The ‘off licence’ use of drugs is common in the UK. One such use is the treatment of bipolar disorder with sodium valproate. This paper reviews the evidence for using the licensed alternative, valproate semisodium, under the headings of licence, efficacy, pharmacokinetics and tolerability.
Background
Valproate (2-propylpentanoic acid) is an anticonvulsant drug used in the treatment of bipolar disorder, although it is only licensed in the UK for the treatment of epilepsy. Valproate is available clinically in a number of forms: these include sodium valproate alone, valproic acid alone, and sodium valproate in combination with valproic acid. In the UK, sodium valproate and valproic acid are available in enteric coated formulations, but this is not the case in the USA. A modified release formulation of a combination of sodium valproate and valproic acid in a 2.3:1 ratio is also available in the UK.
Valproate semisodium (divalproex semisodium in the USA) is a more recent product, marketed in the UK by Sanofi–Synthelabo under the trade name of Depakote. It consists of a compound of sodium valproate and valproic acid in a 1:1 molar relationship in an enteric coated form. This compound dissociates to release valproate ions in the gastrointestinal tract (Food and Drug Administration, 2002).
Sodium valproate circulates in the plasma as the valproate ion, as do valproic acid and valproate semisodium (Reference Zaccara, Messori and MoroniZaccara et al, 1988; Reference Perry, Bever-Stille and ArndtPerry et al, 2000), and trough valproic acid plasma levels are used to monitor all three. Valproate is protein-bound, with the free fraction concentration-dependent. The exact mechanism of action is not known, but it is thought that valproic acid and its active metabolites are responsible for the antimania activity. The postulated mechanisms of action are potentiation of gamma-aminobutyric acid (GABA), an effect on the protein kinase C pathway or an effect on guanine nucleotide-binding regulatory proteins (G proteins) (Reference Watson, Watterson and LennoxWatson et al, 1998; Reference Brown, Wang and YoungBrown et al, 2000; Reference PeruccaPerucca, 2002).
Comparative data on the cost of UK valproate preparations are shown in Table 1 (Department of Health, 2002; MIMS, 2002).
Table 1. Comparison of valproate semisodium with other preparations
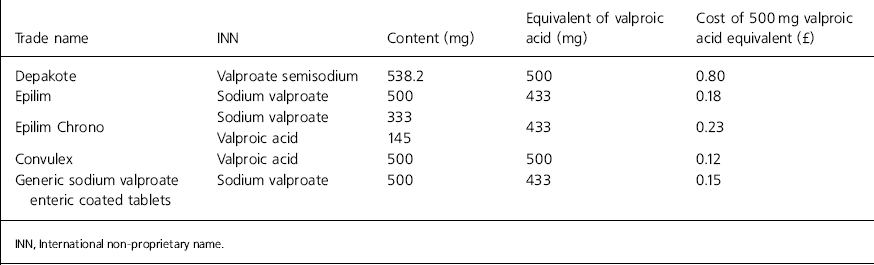
| Trade name | INN | Content (mg) | Equivalent of valproic acid (mg) | Cost of 500 mg valproic acid equivalent (£) |
|---|---|---|---|---|
| Depakote | Valproate semisodium | 538.2 | 500 | 0.80 |
| Epilim | Sodium valproate | 500 | 433 | 0.18 |
| Epilim Chrono | Sodium valproate | 333 | 433 | 0.23 |
| Valproic acid | 145 | |||
| Convulex | Valproic acid | 500 | 500 | 0.12 |
| Generic sodium valproate enteric coated tablets | Sodium valproate | 500 | 433 | 0.15 |
If valproate semisodium and other forms of valproate all act through the final common pathway of the valproate ion, are there any advantages in using the more expensive drug? In order to answer this question we performed a literature search of the Cochrane Library, PsychINFO, Medline (1966 to 2002) and EMBASE (1996 to 2002), using the terms BIPOLAR, MANIA, VALPROATE, VALPROIC ACID, DIVALPROEX, DEPAKOTE and VALPROATE SEMISODIUM.
Results
The results of our research are summarised under the four headings of licence, efficacy, pharmacokinetics and tolerability.
Licence
In the UK, valproate semisodium is the only form of valproate to be licensed for use in bipolar disorder. However, this licence applies only to the acute treatment of mania; the drug is used but not licensed for maintenance treatment of bipolar disorder. Other forms of valproate are used for treatment of bipolar disorder in the UK although this use is ‘off licence’. Psychiatrists in the UK face a dilemma: recognised guidelines (Reference Taylor, McConnell and Duncan-McConnellTaylor et al, 2001) for the treatment of bipolar disorder recommend the use of valproate, but the drug is unlicensed. ‘Off license’ use of drugs by UK psychiatrists is common. Douglas-Hall et al (Reference Douglas-Hall, Fuller and Gill-Banham2001) assessed the scale of ‘ off licence’ prescribing across a large number of psychiatric hospitals - it amounted to 7.5% of all prescribing, and sodium valproate for affective disorder was one of the most common of these prescriptions.
Efficacy
Sodium valproate (Reference Pope, McElroy and KeckPope et al, 1991; Reference Freeman, Clothier and PazzagliaFreeman et al, 1992) and valproate semisodium (Reference Bowden, Brugger and SwannBowden et al, 1994) have both been shown to be effective in the treatment of mania. Only one randomised controlled trial has looked at valproate in the maintenance treatment of bipolar disorder, comparing valproate semisodium with lithium and placebo (Reference Bowden, Calabrese and McElroyBowden et al, 2000). The study failed to show that valproate semisodium or lithium were more efficacious than placebo, but this failure might have been due to the study design (Reference Macritchie, Geddes and ScottMacritchie et al, 2001). Further trials of this type are in progress. A randomised open study found valpromide (a pro-drug of valproate) to be of equal efficacy to lithium in maintenance treatment (Reference Lambert and VenaudLambert & Venaud, 1992). The psychiatric uses of valproate are reviewed by Davis et al (Reference Davis, Ryan and Adinoff2000); this review does not differentiate between semisodium and other forms of valproate other than when referring to tolerability and side-effects.
Pharmacokinetics
It is commonly held that valproate semisodium has different pharmacokinetic properties from those of enteric coated sodium valproate. Evidence for this can be seen in the recent balance trial protocol (available on the internet at http://www.psychiatry.ox.ac.uk/balance). The balance trial is a UK multi-centre comparison of lithium, valproate, and lithium plus valproate in the maintenance treatment of bipolar disorder; the protocol states: ‘[valproate semisodium] has more favourable pharmacokinetic properties than valproate preparations’. We were unable to find evidence to support such views. Contact with Sanofi-Synthelabo, the UK manufacturers of valproate semisodium, revealed that they also believed that there were differences in simple pharmacokinetic parameters - maximum plasma concentration (Cmax), time to maximum concentration (Tmax) and half-life (t½) - observed between valproate semisodium and sodium valproate at steady state. They were also of the opinion that valproate semisodium produces higher peak plasma levels, and therefore higher intracerebral levels, than similar doses of sodium valproate. We were referred to Sanofi-Synthelabo data on file F90-196, M93-004 and 491.6.020 (GB188) as supporting evidence; however, the third of these appears to be the same as data published by Roberts et al (Reference Roberts, Easter and O'Bryantear1996). Examination of the data shows that the mean Cmax for valproate semisodium at a dose of 500 mg (valproic acid equivalent 500 mg) twice a day is 103 (s.d. 13.5) mg/l. For enteric coated sodium valproate at a dose of 500 mg (valproic acid equivalent 433 mg) twice a day it is 91.33 (s.d. 17.60) mg/l. The Cmax values probably reflect the difference in dose and are not evidence of a difference in pharmacokinetics. The mean Tmax value for valproate semisodium is 3.6 (s.d. 1.1) h and for enteric coated valproate 3.8 h (no s.d. value given). In the case of t½, values are remarkably similar and we would question whether there is any significant difference in clinical practice. There is some limited case report evidence to suggest increased bioavailability of valproate from valproate semisodium compared with the same dose of valproic acid, demonstrated by higher trough plasma valproate levels (Reference Demoulin and LandryDemoulin & Landry, 2000).
It is worth noting that other factors can have a major effect on the Tmax and Cmax of valproate preparations: the Tmax can be delayed by about 1 h by administration with food (Sanofi-Synthelabo, 2001), and both parameters are subject to substantial diurnal variation, possibly related to gastric emptying rates (Reference Roberts, Easter and O'BryantearRoberts et al, 1996).
Tolerability
A common opinion is that valproate semisodium has less severe side-effects, and is therefore better tolerated, than enteric coated sodium valproate. This is probably based on the review by Davis et al (Reference Davis, Ryan and Adinoff2000), who noted that valproate semisodium was better tolerated than valproic acid, with patients less likely to experience gastrointestinal side-effects. They quote Zarate et al (Reference Zarate, Tohen and Narendran1999) as evidence; similar findings were reported by Brasfield (Reference Brasfield1999). Both of these studies looked at patient populations in the USA and compared enteric coated valproate semisodium with non-enteric coated valproic acid. It is our view that these studies cannot be used to substantiate claims of improved tolerability of valproate semisodium in the UK where, with the exception of crushable sodium valproate, all solid forms of sodium valproate and valproic acid are enteric coated to reduce gastrointestinal side-effects.
Conclusion
The UK Medicines Control Agency favours the use of licensed drugs over unlicensed alternatives and this is an argument in favour of using valproate semisodium in acute mania as opposed to enteric coated valproate. The argument no longer holds once treatment becomes maintenance. The question of licensed v. unlicensed prescribing needs to be considered in the light of limited health care budgets.
There is no trial that directly compares valproate semisodium with enteric coated sodium valproate in terms of efficacy in bipolar disorder or tolerability. At present, the evidence base does not substantiate claims that valproate semisodium is better tolerated or more efficacious in the treatment of acute mania than enteric coated sodium valproate. The study by Bowden et al (Reference Bowden, Calabrese and McElroy2000), despite its design limitations, is the only randomised controlled trial of maintenance treatment, and here valproate semisodium did not differ from placebo.
The pharmacokinetics of valproate semisodium and enteric coated sodium valproate are remarkably similar and we could find no evidence to support any significant clinical difference. However, it is important to note that valproate semisodium 500 mg tablets contain 15.5% more valproic acid equivalent than sodium valproate 500 mg tablets (Table 1).
A direct comparison of valproate semisodium and enteric coated valproate is required before the conclusions drawn from US data on the improved tolerability of valproate semisodium over other forms of valproate can be applied in the UK, where most other forms of valproate are enteric coated.
Declaration of interest
None.




eLetters
No eLetters have been published for this article.