Hepatitis C virus (HCV) infection is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. HCV is classified into several genotypes and sub-genotypes with varying prevalence rates in different regions of the world [Reference Simmonds1]. In Japan, the majority of individuals with HCV infection have genotype 1b [Reference McOmish2, Reference Tanaka3], which is one of the sub-genotypes resistant to interferon-based antiviral therapy [Reference Ghany4]. Before the detection of HCV antibodies was established in 1990, the main modes of HCV transmission had been blood transfusion or nosocomial infection. After the discovery of HCV, prevention of HCV transmission through these routes was established and the number of individuals newly infected with HCV rapidly decreased in Japan [Reference Moriya5]. Although decreases in the number of individuals with HCV infection have been reported, changes in the distribution of HCV genotype over time have not been studied. In the present study, we investigate changes in HCV genotype distribution based on the birth year of HCV-infected individuals in Japan. We analysed individuals from three different areas of the country to clarify whether any changes in HCV genotype distribution constitute a nationwide trend.
HCV genotype was analysed in a total of 5515 HCV-infected Japanese individuals at three institutions located in different parts of Japan (Fig. 1): Ogaki Municipal Hospital (institution A; Ogaki City, located in central Japan, 3269 individuals), Kagawa Prefectural Central Hospital (institution B; Takamatsu City, located on Shikoku Island, western Japan, 1421 individuals), and Shinmatsudo Central General Hospital (institution C; Matsudo City, near Tokyo, 825 individuals). These individuals received regular follow-up at one of the institutions between 1991 and 2013. HCV infection was confirmed by both positive serum HCV antibody (Architect Anti-HCV; Abbott Laboratories, USA) and serum HCV RNA using a real-time polymerase chain reaction (PCR)-based method (COBAS AmpliPrep/COBAS TaqMan HCV test; Roche Molecular Systems, USA; lower limit of detection, 1·2 log10 IU/ml). HCV genotype was assessed using PCR methods to amplify the core gene sequences using genotype-specific primers [Reference Ohno6]. Genotype was classified as 1a, 1b, 2a, 2b, 3a, or mixed. Review and analysis of clinical data including patient's birth year, age, sex, and HCV genotype were approved by the Institutional Review Board of each institution.

Fig. 1. Location of the three Japanese institutions (liver centres) participating in the study. A, Ogaki Municipal Hospital; B, Kagawa Prefectural Central Hospital; C, Shinmatsudo Central General Hospital.
Trends for changes in the percentage of each HCV genotype according to birth year were evaluated with the Cochran–Armitage test. All P values were two-tailed, and P <0·05 was considered statistically significant.
All individuals studied were of Japanese ethnicity and no immigrants were included. The HCV-infected individuals comprised of 1790 males and 1479 females with a mean age of 67·1 ± 13·3 years at institution A; 729 males and 692 females with a mean age of 62·0 ± 12·7 years at institution B; and 419 males and 406 females with a mean age of 66·6 ± 13·3 years at institution C. The distribution of HCV genotypes in all patients was: institution A [genotype 1b (64·3%), genotype 2a (27·0%), genotype 2b (7·7%), and other (including genotypes 1a, 3a, and mixed) (1·0%)]; institution B [genotype 1b (62·3%), genotype 2a (26·5%), genotype 2b (10·3%), and other (including genotypes 1a and 3a) (1·0%)]; and institution C [genotype 1b (70·8%), genotype 2a (17·6%), genotype 2b (10·3%), and other (including genotypes 3a and mixed) (1·3%)].
At all three institutions, the number of individuals with HCV infection who were under follow-up at the institutions decreased over time starting with birth years 1930–1939 at institution A and 1939–1940 at institutions B and C (Fig. 2). Figure 3 shows the distribution of HCV genotypes based on patient's birth year at each institution. There is a trend of HCV genotype 1b decreasing over time (P<0·0001 for all three institutions). The percentage of HCV genotype 1b was <50% in individuals born after 1970. The percentage of HCV genotype 2a was constant except for individuals born before 1929. By contrast, the percentage of HCV genotype 2b increased during the period studied (P<0·0001 for institutions A and B, and P = 0·0004 for institution C).

Fig. 2. Changes in the number of hepatitis C virus-infected individuals under follow-up at institutions based on birth year at (a) Ogaki Municipal Hospital, (b) Kagawa Prefectural Central Hospital, and (c) Shinmatsudo Central General Hospital.
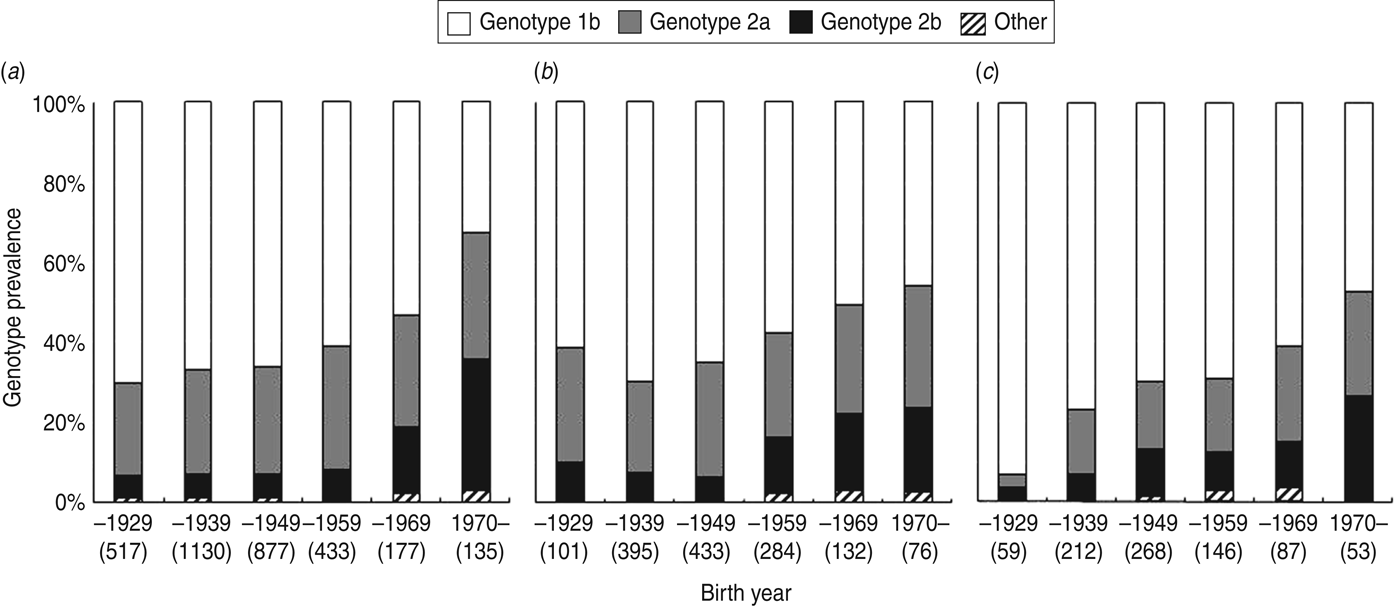
Fig. 3. Changes in hepatitis C virus (HCV) genotype distribution based on birth year of HCV-infected individuals at (a) Ogaki Municipal Hospital, (b) Kagawa Prefectural Central Hospital, and (c) Shinmatsudo Central General Hospital. The number of individuals born during each time period is indicated in parentheses. ‘Other’ includes genotypes 1a, and 3a, as well as mixed genotypes.
This study showed that the HCV genotype distribution changed markedly over time in Japan. The trend was confirmed at three large institutions from different parts of the country and seems to be nationwide. The percentage of individuals with HCV genotype 1, which is a major HCV genotype worldwide [Reference McOmish2], is decreasing and is found in <50% of individuals born after 1970. A decrease in the prevalence of patients with HCV genotype 1b has also been reported in the USA and Europe in studies with a smaller number of subjects [Reference Rosen7–Reference Chlabicz9].
Several studies have reported that HCV genotypes are different between individuals with HCV acquired through transfusions or medical procedures and individuals with HCV acquired through other transmission route, including intravenous drug use or tattooing [Reference Bourliere8, Reference Mathei10–Reference Chung, Ueda and Kudo13]. In Japan, HCV genotype 1b is associated with the former route of transmission, whereas other genotypes, i.e. genotypes 2a and 2b, are associated with the latter route [Reference Chung, Ueda and Kudo13]. With the establishment of methods to prevent HCV transmission during blood transfusions and medical procedures in developed countries, the routes of HCV transmission other than transfusions or medical procedures have become the main routes of HCV infection. Indeed, among 135 individuals with a birth year of 1970 or later at institution A, 29 (21·5%) had a history of intravenous drug use and 33 (24·4%) had a history of tattooing. By contrast, no individuals had a history of blood transfusion. Although the accurate route of HCV transmission was unclear in most individuals in the present study, these changes in the common routes of HCV transmission may be associated with changes in HCV genotype distribution over time. Further studies will be necessary to clarify why the percentage of HCV genotype 2b increased in Japan.
There are several limitations to this study. HCV genotype distribution was analysed based on the birth year of infected individuals instead of the year of HCV infection since there was not enough available information to accurately determine the year of HCV infection. However, results with a large study population from different parts of Japan can be indicative of changes in HCV genotype distribution. The HCV-infected individuals analysed were those who received regular follow-up and may not reflect the HCV genotype distribution of the entire Japanese HCV-infected population. Better nationwide surveillance of HCV that includes data on genotype determination is required to confirm these observed changes in HCV genotype distribution over time.
In conclusion, significant changes in HCV genotype distribution over time were observed in HCV-infected Japanese individuals in various regions of Japan. HCV genotype 1b may no longer be the predominant genotype of HCV in Japan in the future. These changes may also be occurring in other countries throughout the world, especially developed countries. Reappraisal of HCV genotype distribution will be necessary over time. Recent new antiviral drugs for the treatment of HCV, including direct-acting antivirals, mainly target HCV genotype 1 with some exceptions. However, other genotypes may become dominant in various populations of HCV-infected individuals.
DECLARATION OF INTEREST
None.






