one was undetermined. Active screening was conducted in three of the six facilities where secondary cases arose. We did not find any statistical association between the presence of infectious cases at the facility and the occurrence of secondary cases, but numbers were small.
Table 2. List of incidents involving at least one secondary case in children aged ⩽5 years in childcare facilities where at least one infectious case was in attendance, England, 2010–2011
Assuming an average number of contacts between 10 and 30 in the 83 facilities that had at least one infectious case, we estimated the proportion of secondary cases in children exposed to primary or co-primary cases within childcare facilities with at least one infectious case to vary between 2·4% and 0·6%, respectively.
DISCUSSION
Children aged ⩽5 years attending childcare facilities shed VTEC for a median duration of approximately 1 month, with younger children shedding for slightly longer. The median duration of exclusion was 39·5 days. There was a greater proportion of older children in the group whose shedding duration was unknown (39% aged ⩾5 years) compared to those where shedding duration was known (19% aged ⩾5 years) (P < 0·05). Therefore our overall shedding duration may be an overestimate. However, assuming that the children with missing data shed for a duration consistent with the median for their age group, the estimated overall median shedding duration (30·5 days) was very similar to our result of 31 days. About half the children for whom this information was available had attended their childcare facility while infectious for a median of 2 days. Secondary cases were reported in 7% of facilities attended by infectious primary cases, but not all of these were considered to be due to transmission within the childcare facility itself. Difficulty in implementing exclusion was experienced in nearly a third of cases. Parental anxiety and dissatisfaction with control measures is a key issue, as it may reduce compliance, with the child being sent to alternative childcare providers. Clear communication with parents is required regarding expected duration of shedding and the rationale for exclusion. The sustainability and acceptability of control measures is an important aspect to consider when formulating guidance and evaluating its impact.
Few studies have estimated the duration of shedding in young children in childcare facilities, but our estimate appears higher than those previously reported. The median duration of shedding in children aged <10 years has been reported as between 13 and 21 days in earlier studies from USA, Norway and Germany [Reference Belongia10, Reference Wahl11, Reference Karch17]. A recent study reported higher estimates, with a median shedding duration of 30·5 days in adults and children with E. coli O26:H11 infection [Reference Brown18], which is similar to our finding. However, in our study, 24% of cases continued to shed for ⩾6 weeks, which has rarely been reported before. Duration of shedding is affected by the frequency and method of testing; more sensitive methods are likely to result in an apparently longer shedding duration [Reference Karch17, Reference Chapman, Wright and Siddons19]. Other factors, such as young age [Reference Boyce, Swerdlow and Griffin20, Reference Pai21] or severe illness [Reference Karch17], may also result in longer shedding duration. The effect of antibiotics on shedding duration is not consistent throughout the literature. Most countries advise against antibiotic treatment due to previously reported increased risk of HUS [Reference Wong22, Reference Pavia23] but several studies have refuted this association [Reference Ikeda24–Reference Safdar26]. A recent study following the German Shiga-toxigenic E. coli (STEC) outbreak in 2011 reported that treatment with azithromycin was associated with a lower frequency of long-term STEC O104:H4 carriage [Reference Nitschke27] but the value and safety of late-in-illness treatment has not been established [Reference Seifert and Tarr28].
Data on person-to-person transmission during VTEC outbreaks in childcare facilities is scarce. A systematic analysis of published E. coli O157 outbreaks from 1982 to 2006 in Great Britain, Ireland, Scandinavia, Canada, the USA and Japan included 90 outbreaks, of which 69 (76·7%) had secondary cases. Person-to-person transmission occurring at home or in childcare facilities was described for 60% and 14% of these, respectively [Reference Snedeker29]. Most transmission studies focus on households, where attack rates have been estimated to be between 4% and 15% [Reference Parry and Salmon30]. Within childcare facilities, attack rates ranging from 3% to 38% with a median of 22% have been documented [Reference Al-Jader31–Reference Reida33], which are greater than our estimate of 0·6% to 2·4%. Variable implementation of control measures, including exclusion of cases until stool clearance, could account for some of the difference in attack rates in our study compared to those in the literature. Other possible reasons for this difference include investigation and publication bias whereby only large outbreaks are published. Our analysis records events where no further person-to-person transmission occurred, which is the situation in more than 92% of the childcare facilities with at least one infectious primary case in attendance. In addition, asymptomatic cases are not captured systematically in our analysis as active screening of contacts is not routinely performed in facilities following a single case. Facility-based risk factors also impact upon the risk of transmission, e.g. hygiene practices and facilities, staff training and knowledge, effective policies for excluding children with diarrhoea, food safety and feeding practices [Reference Gilbert34].
Both the design of the study and current VTEC management practices influence the accuracy of our estimates. Shedding duration may be overestimated, as those shedding for shorter periods are more likely to be culture negative on initial testing. However, this feature is common to other studies; therefore our results should be comparable.
For our transmission estimates, we have included all incidents that involved multiple cases at a childcare facility but the true mode of transmission may be uncertain. In some of these incidents, transmission may have occurred within a household (e.g. in siblings) or other places where children mix socially, resulting in an overestimate of transmission at childcare facilities. We also included asymptomatic cases when screening was performed. As universal screening is not routinely performed, asymptomatic cases may be misclassified as exposed non-cases in childcare facilities resulting in an underestimation of transmission. We did not examine the timing of interventions and how control measures such as alerting letters, facility closure or improving personal hygiene may have affected the transmission estimates. The low proportion of transmission events seen may have been due in part to the current exclusion policy. However, it is known that for every child with confirmed VTEC infection, there will be other undiagnosed cases continuing to attend childcare facilities [Reference Tam35]. This has to be taken into consideration when assessing the value of exclusion of clinically recovered, prolonged shedders. In addition, although transmission from asymptomatic shedders remains a possibility, they may not be as infectious as previously thought [Reference Vogelsang and Pulz36].
CONCLUSIONS
Our study provides estimates of both the duration of shedding of VTEC in children aged ⩽5 years, and the risk of transmission within childcare facilities in England. Our findings highlight the considerable proportion of children who attend childcare facilities while infectious, and the prolonged shedding which causes disruption to families. We recommend that parents are given clear information regarding the possible duration of shedding and the stool sampling strategy in order for them to prepare accordingly. The risk of transmission to other children within childcare facilities in England appears relatively low, which may be attributable to control measures such as exclusion of symptomatic children and enhanced hygiene. However, the additional benefit from continued exclusion of recovered, prolonged shedders is not clear. Prospective studies are needed to gather additional evidence on the risk of transmission within childcare facilities taking into account timing of interventions, and the role of asymptomatic prolonged shedders [Reference Swerdlow and Griffin37].
Current guidelines focus on excluding presumptive cases until two negative stool specimens are obtained. The prolonged duration of shedding and the low number of transmission events should be taken into account when updating guidelines. The public health management of VTEC cases should continue to promote universal approaches, including exclusion for all cases of diarrhoeal illness until symptom-free for 48 h and good hand hygiene at all times. Supervised return of prolonged shedders to childcare facilities could be considered if evidence of low transmission is confirmed.
ACKNOWLEDGEMENTS
The authors thank all 25 HPUs for contributing data; Helen Maguire (Consultant Epidemiologist, HPA London), for critical comments on the manuscript; Naomi Launders and Katy Harker (HPA Colindale), for providing the national VTEC data; and Nick Andrews for statistical advice.
DECLARATION OF INTEREST
None.






