1. Introduction
Since its inception two decades ago (in 1996, [Reference Yung, McGorry, McFarlane, Jackson, Patton and Rakkar1]), the ultra high-risk (hereafter UHR) state for psychosis quickly became increasingly influential in the field of psychiatry. The UHR state is defined on the basis of three inclusion criteria which have been validated internationally [Reference Fusar-Poli, Cappucciati, Rutigliano, Schultze-Lutter, Bonoldi and Borgwardt2]: attenuated psychotic symptoms (APS), brief and limited intermittent psychotic symptoms (BLIPS) and genetic risk and deterioration syndrome (GRD) [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3]. This has led to specialist UHR care provision being recognized as an important component of clinical services for early psychosis intervention [Reference England4, 5] (e.g. NICE guidelines [Reference Nice6]; recent NHS England Access and Waiting Time [AWT] standard [Reference England4], DSM-5 diagnostic manual) [Reference Fusar-Poli, Carpenter, Woods and McGlashan7]. Accumulating evidence has confirmed that help-seeking individuals meeting UHR criteria have an enhanced risk of developing psychotic disorders – mostly schizophrenia spectrum disorders [Reference Fusar-Poli, Bechdolf, Taylor, Bonoldi, Carpenter and Yung8] – within a relatively short period of time. The transition to psychosis in UHR individuals is most likely to occur within the first 2 years after presentation to clinical services, with 25% of transitions occurring by 106 days and 50% by 240 days [Reference Kempton, Bonoldi, Valmaggia, McGuire and Fusar-Poli9, Reference Fusar-Poli and Schultze-Lutter10]. The risk of transition to psychosis accumulates to 29% (95% CI, 23–36) at 2 years [Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton and Valmaggia11]. After this phase, the speed of psychosis progression tends to plateau from the third year, reaching approximately 35% after 10 years [Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton and Valmaggia11]. This risk is significantly higher than the risk of psychosis of 0.0317 per 100 person-years (95% CI: 0.025–0.041) [Reference Kirkbride, Errazuriz, Croudace, Morgan, Jackson and Boydell12] observed in the general population. Consequently, UHR individuals have a 2-year relative risk (RR) for developing psychosis of 460, as compared to the general population (29%/0.063%). The risk is higher even when restricting the comparison to UHR subjects meeting only attenuated psychosis symptoms (APS) criteria, which have a 2-year risk of transition of 0.16 (95% CI 0.13–0.19 [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3], RR = 254). There is also converging evidence suggesting that the APS and the BLIPS subgroups of the UHR show an increased risk of psychosis as compared to subjects seeking help at clinical services but not meeting UHR criteria (i.e. UHR−) [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3]. Conversely, there is no evidence indicating that UHR individuals are at risk of developing other non-psychotic disorders. Although a substantial proportion those not developing psychosis would present with persistent symptoms or associated comorbid disorders [Reference Simon, Borgwardt, Riecher-Rossler, Velthorst, de Haan and Fusar-Poli13], the vast majority of these problems had already been present at baseline [Reference Rutigliano, Valmaggia, Landi, Frascarelli, Cappucciati and Sear14, Reference Lin, Wood, Nelson, Beavan, McGorry and Yung15]. Furthermore, understanding whether the UHR status delineates specific risk for developing non-psychotic mental disorders necessarily relies upon the comparing of incident rates of non-psychotic mental disorders in UHR versus a control group not at risk for psychosis. The only available study confirmed that, compared to a control group, UHR individuals are not at higher risk of developing non-psychotic mental disorders [Reference Webb, Addington, Perkins, Bearden, Cadenhead and Cannon16].
The risk factors accounting for such substantial risk accumulation (RR = 460) are undetermined. The most validated model to understand the aetiology of psychosis is based on genetic and environmental risk factors and their interaction [Reference van Os, Rutten and Poulton17], likely involving epigenetic mechanisms [Reference Millan18]. Because UHR individuals are at enhanced risk of psychosis but not of non-psychotic disorders, the current review focus on risk factors for psychosis that have been widely established in the available literature. According to these premises, UHR individuals are likely to show a heightened vulnerability because of accumulating genetic and/or environmental risk factors for psychosis. Indeed, several original studies have investigated the association of established risk factors for psychosis and the UHR state. However, the findings are sparse and often conflicting. For example, some studies showed that UHR individuals have been more exposed to traumatic events than controls [Reference Stowkowy, Liu, Cadenhead, Cannon, Cornblatt and McGlashan19, Reference Palmier-Claus, Berry, Darrell-Berry, Emsley, Parker and Drake20], while others found no differences [Reference Papmeyer, Wursch, Studerus, Stieglitz and Riecher-Rossler21].
To address these inconsistencies and to improve current knowledge of risk enrichment in the UHR state, this systematic review investigates the association of established genetic and environmental risk factors for psychosis and the UHR state. We first test the hypothesis that these risk factors are more likely to affect UHR individuals, compared to control groups, accounting for increased vulnerability to psychosis observed in these samples, compared to controls (RR = 460). We then investigate the specific impact of each risk factor by providing a quantitative analysis of the strength of the association between specific risk factors for psychosis and the UHR state.
2. Materials and methods
2.1. Search strategy
Two independent investigators (SDS, MT) conducted two-step literature searches. First, the Web of KnowledgeSM database was searched, incorporating both the Web of ScienceSM and Medline®. The search was extended until 1st of June 2016, including English language abstracts only. The electronic database searches used several combinations of the search terms “UHR”, “psychosis risk”, “ultra high risk”, “at risk mental state”, “subclinic* psychosis”, “earl* psychosis”, “prodrom* psychosis”, “psychosis onset”, with specific keywords relating to the type of the diverse risk factors of interest (eTable 1), and refined by the topic “research” in the Web of KnowledgeSM database. Second, a manual search of the reference lists of retrieved articles was performed. The abstracts of the articles identified through these two steps were then screened in relation to the selection criteria. The full text of the remaining articles were then assessed for eligibility, following the MOOSE checklist (eTable 2) [Reference Stroup, Berlin, Morton, Olkin, Williamson and Rennie22].
2.2. Selection criteria
Studies were eligible for inclusion when the following criteria were fulfilled:
• an original article, written in English;
• inclusion of UHR individuals, defined according to established international criteria (i.e. Comprehensive Assessment of At Risk Mental State [CAARMS]; Brief Psychiatric Rating Scale [BPRS]; Structured Interview for Psychosis-Risk Syndrome [SIPS]; Basel Screening Instrument for Psychosis [BSIP]) [Reference Yung, Phillips, Yuen and McGorry23–Reference Riecher-Rossler, Aston, Ventura, Merlo, Borgwardt and Gschwandtner25];
• inclusion of a comparison group of controls (healthy or UHR− or local general population);
• cohort studies and case-control studies (in line with previous meta-analyses of risk factors [Reference Huxley, Filion, Konety and Alonso26]) investigating risk factors in UHR individuals as part of the primary or secondary study’s aims;
• reported sufficient meta-analytical data to perform the statistical analyses. When data were not available, the corresponding author was contacted and invited to send additional information.
Exclusion criteria were:
• abstracts, pilot datasets and manuscripts in languages other than English;
• studies that did not employ internationally validated definitions for UHR;
• studies acknowledging that their datasets were completely included in other larger samples;
• Randomized Controlled Trials;
• studies that did not investigate risk factors in UHR samples as part of the primary or secondary study’s aims;
• studies that could not provide meta-analytical data;
• studies addressing biomarkers of psychosis.
The literature search was summarized according to the PRISMA guidelines [Reference Moher, Liberati, Tetzlaff, Altman and Group27].
2.3. Definition of risk factors
Because the ARMS predicts psychosis but not non-psychotic disorders [Reference Webb, Addington, Perkins, Bearden, Cadenhead and Cannon16], we focused only on the association between risk factors for psychosis and the ARMS, compared to controls. According to the WHO, a risk factor is any attribute, characteristic or exposure of an individual that increases the likelihood of developing a disease or injury [28]. Therefore, in our case, a risk factor for psychosis should increase the likelihood of developing psychosis. Accordingly, the risk factors for psychosis considered in the present manuscript were identified on the basis of previous published evidence showing a significant association with established psychotic disorders (i.e. the 95% CIs of association measures should not include 1). A qualitative summary table of association measures for each risk factor considered in the current review was produced for descriptive purposes. Two additional methodological considerations relate to the nature and exposure to risk factors in UHR individuals. First, although risk factors can be either causal or correlational, pathophysiology of psychosis is unknown and there are no causal risk factors as such. Consequently, all the included risk factors are correlational. Second, exposure to risk factors was checked against a reference index, which was the time of the first UHR diagnosis. However, temporality is impossible to define with certainty in case-control or cross-sectional designs of later and antecedents risk factors. Therefore, it is possible that some of the later and antecedents risk factors may actually represent baseline characteristics of the UHR state. We included both genetic and environmental risk factors of psychosis. The environmental risk factors were clustered as previously described [Reference Matheson, Shepherd, Laurens and Carr29–Reference Dean and Murray32]: sociodemographic and familiar risk factors, prenatal/perinatal risk factors, later risk factors and antecedents. Biomarkers (e.g. neurocognitive markers) were not included as exposure is undetermined and because these have been reviewed elsewhere [Reference Prata, Mechelli and Kapur33, Reference Fusar-Poli, Deste, Smieskova, Barlati, Yung and Howes34].
2.4. Recorded variables
Data extraction was independently performed by two investigators (SDS, MT). To estimate the primary outcome variable, we extracted the number of cases and non-cases across UHR and comparison groups (controls, C). When this data was not available (e.g in case of continuous risk factors), we extracted the mean value and standard deviation (SD) of the risk factor of interest across cases and non-cases. To estimate the impact of potential confounders, we further collected the following variables: type of UHR psychometric instrument, age, sex, year of publication and quality of study. Quality assessment was performed with an adapted version of the Newcastle-Ottawa Scale (NOS) for the evaluation of non-randomized studies. This tool has been adopted in recent meta-analyses [Reference Mertz, Kim, Johnstone, Lam, Science and Kuster35].
2.5. Systematic review
The studies were systematically discussed across each risk factor domain as described above.
2.6. Meta-analysis
The primary purpose of the meta-analysis was to quantitatively determine if there was an association between genetic and/or environmental risk factors of psychosis and the UHR state. The main effect size measure was the odds ratio (OR), which was estimated through the number of cases and non-cases across UHR and healthy controls (HC). When raw counts of cases and non-cases across UHR or HC were not available (e.g. continuous risk factors), we used the mean value and the SD of each risk factor in each group. These were transformed into OR with the method described by Hasselblad and Hedges [Reference Hasselblad and Hedges36]. Meta-analysis was performed with the “metan” [Reference Harris, Bradburn, Deeks, Harbord, Altman and Sterne37] function in Stata. Heterogeneity among study point estimates was assessed using Q statistics with the proportion of the total variability in the effect size estimates being evaluated with the I2 index [Reference Lipsey and Wilson38], which does not depend upon the number of studies included. As meta-analysis of observational studies is supposed to be characterized by significant heterogeneity, the DerSimonian and Laird random-effects models were used [Reference Harris, Bradburn, Deeks, Harbord, Altman and Sterne37]. Publication biases were assessed with the “metafunnel” function of Stata which produced funnel plots for assessing small-study reporting bias in meta-analysis [Reference Sterne, Egger and Smith39] and with the “metatrim” [Reference Duval and Tweedie40] function of Stata. In the case of more than two studies per group, we further performed the Egger test to estimate publication biases with the “metabias” function of Stata [Reference Harbord, Harris and Sterne41]. The impact of quality of studies (NOS) on meta-analytical estimates was assessed using meta-regression analyses with the “metareg” [Reference Harbord and Higgins42] function of Stata. The meta-regressions were conducted when at least 10 studies were available for each risk factor [Reference Borenstein, Hedges, Higgins and Rothstein43]. In the case of significant meta-analytical findings not affected by publication biases, sensitivity analyses were additionally conducted to investigate the robustness of the results. The influence of each single study on the overall meta-analytical estimates was investigated by omitting one study at a time, using Stata’s user-written function, “metaninf” [Reference Steichen44, Reference Peters, Sutton, Jones, Abrams and Rushton45].
3. Results
3.1. Database
The initial literature search (PRISMA Fig. 1) uncovered 44 independent articles, comprising a total of 170 independent datasets that were grouped into 54 risk factors. There were 18 studies employing the CAARMS, 25 employing the SIPS/SOPS, one employing the BPRS and one employing the BSIP. The mean age of the patients and controls was 21.4 years and 21.9 years, respectively. With the exception of four studies using UHR− samples [Reference Rosen, Miller, D’Andrea, McGlashan and Woods46–Reference Masillo, Valmaggia, Saba, Brandizzi, Lindau and Solfanelli49], all the other studies recruited healthy controls as comparison group. The type of risk factor, risk factor domain, sample size, type of UHR psychometric instrument are detailed in Table 1. Independent evidence that each of these risk factors is significantly associated with an increased risk of psychosis is qualitatively summarized in the eTable 3.
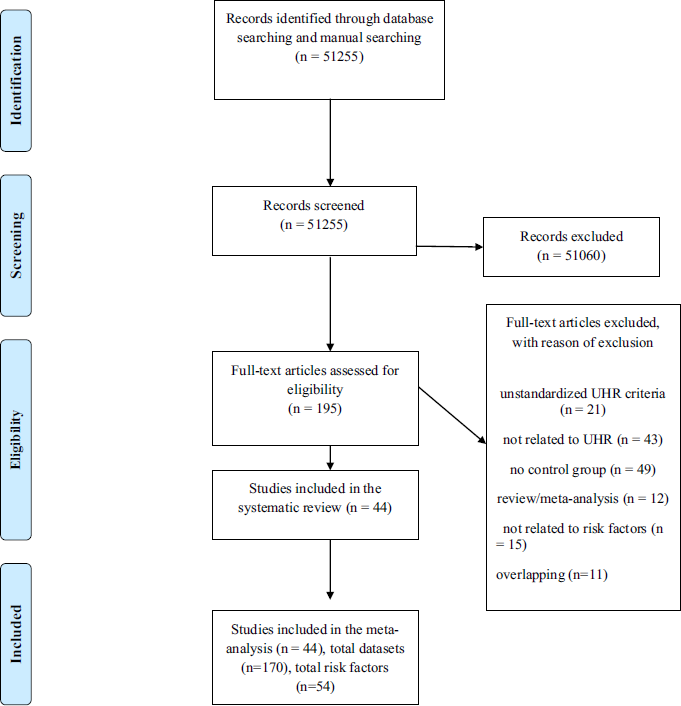
Fig. 1 PRISMA flow diagram.
Table 1 Studies included in the systematic review.
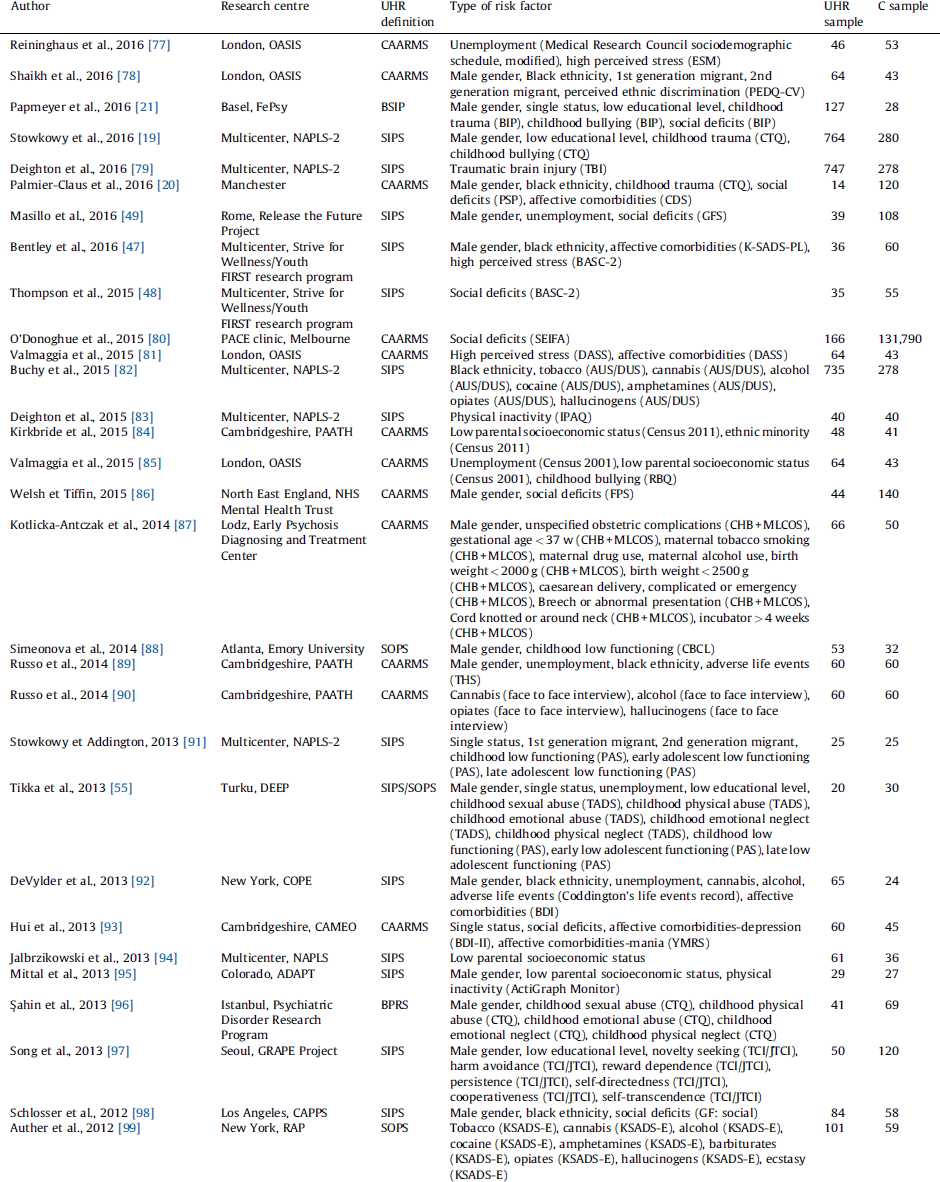

ADAPT: Adolescent Development and Preventive Treatment research program; AUS/DUS: Alcohol and Drug Use Scale; BASC-2: Behaviour Assessment System for Children: Second Edition; BDI: Beck Depression Inventory; BDI-II: Beck Depression Inventory: version II; BIP: Basel Interview for Psychosis; BPRS: Brief Psychiatric Rating Scale; BSIP: Basel Screening Instrument for Psychosis; CAARMS: Comprehensive Assessment of At-Risk Mental State; CAPPS: Staglin Music Festival Center for the Assessment and Prevention of Prodromal States; CARE: Cognitive Assessment and Risk Evaluation Program; CBCL: Child Behaviour Checklist; CDS: Calgary Depression Scale; CHB: Child’s Health Book; COPE: Center of Prevention and Evaluation; CTQ: Childhood Trauma Questionnaire; DASS: Depression, Anxiety and Stress Scale; DEEP: Detection of Early Psychosis Project; ESM: Experience Sampling Method; FePsy: Fruherkennung von Psychosen; FIGS: Family Interview for Genetic Studies; FPS: Family Perceptions Scale; GFS: Global Functioning Social Scale; GF: Global Functioning Scale: social; GRAPE: Green Programme for Recognition and Prevention of Early Psychosis; IPAQ: International Physical Activity Questionnaire; JTCI: Junior Temperament and Character Inventory; KSADS-E: Kiddie Schedule for Affective Disorders and Schizophrenia – Epidemiologic Version; K-SADS-PL: Kiddie Schedule for Affective Disorders and Schizophrenia: Present and Lifetime Version; MLCOS: Murray-Lewis Obstetric Complications Scale; NAPLS: North American Prodrome Longitudinal Study; NAPLS-2: North American Prodrome Longitudinal Study 2; OASIS: Outreach and Support in South London; PAATH: Prospective Analysis of At-Risk-Mental-States and Transitions into PsycHosis; PAS: Cannon-Spoor Premorbid Adjustment Scale; PEDQ-CV: Perceived Ethnic Discrimination Questionnaire-Community Version; PEPP-Montreal: Prevention and Early Intervention Program for Psychoses; PSP: Personal and Social Performance Scale; PSS: Perceived Stress Scale; RAP: Recognition and Prevention; RBQ: Retrospective Bullying Questionnaire; SAICA: Social Adjustment Inventory for Children and Adolescent; SAS-SR: Social Adjustment Scale – Self Rated; SCID-I/P: DSM-IV Axis I disorders – patient edition; SEIFA: Socioeconomic Indexes for Areas; SFS: Social Functioning Scale; SIPS: Structured Interview for Prodromal Syndromes; SOPS: Scale of Prodromal Symptoms; SYC: Seoul Youth Clinic; TADS: Trauma and Distress Scale; TBI: Traumatic Brain Injury Interview; TCI: Temperament and Character Inventory; THS: Trauma History Screen; TICS: Trier Inventory for the Assessment of Chronic Stress.
3.2. Systematic review
Results of the systematic review are appended supplementary in the eResults section.
3.3. Meta-analysis
3.3.1. Study selection for the meta-analysis
There were not enough studies (i.e. less than 2 studies) to perform a meta-analysis of the following risk factors: gestational age < 37 weeks, birth weight < 2000 g, birth weight < 2500 g, breech presentation, cord knotted or around neck, incubator > 4 weeks, ethnic minorities, perceived ethnic discrimination, traumatic brain injuries, barbiturates use, ecstasy use, novelty seeking, harm avoidance, reward dependence, persistence, self-directness, cooperativeness, self-transcendence and family history of non-psychotic disorders (Table 2). For comprehensiveness, we reported the OR of these single studies along with their 95% CI.
Table 2 Meta-analytical evidence for the association of environmental risk factors for psychosis and the UHR state. When a single study per group is available, the individual effect size is reported for descriptive purposes. There was no assumption that these risk factors were of causal nature or that they all are independent from each other.
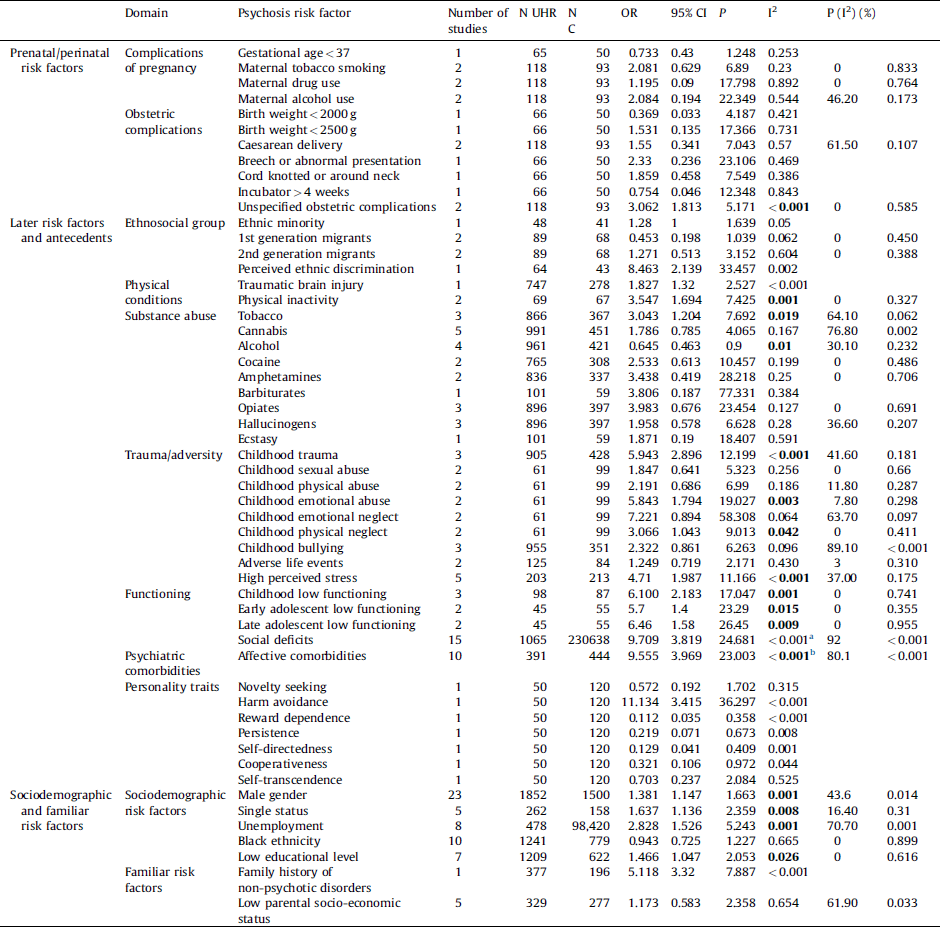
In bold, significant meta-analytical results corrected for potential publication biases.
a Adjusted OR for publication biases: 0.413 (95% CI 0.001–1.439).
b Adjusted OR for publication biases: 1.371 (95% CI 1.014–2.264).
3.3.2. Environmental risk factors for psychosis in UHR samples
3.3.2.1. Prenatal/perinatal risk factors
There was meta-analytical evidence for a significant association between unspecified obstetric complications and the UHR state (OR = 3.06). No significant association was observed for maternal tobacco smoking, alcohol, aspecific drug use and caesarean delivery.
3.3.2.2. Later risk factors and antecedents
There was meta-analytical evidence for a significant association between tobacco use and the UHR state (OR = 3.04). There was also meta-analytical evidence that the UHR individuals were less likely to use alcohol than controls (OR = 0.65) and more likely to be physically inactive (OR = 3.54). There was strong meta-analytical evidence that aspecific childhood trauma was associated with the UHR state (OR = 5.94) as well as childhood emotional abuse (OR = 5.84), childhood physical neglect (OR = 3.07) and high perceived stress (OR = 4.71). General childhood low functioning (OR = 6.1), early adolescent low functioning (OR = 5.7) and late adolescent low functioning (OR = 6.46) were strongly associated with the UHR state. Social deficits (OR = 9.71) and affective comorbidities (OR = 9.56) appeared strongly associated with the UHR state, but there were significant publication biases (see below).
There was no meta-analytical evidence that cannabis, cocaine, amphetamine, opiates, hallucinogens use, 1st or 2nd generation migrant status, childhood sexual abuse, childhood physical abuse, childhood emotional neglect, adverse life events, childhood bullying were associated with the UHR state.
3.3.2.3. Sociodemographic and familiar risk factors
There was meta-analytical evidence that male gender (OR = 1.38), single status (OR = 1.64), unemployment (OR = 1.53) and low educational level (OR = 1.47) were associated with the UHR state. Conversely, there was no meta-analytical evidence that the UHR samples were more likely to be of black ethnicity or to have a low parental socioeconomic status.
3.3.3. Publication bias
There were indications of publication biases with respect to social deficits (Egger = 0.060) and presence of affective comorbidities (Egger = 0.018). The trim and fill method adjusted meta-analytical estimate for social deficits was non-significant 0.413 (95% CI 0.001–1.439), while it survived for affective comorbidities: 1.371 (95% CI 1.014–2.264). There were no publication biases for the other risk factors associated with the UHR state.
3.3.4. Quality assessment
Quality assessment with the NOS is appended in the supplementary eTable 4. Meta-regression analyses showed no impact of NOS on OR estimates of social deficits (β = −0.069, SE = 0.407, 95% CI −0.950–0.811, P = 0.867), affective comorbidities (β = 0.291, SE = 0.392, 95% CI −0.614–1.117, P = 0.479), male gender (β = 0.0818, SE = 0.101, 95% CI −0.127–0.292, P = 0.426), black ethnicity (β = 0.145, SE = 0.144, 95% CI −0.187–0.478, P = 0.342).
3.3.5. Sensitivity analyses
Sensitivity analyses of meta-analytical estimates uncovered no outliers and confirmed the robustness of the results.
4. Discussion
This is the first systematic review to comprehensively explore why UHR individuals have an enhanced vulnerability to psychosis compared to matched controls (RR = 460), by investigating the association between established genetic and environmental risk factors for psychosis and the UHR state. We found 44 studies and a total of 170 independent datasets investigating risk factors for psychosis in UHR individuals. There were no studies reporting on genetic risk factors for psychosis in UHR subjects that fulfilled our inclusion criteria. There was substantial research into sociodemographic risk factors for psychosis and to a lesser extent into later risk factors and antecedents, while data on prenatal/perinatal risk factors were relatively scarce. The meta-analysis indicated that UHR individuals were more likely to experience obstetric complications, use tobacco, have physical inactivity, childhood trauma/emotional abuse/physical neglect, high perceived stress, childhood and adolescent low functioning, affective comorbidities, be of male gender, single status, unemployment and have low educational level as compared to controls.
First, our systematic review uncovered no studies investigating the association of genetic risk factors of psychosis and the UHR state. This is surprising, given that the genetic and deterioration syndrome (GRD) subgroup is one of the three core inclusion criteria defining an UHR state [Reference Yung, Phillips, Yuen and McGorry23]. However, the lack of research for prominent genetic risk factors in UHR samples is in line with recent evidence suggesting that in UHR subjects trait risk factors are of less clinical relevance than state risk factors. For example, a recent meta-analysis has shown that the GRD subgroup is not actually indexing an enhanced vulnerability for psychosis, with only modest transitions risks of 5% at 3 years follow-up that were comparable to the control group [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3]. The GRD construct also lacks epidemiological validation (prevalence for the APS and BLIPS subgroups but not GRD has been reported in the general population [Reference Kelleher, Murtagh, Molloy, Roddy, Clarke and Harley50]). Research into genetic risk factors of psychosis in this field may be further hindered by small statistical power associated with the low frequency of the GRD subgroup, which accounts for only 5% of the UHR cases at intake [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3]. Conversely, the BLIPS subgroup expressing extreme state risk factors (as opposed to genetic trait factors characterizing the GRD) for psychosis [Reference Fusar-Poli, Cappucciati, Bonoldi, Hui, Rutigliano and Stahl51] displays a distinctive prognosis with higher transition risks (e.g. 39% vs 19% at 24 months) compared to the APS and GRD subgroups [Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington and Nelson3]. On the basis of the available evidence, it is therefore possible to conclude that UHR samples have an increased vulnerability for psychosis mostly because of state risk factors that are associated with environmental exposures, the role of genetic (and epigenetic) risk factors awaiting elucidation.
Second, our systematic review, refined by quantitative synthesis, has identified a set of specific risk factors for psychosis that may be associated with an increased vulnerability in UHR individuals. For example, although research into prenatal/perinatal risk factors among UHR subjects is still in its infancy, we found that UHR individuals were more likely to have had general obstetric complications as compared to controls (OR = 3.06). Perinatal insults have been consistently associated with psychotic disorders (eTable 3); they may impact brain developmental processes including neurogenesis, neuronal proliferation and migration, synaptogenesis, gliogenesis and subcortical myelination, and may represent the first-wave hits for the onset and progression of psychosis (see Fig. 2 below here [Reference Millan, Andrieux, Bartzokis, Cadenhead, Dazzan and Fusar-Poli52]). Although the most compelling potential interventions to prevent psychosis currently address the UHR state, which usually identify subjects in the 2 years preceding a first episode of psychosis, perinatal preventative treatments are on the horizon. For example, perinatal dietary supplementation with phosphatidylcholine in healthy pregnant women, starting in the second trimester, ameliorates electrophysiological biomarkers of psychosis in infants at 13 weeks [Reference Ross, Hunter, McCarthy, Beuler, Hutchison and Wagner53].
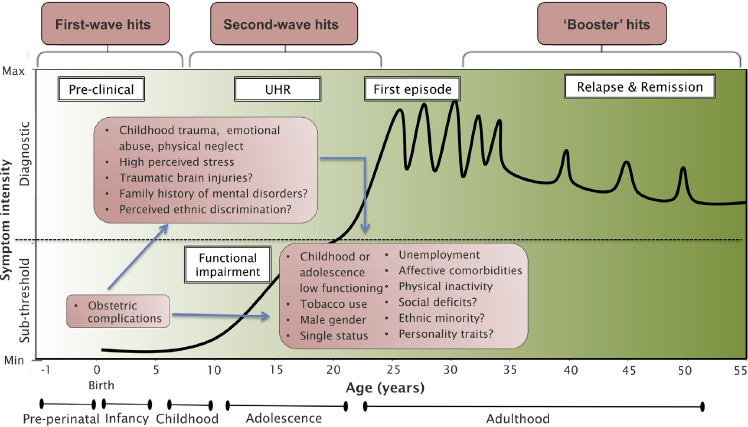
Fig. 2 Putative model of the onset and progression of psychosis in relation to risk factors and developmental processes affected by the disorder. The diagnosis of psychosis, which operationally corresponds to the first episode of psychosis, is usually made in young adults. Diagnosis generally follows an UHR phase in which attenuated psychotic symptoms, functional impairment and help-seeking behavior are apparent. Once diagnosed, psychosis follows a fluctuating course punctuated by acute exacerbation of psychotic crises superimposed upon a background of poorly controlled negative, neurocognitive and social cognitive symptoms. Many environmental risk factors have been incriminated during both the perinatal (first wave) period and during adolescence (second wave), on top of baseline genetic load. Throughout the disorder, additional adverse environmental events can trigger crises (booster hits). The pink boxes represent the putative risk factors for psychosis associated with an UHR state as identified by our systematic review and meta-analysis. There was no assumption that these risk factors were of causal nature or that they all are independent from each other. Furthermore, certain second-wave hits may actually represent outcomes of first-wave hits (blue arrows).
Adapted from [Reference Millan, Andrieux, Bartzokis, Cadenhead, Dazzan and Fusar-Poli52].
Our review further showed that second-wave hits occurring during childhood and adolescence of UHR samples were relatively more investigated, including traumatic events (childhood trauma/emotional abuse/physical neglect, high perceived stress) and low childhood and adolescent functioning (general childhood, early and late adolescent low functioning). All of these factors have been consistently associated with an increased risk of psychotic disorders (eTable 3). The magnitude of association between traumatic events and the UHR was strong, with meta-analytical ORs of up to 5.9 (Table 2). A previous meta-analysis investigating the prevalence of childhood trauma in UHR individuals confirmed a high mean prevalence rate of 86.8% (95% CI 77%–93%) [Reference Kraan, Velthorst, Smit, de Haan and van der Gaag54]. Traumatic events are thus highly prevalent and strongly associated with the UHR state as compared to controls, impacting on the severity of attenuated psychotic symptoms [Reference Tikka, Luutonen, Ilonen, Tuominen, Kotimaki and Hankala55]. A recent study showed that traumatic events additionally impact the functional level of UHR subjects [Reference Yung, Cotter, Wood, McGorry, Thompson and Nelson56]. In line with this finding, we found that UHR individuals were more likely to be functionally impaired during their early and late adolescence. The magnitude of the effect size was the strongest across all risk factors examined in our analysis (5.7 to 6.4, see Table 2). The finding of an impaired functional level in the UHR state is not novel and it is an established marker of the condition as well of its longitudinal course [Reference Fusar-Poli, Rocchetti, Sardella, Avila, Brandizzi and Caverzasi57]. The current review adds on these previous findings by showing that an early functional impairment during adolescence may represent a consistent second-wave hit for the constitution of an UHR state, in line with studies showing that functional impairment can precede psychosis by about 15 years [Reference Velthorst, Reichenberg, Kapara, Goldberg, Fromer and Fruchter58]. Traumatic events and low adolescent functioning may be clinically associated with the development of affective dysregulation and mood comorbidities. Indeed, we confirmed the previous prevalence finding of high rates of depressive and anxiety disorders in UHR subjects [Reference Fusar-Poli, Nelson, Valmaggia, Yung and McGuire59]. These findings are in line with the theory of an affective pathway to psychosis after exposure to traumatic events, with mood symptoms as a main connective component [Reference Isvoranu, van Borkulo, Boyette, Wigman, CH and Borsboom60]. From a psychopathological perspective, affective dysregulation lies at the heart of psychosis onset and may be associated with the neurobiological alterations observed in UHR individuals [Reference Mishara and Fusar-Poli61].
In contrast, we found no convincing evidence that the UHR state was associated with illicit substance use. UHR subjects were more likely to use tobacco and less likely to use alcohol than controls. The impact of tobacco use as a risk factor for psychosis is an emerging theme (as it is physical inactivity) [Reference Gurillo, Jauhar, Murray and MacCabe62] and it is less established than other traditional risk factors [Reference Kumar63] (eTable 3). Our systematic review found no evidence that UHR subjects were more likely to use cannabis than controls, in line with studies indicating that cannabis use or cannabis use disorders are not related to more severe attenuated positive psychotic symptoms at baseline [Reference Auther, Cadenhead, Carrion, Addington, Bearden and Cannon64]. There is specific evidence that the lack of association between cannabis misuse and transition to psychosis in UHR samples might be confounded by alcohol use [Reference Auther, Cadenhead, Carrion, Addington, Bearden and Cannon64]. Owing to the observed interaction between alcohol and cannabis abuse and to small to medium sample size of these meta-analyses, these findings should be interpreted cautiously and further research on these risk factors is needed.
Among sociodemographic and familial risk factors, we found that UHR individuals were more likely to be male than controls. Male gender has been consistently associated with an increased risk of psychosis [Reference Kirkbride, Errazuriz, Croudace, Morgan, Jackson and Boydell12] (eTable 3). Within the UHR population, it has been reported that males are more likely to have poorer baseline functioning [Reference Salokangas, Nieman, Heinimaa, Svirskis, Luutonen and From65] and that poorer baseline functioning predicts later transitions in males [Reference Walder, Holtzman, Addington, Cadenhead, Tsuang and Cornblatt66]. The male gender has also been associated with a prolonged duration of untreated UHR symptoms [Reference Chon, Lee, Kim, Huh, Park and Lee67], which in turn is associated with higher risk of transition [Reference Nelson, Yuen, Wood, Lin, Spiliotacopoulos and Bruxner68]. Additional evidence linking male gender and vulnerability to psychosis in UHR samples comes from meta-analyses suggesting that changes in the proportion of males accessing UHR services may contribute to the declining transition risks observed over the recent years [Reference Wilson, Patel and Bhattacharyya69]. We further found that UHR individuals were more likely to be single, unemployed and with low educational levels as compared to controls, all established risk factors for psychosis (eTable 3). Marital status is a significant protective factor for health related outcomes in several physical conditions (including severe disorders such cancer) [Reference Li, Gan, Liang, Li and Cai70], and single status has been specifically associated with an increased risk of psychosis [Reference Pelayo-Teran, Perez-Iglesias, Ramirez-Bonilla, Gonzalez-Blanch, Martinez-Garcia and Pardo-Garcia71] and with social dysfunction in schizophrenic patients [Reference Xue-jie, Jing-hua, Jun-biao, Kai-ping, Fang and Xiao-hui72]. Similarly, unemployed status and low educational level have been shown to be associated with an increased risk of psychosis, in particular in males [Reference Pelayo-Teran, Perez-Iglesias, Ramirez-Bonilla, Gonzalez-Blanch, Martinez-Garcia and Pardo-Garcia71]. There is also specific evidence for unemployment to predict psychosis onset within UHR samples [Reference Fusar-Poli, Byrne, Valmaggia, Day, Tabraham and Johns73]. Overall, these results suggest a potential pathological association between negative social factors and an increased vulnerability to psychosis. These negative social factors are also likely to reflect the impact of accumulating traumatic events in UHR individuals, which in turn may lead to mood dysregulation and affective comorbidities, as tentatively illustrated in Fig. 2.
These assumptions, however, remain speculative, as our study is not designed to investigate causal interactions between different environmental risk factors for psychosis. As we have acknowledged for the definition of risk factors, association does not necessarily imply causation and because the pathophysiology of psychosis is unknown, there are no causal risk factors for psychosis as such. Temporality is also impossible to define with certainty in case-control or cross-sectional designs but they have the advantage of examining remote exposures and have been used in previous meta-analyses of risk factors for medical conditions [Reference Taylor, Wells and Smolak74]. The evidence that each risk factor considered in the current meta-analysis is actually increasing the likelihood of psychosis is therefore based on previous evidence published in patients with a frank psychotic disorder (eTable 3). Furthermore, large-scale prospective cohort studies of epidemiological validity are not feasible in this field because UHR samples are defined not only by the UHR criteria but also by heterogeneous and idiosyncratic recruitment procedures that are not easily replicable [Reference Fusar-Poli, Schultze-Lutter, Cappucciati, Rutigliano, Bonoldi and Stahl75]. At the same time, the use of UHR instruments in other non help-seeking samples in the general population is not informative, yielding minimal transition risks that are likely to reflect a different underlying composition of risk factors for psychosis [Reference Fusar-Poli, Cappucciati, Rutigliano, Schultze-Lutter, Bonoldi and Borgwardt2]. Another limitation is that our review focused only on well-known risk factors associated with psychosis. Therefore, we cannot exclude that there may be additional different risk factors that are specifically associated with an ARMS but not with established psychosis. It is also possible that the risk factors for psychosis identified at the univariate meta-analysis may not be all independent and that some of them may actually interact. Multivariable analyses in this database were not possible because the data were too sparse. Also, because of the lack of specific data, we were unable to address if environmental risk factors for psychosis differed across UHR subgroups (i.e. BLIPS vs GRD vs APS) or across UHR instruments (i.e. CAARMS vs SIPS). Similarly, it is important to acknowledge that some of the negative findings should be interpreted cautiously, because the power of some meta-analyses (e.g. migration status and bullying) was rather limited. In addition, our primary aim was to deconstruct the enhanced vulnerability to psychosis of UHR individuals (RR = 460), compared to matched controls. Consequently, we did not specifically investigate the association between potential risk factors and the onset of psychosis within UHR samples. Reviewing predictors of psychosis within UHR samples would require a different methodological approach focused on predictive models, which has been explored in a recent independent study from our group [Reference Schmidt, Cappuciati, Radua, Rutigliano, Rocchetti and Dell’Osso76].
5. Conclusion
The increased vulnerability of UHR subjects for psychosis can be attributed to diverse environmental risk factors. Conversely, the relevance of genetic (and epigenetic) risk factors for UHR subject conversion to psychosis remains to be elucidated. UHR subjects are more likely to show obstetric complications during the prenatal/perinatal period. During childhood or adolescence, traumatic events coupled with high-perceived stress may result in affective dysregulation, low adolescent functioning and significant affective comorbidities. Sociodemographic risk factors such as male gender, single status, unemployment and low educational level may further accumulate in UHR individuals, accounting for their enhanced risk of developing psychosis. These findings provide a framework for further study of the possible relationship between risk factors and UHR.
Financial support
This study was supported in part by a 2014 NARSAD young investigator award to Paolo Fusar-Poli.
Disclosure of interest
The authors declare that they have no competing interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eurpsy.2016.09.003.







Comments
No Comments have been published for this article.