Introduction
Major depressive disorder (MDD) is a common mental disorder in adolescents with an estimated 1-year prevalence higher than 4% in mid to late adolescence.Reference Costello, Egger and Angold1, Reference Jane Costello, Erkanli and Angold2 MDD in adolescents is a major risk factor for suicide and may lead to serious social and educational impairments and an increased rate of substance misuse and psychiatric comorbidities in adulthood.Reference Thapar, Collishaw, Pine and Thapar3 Therefore, MDD in adolescents must be identified early for timely intervention. Several indicators can be used to identify adolescents who are at risk of developing MDD; these indicators include being female, having a family history of depression (FHD), having a history of family conflict, having experienced childhood abuse or neglect, having low socioeconomic status, and having poor academic performance.Reference Siu4
Despite being a risk factor for suicide, antidepressants remain a key component of the treatment of moderate-to-severe MDD in adolescents.Reference Cousins and Goodyer5 In a recent meta-analysis of 17 randomized control trials including 2537 children and adolescents with MDD, antidepressants were shown to have significantly positive effects on functioning.Reference Teng, Zhang and Yin6 A further subgroup analysis demonstrated that second-generation antidepressants (such as selective serotonin reuptake inhibitors [SSRIs]), but not traditional tricyclic antidepressants, led to significant improvements in functioning.Reference Teng, Zhang and Yin6 Similarly, another meta-analysis demonstrated that fluoxetine (an SSRI, alone or in combination with cognitive behavioral therapy) had beneficial effects on the management of MDD in children and adolescents.Reference Zhou, Teng and Zhang7 However, the therapeutic effects of antidepressants might vary between individuals; initial treatment with an SSRI failed to produce a satisfactory clinical outcome in approximately one-third of adolescents with MDD.Reference Brent, Emslie and Clarke8 Some of the indicators that have been associated with favorable antidepressant treatment include having less chronic depression, higher functioning, lower suicide intent, and fewer signs of melancholic feature.Reference Curry, Rohde and Simons9 Brent et al. showed that for those with a poor response to initial antidepressant treatment, a switch to another SSRI was as effective as a switch to venlafaxine, a serotonin and norepinephrine reuptake inhibitor (SNRI).Reference Brent, Emslie and Clarke8
MDD is a complex psychiatric disorder involving both environmental and genetic indicators. Studies have demonstrated that an individual with FHD is more likely to develop depression due to innate vulnerabilities related to the genetic structure and function of the brain.Reference Levinson10 For instance, studies have shown that the first-degree offspring of patients with depression have a 2 to 3 times greater risk of developing depression.Reference Sullivan, Neale and Kendler11 In addition, an individual with FHD was more likely to have an earlier onset of MDD,Reference Azorin, Belzeaux, Fakra, Hantouche and Adida12 more likely to have chronic or recurrent depression,Reference Hardeveld, Spijker and De Graaf13, Reference Patten, Wang, Williams, Lavorato, Khaled and Bulloch14 and more likely to have psychiatric comorbidities.Reference Azorin, Belzeaux, Fakra, Hantouche and Adida12
MDD has a high tendency toward coaggregation within a family and may also be an independent predictor of other mental disorders within a given family. For example, parental psychiatric disorders were associated with increased risks of within-disorder transmission for attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), MDD, and bipolar disorder (BD).Reference Liang, Bai and Hsu15 In addition, parental psychiatric disorders were associated with increased risks of cross-disorder transmission to offspring.Reference Liang, Bai and Hsu15 Despite the high comorbidity between MDD and other major psychiatric disorders, few population-based studies have examined the likelihoods of the mental disorders in parents of probands with MDD.
In this study, we used data from the Taiwan National Health Insurance Research Database (NHIRD), which contains detailed registry and claims data for all residents of Taiwan. We examined the association between adolescents with MDD and the likelihood of parental psychiatric disorders. First, we investigated the likelihood that the parents of adolescents with MDD had psychiatric disorders (MDD, BD, schizophrenic disorder [SZ], alcohol use disorder, and substance use disorder). Then, we further hypothesized that the parent’s sex and the response to antidepressant treatment predicted the likelihood of parental psychiatric disorders.
Methods
Data source
The Taiwan NHIRD is audited and released by the National Health Research Institute (NHRI) for scientific and study purposes upon the formal application. Claims data of individuals included in the NHIRD are anonymous to maintain the privacy. Comprehensive medical information about the insured patients, such as demographics (birthdate, sex, and residence) and clinical visits (dates and diagnoses), is available in the database. Following Chen et al.’s and Cheng et al.’s methods,Reference Chen, Hsu and Huang16, Reference Cheng, Chang and Chen17 the recorded family kinships in the NHIRD were used for genealogy reconstruction, and the family triads (father, mother, and child) were identified. The diagnostic codes used were based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The NHIRD has been used extensively in many epidemiologic studies in Taiwan.Reference Chen, Hsu and Huang16–Reference Wang, Cheng and Bai18 This study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Study and control groups
Adolescents aged 12–19 years who were diagnosed with MDD (ICD-9-CM codes: 296.2 and 296.3) by board-certified psychiatrists between 2001 and 2011 were included, and were classified as antidepressant responsive or antidepressant resistant according to their response to antidepressant treatment during the 1-year follow-up period after depression diagnosis.Reference Chen, Chen, Bai, Chen, Chen and Su19, Reference Li, Bai and Huang20 An adequate trial of antidepressant treatment was defined as use of an antidepressant within its therapeutic dosage range (exp., fluoxetine ≥ 20 mg/day) for ≥60 consecutive days.Reference Chen, Chen, Bai, Chen, Chen and Su19, Reference Li, Bai and Huang20 Patients who remained on a single antidepressant were defined as the antidepressant-responsive depression group; those who changed the antidepressant treatment regimen 2 or more times were defined as the antidepressant-resistant depression group. In addition, adolescents with antidepressant-resistant depression were further divided to 2 subgroups: only resistant to SSRIs and additionally resistant to non-SSRIs groups. SSRIs include fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram, and escitalopram. Non-SSRIs include SNRIs (venlafaxine, duloxetine, and milnacipran), norepinephrine–dopamine reuptake inhibitor (bupropion), and mirtazapine. Antidepressant-responsive and antidepressant-resistant groups were further matched (4:1) based on age, age of depression diagnosis, sex, residence, and family income. The age-, sex-, family income-, and residence-matched (1:4) control cohort was randomly identified as the control group after eliminating the study cases and those who had any diagnostic code of severe mental disorders (ICD-9-CM codes: 295 and 296) in the database.
Outcome and confounder assessment
Diagnoses of parental mental disorders, including SZ, BD, MDD, alcohol use disorder, and substance use disorder, were assessed between groups. In addition, to ensure the diagnostic validity, the diagnoses of above mental disorders were given by board-certified psychiatrists at least twice. Income level (levels 1–3 per month: ≤19 000 New Taiwanese Dollars [NTD], 19 001–42 000 NTD, and ≥42 001 NTD) and urbanization level of residence (levels 1–5, most to least urbanized) were regarded as the proxies for healthcare availability in Taiwan.Reference Liu, Hung, Chuang, Chen, Weng and Liu21
Statistical analysis
Regarding between-group comparisons, the F-test was used for continuous variables and Pearson’s X Reference Jane Costello, Erkanli and Angold2-test for nominal variables, where appropriate. Logistic regression analyses with adjustment of demographic characteristics (age, sex, income, and residence) were performed to investigate the likelihoods of paternal and maternal mental disorders between adolescents with antidepressant-resistant and antidepressant-responsive depression and the control group. Furthermore, we assessed the association between treatment resistance to SSRIs only or additionally to non-SSRIs (SNRIs, bupropion, or mirtazapine) and the risks of parental mental disorders. A 2-tailed P-value of less than .05 was considered statistically significant. All data processing and statistical analyses were performed with Statistical Package for Social Science (SPSS) version 17 software (SPSS Inc.) and Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Results
We included 1758 adolescents with antidepressant-resistant depression, 7032 adolescents with antidepressant-responsive depression, and 7032 matched controls (Table 1). Three groups had similar age at enrollment, sex distribution, level of urbanization, and income-related insured amount (all P > .05). Among 1758 adolescents with antidepressant-resistant depression, 887 subjects were only resistant to SSRIs and 871 were additionally resistant to non-SSRIs (Table 1).
Table 1. Demographic Characteristics between Adolescents with Treatment-Responsive vs. Treatment-Resistant Depression

a SNRIs, bupropion, and mirtazapine.
Abbreviation: NTD, New Taiwan dollar; SD, standard deviation; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Table 2 shows the risks (shown as odds ratio with 95% confidence interval) of 5 psychiatric disorders in parents of probands in the antidepressant-responsive depression and antidepressant-resistant depression groups compared with the parents of the control group, with adjustment for demographic characteristics. The parents of adolescents with MDD who were antidepressant resistant had the highest likelihood to be diagnosed with BD (3.82, 2.54–5.77) and MDD (3.53, 3.01–4.15) compared with the parents of adolescents who were antidepressant responsive (BD: 2.67, 1.91–3.73; MDD: 2.81, 2.48–3.17) and the parents of the control group. The parents of the adolescents with MDD who were antidepressant responsive or antidepressant resistant had the higher likelihood to be diagnosed with SZ (2.38, 1.71–3.31; 2.14, 1.34–3.43), alcohol use disorder (2.10, 1.66–2.67; 1.93, 1.37–2.73), and substance use disorder (2.72, 2.08–3.56; 2.42, 1.66–3.52) compared with the parents of the control group (Table 2).
Table 2. Risk of Parental Mental Disorder between Adolescents with Treatment-Responsive vs. Treatment-Resistant Depression
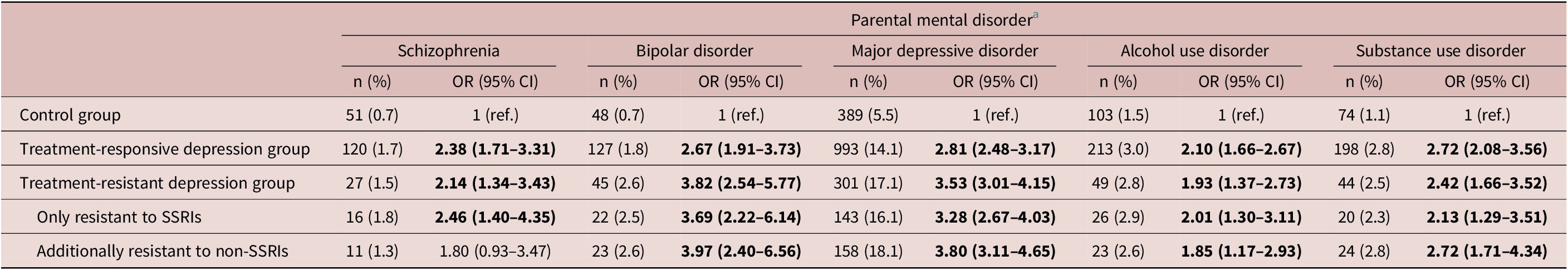
Note. Bold type indicates statistical significance.
Abbreviation: CI, confidence interval; OR, odds ratio; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
a Adjusting for demographic characteristics.
Furthermore, the parents of the adolescents who were additionally resistant to non-SSRIs had a slightly trend of increased likelihood to be diagnosed with BD (3.97, 2.40–6 vs. 3.69, 2.22–6.14) and MDD (3.80, 3.11–4.65 vs. 3.28, 2.67–4.03) compared with the parents of those who were only resistant to SSRIs (Table 2). Finally, both fathers and mothers of the antidepressant-responsive and the antidepressant-resistant depression group had a higher likelihood to be diagnosed with these 5 psychiatric disorders than the parents of the control group (Table 3).
Table 3. Risk of Paternal and Maternal Mental Disorders between Adolescents with Treatment-Responsive vs. Treatment-Resistant Depression

Note. Bold type indicates statistical significance.
Abbreviation: CI, confidence interval; OR, odds ratio.
aAdjusting for demographic characteristics.
Discussion
To the best of our knowledge, the present nationwide study is the largest one to have assessed the likelihood of parental psychiatric disorders in probands with MDD. We found that the parents of the adolescents with MDD were more likely to be diagnosed with not only MDD but also BD, SZ, alcohol use disorder, and substance use disorder than the parents of the control group. In a recent Danish registry study, Thorup et al. demonstrated that the offspring of parents with a severe mental illness were more likely to be diagnosed with any child and adolescent mental disorder than the offspring of parents without a severe mental illness.Reference Thorup, Laursen and Munk-Olsen22 Findings from Thorup et al. and the current study support the notion that FHD can be used as an indicator to predict both within-disorder and cross-disorder transmission in families.Reference van Santvoort, Hosman, Janssens, van Doesum, Reupert and van Loon23
Various psychiatric disorders have been shown to have a common pathogenesis in molecular studies. A genome-wide meta-analysis of 232 964 cases (ADHD, ASD, BD, MDD, and SZ) and 494 162 controls revealed 109 loci associated with at least 2 psychiatric disorders, including 23 loci with pleiotropic effects on 4 or more disorders and 11 loci with antagonistic effects on multiple disorders.24 The study also identified at least 2 groups of disorders based on shared genomics: one comprising mood and psychotic disorders (MDD, BD, and SZ), and the second comprising 2 neurodevelopmental disorders (ADHD and ASD).24 We found that the parents of adolescents with MDD were not only more likely to be diagnosed with MDD, BD, and SZ, but were also more likely to be diagnosed with alcohol use disorder and substance use disorder than the parents of the control group. This is consistent with a report from Lieb et al. that found that the offspring of parents with MDD were more likely to use substances than the offspring of parents without MDD.Reference Lieb, Isensee, Höfler, Pfister and Wittchen25 Furthermore, a genetic segregation analysis found that a major locus that contributes to the expression of alcohol and other substance use disorders within families can be used to identify probands with recurrent, early-onset MDD.Reference Maher, Marazita, Zubenko, Kaplan and Zubenko26 Various psychiatric disorders have also been shown to have a common pathogenesis in neuroimaging studies. In a functional magnetic resonance imaging study of patients with MDD, BD, and SZ, transdiagnostic dysconnectivities were identified within somatomotor and salience networks and between subcortical-limbic and subcortical-dorsal attention networks.Reference Huang, Luo and Palaniyappan27 Another systematic review of 401 neuroimaging studies found that the concentrations of glutamate-glutamine and white matter abnormalities were commonly found in patients with MDD, BD, and SZ.Reference Luttenbacher, Phillips and Kazemi28
We performed a subgroup analysis where the sample was segmented by response to antidepressant treatment and found that parents of adolescents with a poor response to antidepressant treatment were more likely to be diagnosed with MDD and BD than parents of adolescents with a good response to antidepressant treatment. In addition, parents of the individuals who were resistant to both SSRIs and non-SSRIs were more likely to be diagnosed with MDD than parents of the individuals who were only resistant to SSRIs. Adolescents with a poor response to antidepressant treatment reported FHD more frequentlyReference Jaffe, Rive and Denee29 and were more likely to have a change in diagnosis from MDD to BDReference Li, Bai and Huang20 than adolescents with a good response to antidepressant treatment. A study in Germany found that parental MDD was associated with an earlier onset and a worse prognosis (severity, impairment, and recurrence) of MDD in offspring.Reference Lieb, Isensee, Höfler, Pfister and Wittchen25 Our findings imply that adolescents with MDD who have a poor response to antidepressant treatment have a higher genetic load for mood disorders than those who have a good response to antidepressant treatment; however, further research is required.
We performed a subgroup analysis where the sample was segmented by sex. The results illustrated in Table 3 show that mothers of adolescents with MDD were more likely to be diagnosed with BD or MDD than fathers. Substantial evidence supports the genetic and sex interaction effect in MDD. A Swedish national twin study of lifetime MDD tested whether genetic risk factors are the same in the 2 sexes,Reference Kendler, Gatz, Gardner and Pedersen30 and it found that the heritability of liability to MDD was significantly higher in women (42%) than in men (29%).Reference Kendler, Gatz, Gardner and Pedersen30 Reporting a similar finding, another twin study suggested that genetic factors play a greater role in the etiology of MDD in women than in men and that the genes that influence risk for MDD in the 2 sexes are correlated but are probably not completely the same.Reference Kendler, Gardner, Neale and Prescott31
The findings from this study have important clinical implications. Parents of adolescents with MDD, especially adolescents with a poor response to antidepressant treatment, should receive a thorough assessment to rule out psychiatric disorders; this is because parents with psychiatric disorders may lack the ability to cope with the psychiatric disorders of their children. Evidence suggests that offspring with psychiatric disorders are better off once their mothers have recovered from depression.Reference Swartz, Cyranowski, Cheng and Amole32 Parental substance abuse has been found to be associated with suicide intent in offspring.Reference Oladeji and Gureje33 Furthermore, analysis of the association between parental psychiatric disorders and the prevalence of work disabilities in offspring suggests that parental psychiatric disorders influence an offspring’s ability to work and other social factors.Reference Halonen, Merikukka and Gissler34
The strength of our study lies in our use of a nationwide dataset with data on many adolescents with MDD and the psychiatric diagnoses of their parents. Furthermore, psychiatric disorders were diagnosed by board-certified psychiatrists. However, our study has some weaknesses that limit the generalizability of our results. First, the prevalence of MDD in adolescents in the present study was significantly lower than those in Western countries.Reference Costello, Egger and Angold1, Reference Jane Costello, Erkanli and Angold2 We might have underestimated the prevalence of MDD in adolescents in Taiwan because only those who seek medical services are included in the database. Second, a growing number of studies indicate that the genetic basis for the propensity for psychiatric disorders vary depending on ethnicity.Reference Chen, Wang, Poland and Lin35 Because the individuals in the study were Taiwanese, further investigation is required to determine whether the results may be generalized to other ethnic groups. Third, certain confounding factors, such as education level, lifestyles, and environmental information, were unavailable in the NHIRD. Without this information, we were unable to assess their influence.
In conclusion, parents of adolescents with MDD are more likely to have a diagnosis of MDD, BD, SZ, or alcohol use disorder and substance use disorder than parents of adolescents without MDD. The parent’s sex and response to antidepressant treatment may affect the within-disorder transmission of MDD. Our findings further support the within-disorder transmission as well as the cross-disorder transmission of psychiatric disorders. Further cross-diagnostic research should be conducted to determine the common pathogenesis among these psychiatric disorders. In addition, the mental health statuses of parents and children have a multifaceted relationship. Parents who have their own mental health disorders may have more difficulty providing care for their children than parents without mental health disorders. Identifying the mental health of parents of adolescents with MDD, especially adolescents with a poor response to antidepressant treatment, and ensuring that parents get the support they need are critical for the treatment of adolescent depression.
Acknowledgements
The authors thank Mr. I-Fan Hu, MA (Courtauld Institute of Art, University of London; National Taiwan University) for his friendship and support. Mr. Hu declares no conflicts of interest.
Data availability statement
The NHIRD was released and audited by the Department of Health and Bureau of the NHI Program for the purpose of scientific research (https://nhird.nhri.org.tw/). The NHIRD can be obtained through the formal application that is regulated by the Department of Health and Bureau of the NHI Program.
Financial support
The study was supported by grants from Taipei Veterans General Hospital (V111C-010, V111C-040, and V111C-029), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22, and CI-110-30), and Ministry of Science and Technology, Taiwan (MOST110-2314-B-075-026, MOST110-2314-B-075-024-MY3, MOST 109-2314-B-010-050-MY3, MOST111-2314-B-075-014-MY2, and MOST 111-2314-B-075-013). The funding source had no role in any process of our study.
Author contributions
M.-H.C. and S.-J.T. designed and conducted the clinical trials, and drafted the first version of manuscript; M.-H.C. performed the formal analysis; Y.-M.B., J.-W.H., K.-L.H., T.-P.S., and T.-J.C. performed literature search and reviewed the manuscript. All authors contributed substantially to the manuscript and approved the final manuscript for submission. All authors are responsible for the integrity, accuracy, and presentation of the data.
Disclosures
The authors do not have anything to disclose or declare any conflicts of interest.





