Cancer is a worldwide clinical and public health problem which, in 2013, killed 8·2 million people globally and has become the second leading cause of deaths(1). While the aetiology of cancer is not completely understood, multiple factors including diet, smoking, family history of cancer, ethnicity, genetic factors and nutritional status are postulated to play important roles.
Vitamin A is an essential micronutrient which is derived from dietary sources including animal-based foods or carotenoids from plant-based foods(Reference Krinsky and Johnson2,Reference Harrison3) . Vitamin A plays important roles in cellular growth, differentiation, proliferation and apoptosis(Reference Sporn4,Reference Gudas and Wagner5) . In animal models, vitamin A may suppress carcinogenesis(Reference Sporn4,Reference Altucci and Gronemeyer6) . In humans, plasma retinol is the major circulating form of vitamin A and the most commonly used indicator of vitamin A status(Reference Edem7). To date, a number of studies have been conducted to investigate the association between plasma retinol and the incidence of specific forms of cancer, such as gastric(Reference Abnet, Qiao and Dawsey8–Reference Persson, Sasazuki and Inoue11), oesophageal(Reference Abnet, Qiao and Dawsey8), liver(Reference Lai, Weinstein and Albanes12–Reference Yuan, Gao and Ong14), prostate(Reference Mondul, Watters and Mannisto15,Reference Key, Appleby and Travis16) and breast(Reference Maillard, Kuriki and Lefebvre17–Reference Hu, Wu and Li19) cancer, or total cancer(Reference Willett, Polk and Underwood20–Reference Knekt, Aromaa and Maatela23), but found inconsistent results. Overall, most of the previous prospective studies found that high retinol concentrations were significantly associated with decreased risks of gastric(Reference Abnet, Qiao and Dawsey8,Reference Jenab, Riboli and Ferrari10) , oesophageal(Reference Abnet, Qiao and Dawsey8) or liver cancer(Reference Lai, Weinstein and Albanes12–Reference Yuan, Gao and Ong14). Consistently, Willett et al. (Reference Willett, Polk and Underwood20) also found that although the mean values for retinol were similar for total cancer cases and the matched controls, for gastrointestinal cancer, the retinol levels were substantially lower in cases than their matched controls (P = 0·008). In contrast, a meta-analysis of eleven nested case–control studies(Reference Hu, Wu and Li19) found no significant association between blood retinol and breast cancer. Moreover, a recent meta-analysis(Reference Key, Appleby and Travis16) of fifteen nested case–control studies found that higher retinol level was associated with increased risk of prostate cancer. Taken together, available studies indicate that the beneficial effect associated with higher retinol concentrations was mainly observed for digestive system cancers. This report aimed to address some remaining gaps on this topic. First of all, few previous studies were specifically focused on investigating the shape of the association between plasma retinol levels and incident cancer risk of digestive system v. non-digestive system. Furthermore, few data were available in hypertensive populations, especially in Chinese hypertensive population, who have very different dietary pattern and life style compared with western population. Finally, most previous studies did not fully investigate the modifiers in the association between plasma retinol and cancer risk.
This report analysed the data from the China Stroke Primary Prevention Trial (CSPPT) using a nested cancer case-matched control design. CSPPT showed that the combined use of enalapril and folic acid, compared with enalapril alone, significantly reduced the risk of first stroke among hypertensive adults(Reference Huo, Li and Qin24). A greater beneficial effect was seen in those with hypercholesterolemia(Reference Qin, Li and Spence25), diabetes(Reference Xu, Kong and Xu26), higher total homocysteine (tHcy) levels with CC/CT genotypes(Reference Zhao, Wang and He27) or lower platelet count(Reference Kong, Huang and Zhao28). Moreover, enalapril–folic acid therapy, compared with enalapril alone, can significantly delay the progression of chronic kidney diseases among hypertensive patients with mild-to-moderate chronic kidney diseases(Reference Xu, Qin and Li29), and reduce the mortality risk in hypertensive patients with heavy proteinuria(Reference Li, Qin and Luo30), the development of proteinuria in diabetic patients with hypertension(Reference Li, Liang and Wang31), and the magnitude of the increase of uric acid concentrations in general hypertensive adults(Reference Qin, Li and He32). However, enalapril–folic acid therapy had no significant effect on the risk of new-onset diabetes(Reference Qin, Li and Zhang33) or cancer(Reference Qin, Shen and Zhang34), compared with enalapril alone. In addition, our previous study suggested that elevated tHcy concentrations significantly decreased the antihypertensive effect of the enalapril-based antihypertensive treatment in previously untreated hypertensive patients(Reference Qin, Li and Sun35). Furthermore, there was a significant inverse association between plasma retinol and the risk of first stroke among hypertensive adults(Reference Yu, Zhang and Song36). The present study aimed to examine the relationship of plasma retinol with the incident risk of total cancers and two subtypes: digestive and non-digestive system cancers. Furthermore, this study evaluated possible effect modifiers on the retinol–cancer associations.
Methods
Participants
The study participants for this nested case–control study were drawn from the CSPPT. The methods and major results of the trial have been reported elsewhere(Reference Huo, Li and Qin24). Briefly, the CSPPT was a multi-community, randomised, double-blind clinical trial conducted from 19 May 2008 to 24 August 2013 with 20 702 hypertensive adults in thirty-two communities in China. Eligible participants were men and women aged 45–75 years old who had hypertension, defined as seated resting systolic blood pressure (SBP) of 140 mmHg or higher or diastolic blood pressure (DBP) of 90 mmHg or higher at both the screening and recruitment visits, or were taking antihypertensive medication. The major exclusion criteria included history of physician-diagnosed stroke, myocardial infarction, heart failure, coronary revascularisation or congenital heart disease.
A total of 20 702 eligible participants were randomly assigned, in a 1:1 ratio, to one of two treatment groups: a daily oral dose of one tablet containing 10 mg enalapril and 0·8 mg folic acid (the enalapril–folic acid group); or a daily oral dose of one tablet containing 10 mg enalapril only (the enalapril group). All participants were followed up every 3 months. At each follow-up visit, vital signs, study drug adherence, concomitant medication use, adverse events and possible endpoint events were documented by trained research staff and physicians. All participants were part of the CSPPT (clinicaltrials.gov identifier: NCT00794885). The CSPPT and the present study were approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University, Hefei, China (FWA assurance number FWA00001263). Written, informed consent was obtained from all participants in the CSPPT.
Ascertainment of endpoints
Cancer incidence, a pre-specified endpoint of the CSPPT, was the main outcome in this analysis. Cancer was diagnosed based on either positive pathological findings or specific clinical manifestations. Acceptable evidence for pathological findings included original or photocopied pathological reports and original or photocopied medical records from hospitals in which pathological results were cited. When pathological data were not available, cases were independently reviewed by two oncologists. Cancer was diagnosed only when both physicians made the same clinical diagnosis based on clinical manifestations and examinations.
All cancer events were reviewed and adjudicated by an independent Endpoint Adjudication Committee, whose members were unaware of study-group assignments.
Nested case–control study
During a median treatment duration of 4·5 years, cancer occurred in 116 participants (1·12 %) in the enalapril–folic acid group as compared with 116 participants (1·12 %) in the enalapril group.
Using data from the CSPPT, we conducted a nested case–control study of 232 incident cases and 232 matched controls within this cohort. Controls were randomly chosen from the baseline CSPPT participants who did not develop cancer during the follow-up period and were matched for age (no more than 1 year), sex, treatment group and study site with the cases on a 1:1 ratio. After excluding those with missing retinol data, a total of 231 incident cases, which included 129 cases of digestive cancers (53 oesophageal, forty-three gastric, nineteen colorectal and fourteen other sites) and 102 cases of non-digestive cancers (twenty-seven breast, twenty-six lung and forty-nine other sites), and 231 matched controls were included in the present analysis (Supplementary Fig. S1).
Laboratory assays
Overnight fasting venous blood samples of all participants were obtained from each subject at baseline. Plasma retinol was measured by liquid chromatography with tandem quadrupole mass spectrometers (LC-MS/MS) in a commercial lab (Beijing DIAN Medical Laboratory) from August 2016 to July 2017. The inter-assay CV ranged from 4·66 % to 6·40 %, while the intra-assay CV ranged from 0·54 % to 15·12 %. Serum folate was measured by a commercial laboratory using a chemiluminescent immunoassay (New Industrial) from March 2014 to April 2014. Serum tHcy, fasting lipids and glucose levels were measured using automatic clinical analysers (Beckman Coulter) at the core laboratory of the National Clinical Research Center for Kidney Disease, Nanfang Hospital, Guangzhou, China.
Statistical analysis
We assumed that people with a higher plasma retinol levels represented 25 % of the general population, the estimated OR between the plasma retinol levels and cancer risk was about 0·40(Reference Jenab, Riboli and Ferrari10,Reference Yuan, Gao and Ong14) , the type I error rate was < 0·05 (α = 0·05) and the response rate was 80 %. On the basis of these assumptions, 231 cases have at least 90 % (β = 0·10) power to detect the effect size.
Baseline characteristics are presented as means and standard deviations for continuous variables and percentages for categorical variables. Differences in baseline characteristics between cases and controls were compared using conditional logistic regression for categorical variables and generalised paired t tests for continuous variables. OR of total cancers and cancer subtypes were estimated by modelling plasma retinol as a continuous variable (each 10 μg/dl increment) and as quartiles using conditional logistic regression, without and with adjustment for BMI, smoking status, alcohol drinking status, SBP, DBP, total cholesterol, TAG, HDL-cholesterol, fasting glucose, folate and tHcy at baseline, as well as time-averaged SBP and DBP over the trial period. (To convert retinol in μg/dl to μmol/l, multiply by 0·0349.) In addition, we applied two-piecewise regression models to examine the threshold effect of plasma retinol levels on total cancers and non-digestive cancers using a smoothing function. The threshold level (turning point) for each was determined using likelihood-ratio tests and bootstrap resampling methods.
In the stratified analysis, possible modifications of the association between plasma retinol as a continuous variable (per 10 μg/dl increment) and the risk of digestive cancers were assessed for variables including age (< 60 v. ≥ 60 years), sex, treatment group (enalapril v. enalapril–folic acid), current smoking (no v. yes), current alcohol drinking (no v. yes), SBP (< 160 v. ≥ 160 mmHg), total cholesterol (< 5·2 v. ≥ 5·2 mmol/l) and folate (< 8·6 (median) v. ≥ 8·6 ng/ml) levels at baseline, and time-averaged SBP (< 140 v. ≥ 140 mmHg) over the trial period.
A two-tailed P < 0·05 was considered to be statistically significant in all analyses. Analyses were performed using empower software (www.empowerstats.com, X&Y solutions, Inc.) and R software (http://www.R-project.org/).
Results
Characteristics of the participants
The analysis included 231 cancer cases with complete retinol measurements (Supplementary Fig. S1), which included 129 cases of digestive cancers (fifty-three oesophageal, forty-three gastric, nineteen colorectal and fourteen other sites) and 102 cases of non-digestive cancers (twenty-seven breast, twenty-six lung and forty-nine other sites). The distribution of major cancer subtypes is presented in Supplementary Table S1.
Table 1 shows the baseline characteristics of the cases and controls by total cancers, digestive cancers and non-digestive cancers. Mean values of plasma retinol were lower in patients with digestive cancers (65·8 (SD 25·9) μg/dl) than in control subjects (73·7 (SD 26·4) μg/dl); however, values did not differ significantly between cases and control subjects for total cancers and non-digestive cancers.
Table 1. Baseline characteristics among cancer cases and control subjects
(Mean values and standard deviations; numbers of participants and percentages)
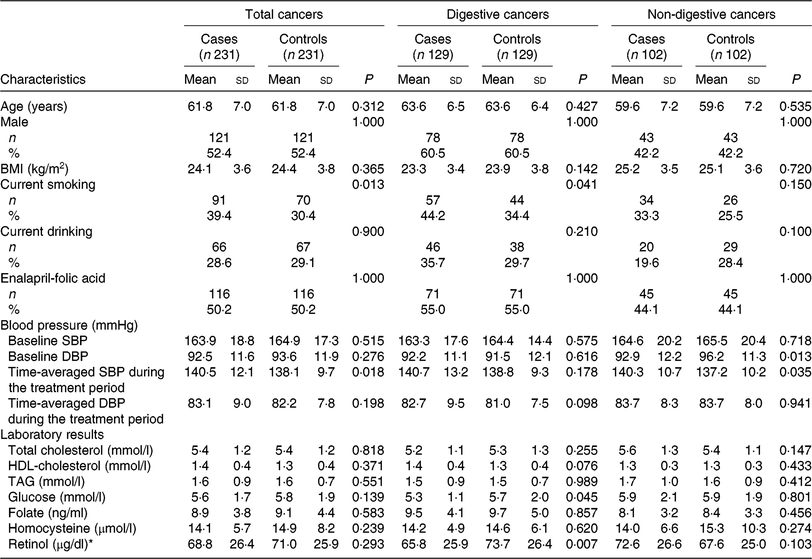
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* To convert retinol in μg/dl to μmol/l, multiply by 0·0349.
Cancer cases were more likely to be smokers and had higher time-averaged SBP over the trial period. However, there were no significant differences between cases and controls regarding other variables, including BMI, alcohol drinking, baseline SBP, total cholesterol, TAG, HDL-cholesterol, tHcy and folate, for total cancers or among the two cancer subtypes.
Association of plasma retinol with the risk of total cancers
Overall, there was a U-shaped association between plasma retinol and total cancers (Fig. 1(a)). For total cancers a turning point of 67·9 μg/dl yielded the best-fitting model in a piecewise regression. The risk of total cancers decreased significantly with each increment of plasma retinol (per 10 μg/dl increases: OR 0·74; 95 % CI 0·60, 0·92) in participants with retinol < 67·9 μg/dl, and increased non-significantly with each increment of plasma retinol (per 10 μg/dl increase: OR 1·11; 95 % CI 0·94, 1·30) in participants with retinol ≥ 67·9 μg/dl (Table 2).
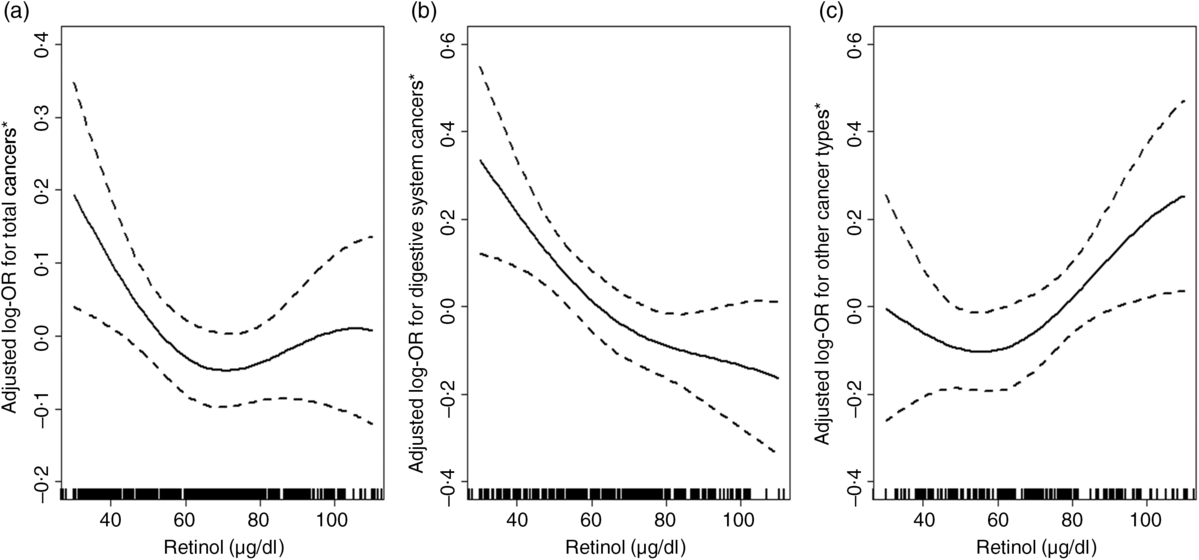
Fig. 1. Relationship of plasma retinol with the risk of total cancers (a), digestive cancers (b) and non-digestive cancers (c). * Adjusted for BMI, smoking status, alcohol drinking status, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, TAG, HDL-cholestrol, fasting glucose, folate, total homocysteine at baseline, as well as time-averaged SBP and DBP over the trial period. To convert retinol in μg/dl to μmol/l, multiply by 0·0349.
Table 2. Threshold effect analyses of retinol levels on the risk of total cancers and non-digestive cancers using two-piecewise regression models
(Odds ratios and 95 % confidence intervals)
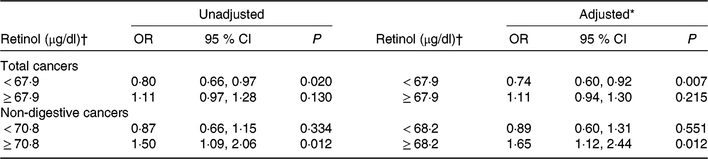
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Adjusted for BMI, smoking status, alcohol drinking status, SBP, DBP, total cholesterol, TAG, HDL-cholesterol, fasting glucose, folate, total homocysteine at baseline, as well as time-averaged SBP and DBP over the trial period.
† To convert retinol in μg/dl to μmol/l, multiply by 0·0349.
Association of plasma retinol with the risk of digestive and non-digestive cancers
The association between plasma retinol and digestive cancers is plotted in Fig. 1(b). There was a significant, inverse association between plasma retinol as a continuous variable and digestive cancers (per 10 μg/dl increases: OR 0·79; 95 % CI 0·69, 0·91). Compared with participants in quartile 1 (< 52·3 μg/dl), a significantly lower risk of digestive cancers was found in participants in quartile 2 (52·3– < 66·4 μg/dl; OR 0·39; 95 % CI 0·16, 0·97), quartile 3 (66·4– < 83·1 μg/dl; OR 0·25; 95 % CI 0·09, 0·67), quartile 4 (≥ 83·1 μg/dl; OR 0·23; 95 % CI 0·08, 0·65; P for trend for quartiles 1–4 = 0·006) and quartile 2–4 ( ≥ 52·3 μg/dl; OR 0·31; 95 % CI 0·13, 0·71) (Table 3). Similar trends were found between plasma retinol and oesophageal cancers (OR 0·58; 95 % CI 0·40, 0·84) or gastric cancers (OR 0·80; 95 % CI 0·62, 1·03) (Supplementary Table S2).
Table 3. Association between baseline retinol levels (per 10 μg/dl increases) and the risk of digestive system cancers
(Odds ratios and 95 % confidence intervals)
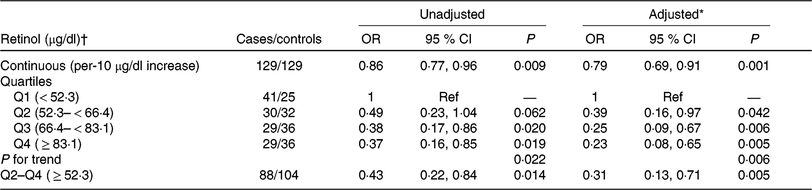
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Adjusted for BMI, smoking status, alcohol drinking status, SBP, DBP, total cholesterol, TAG, HDL-cholesterol , fasting glucose, folate, total homocysteine at baseline, as well as time-averaged SBP and DBP over the trial period.
† To convert retinol in μg/dl to μmol/l, multiply by 0·0349.
However, there was a U-shaped association between plasma retinol and non-digestive cancers (Fig. 1(c)). For non-digestive cancers a turning point of 68·2 μg/dl yielded the best fitting model in a piecewise regression. The risk of non-digestive cancers decreased non-significantly with each increment of plasma retinol (per 10 μg/dl increases: OR 0·89; 95 % CI 0·60, 1·31) in participants with retinol < 68·2 μg/dl; and increased significantly with each increment of plasma retinol (per 10 μg/dl increases: OR 1·65; 95 % CI 1·12, 2·44) in participants with retinol ≥ 68·2 μg/dl (Table 2).
To address the possibility of reverse causation, we performed lag analysis and found similar results as we obtained from the conventional analyses as shown in Supplementary Table S3 for digestive system cancers, and Supplementary Table S4 for non-digestive system cancers and total cancers.
Stratified analyses
Stratified analyses were performed to assess the association between plasma retinol levels (per 10 μg/dl increases) and the risk of digestive cancers (Fig. 2) in various subgroups. None of the variables, including sex, age, treatment group, SBP, current smoking, current alcohol drinking, total cholestrol and folate levels at baseline, as well as SBP over the trial period, significantly modified the protective effects associated with plasma retinol levels (P for all interactions > 0·05).
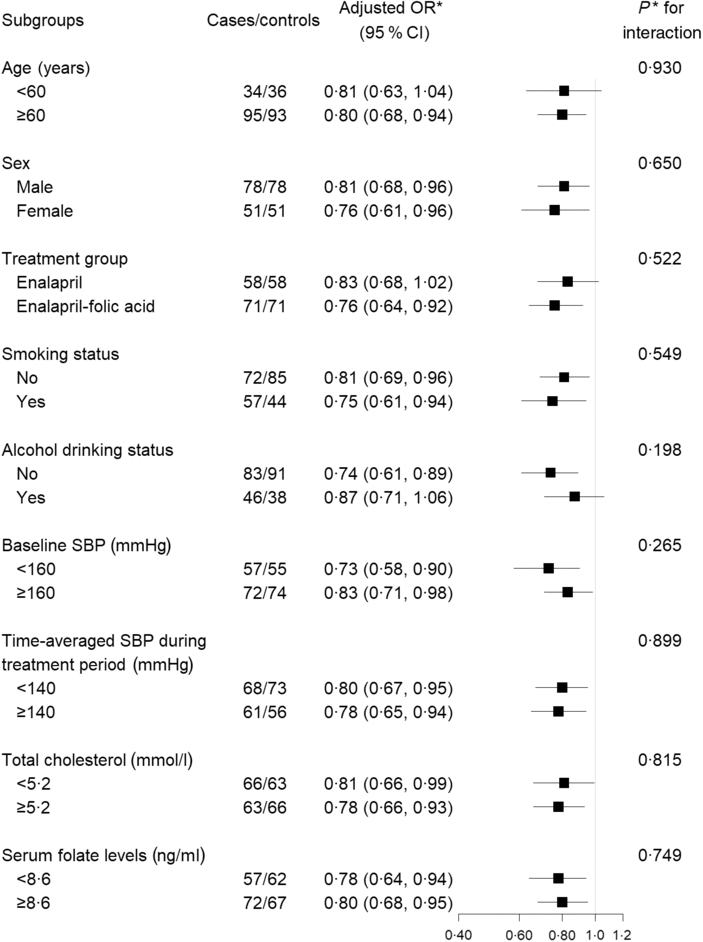
Fig. 2. Stratified analyses of the association between plasma retinol (per 10 µg/dl increment) and the risk of digestive cancers. * Adjusted for BMI, smoking status, alcohol drinking status, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, TAG, HDL-cholesterol, fasting glucose, folate, total homocysteine at baseline, as well as time-averaged SBP and DBP over the trial period. To convert retinol in μg/dl to μmol/l, multiply by 0·0349.
Discussion
This nested case–control study demonstrated a significant inverse dose–response association between plasma retinol and the risk of digestive cancers. However, a U-shaped association between retinol and the risk of non-digestive cancers was observed, with a turning point of retinol at 68·2 μg/dl. Our findings provide some new information regarding the benefit–risk ratio of vitamin A supplementation and cancer risk.
Our findings are in agreement with the results from several previous prospective studies. Two prospective studies, one in Taiwan (fifty cases) and one in Shanghai (213 cases), both observed that men with higher serum retinol had a reduced risk of liver cancer(Reference Yu, Hsieh and Pan13,Reference Yuan, Gao and Ong14) . A Finnish prospective cohort study also found that in male smokers, higher serum retinol levels were associated with a lower risk of incident liver cancer (highest quartile v. lowest quartile of serum retinol: hazard ratio 0·58, 95 % CI 0·39, 0·85, P for trend < 0·001)(Reference Lai, Weinstein and Albanes12). A case–cohort study conducted in Linxian, China, found that serum retinol concentrations were inversely associated with oesophageal cancer risk in male non-smokers (relative risk (RR) 0·79, 95 % CI 0·63, 0·99)(Reference Abnet, Qiao and Dawsey8). It is worth noting that all of these studies were conducted in males only, or only found the protective effect of retinol on the risk of cancer in males. A European prospective study found an inverse association between plasma retinol and gastric cancer risk in males and females, but did not test sex-specific differences(Reference Jenab, Riboli and Ferrari10). The Linxian study reported that the gastric cardia cancer incidence fell 10 % for each quartile increase of serum retinol (RR 0·90; 95 % CI 0·83, 0·99)(Reference Abnet, Qiao and Dawsey8). Our results also showed that there was a strong inverse dose–response association between plasma retinol and the risk of digestive cancers. This association was consistent in males and females, as well as smokers and non-smokers.
In contrast to digestive cancers, our results suggested a U-shaped relationship between plasma retinol levels and total cancers and non-digestive cancers. The β-Carotene and Retinol Efficacy Trial (CARET) found that after an average of 4 years of supplementation, the combination of β-carotene and vitamin A had an adverse effect on the incidence of lung cancer (RR 1·28; 95 % CI 1·04, 1·57), as compared with the placebo group(Reference Omenn, Goodman and Thornquist37). Post-intervention follow-up in CARET participants showed a lung cancer RR of 1·12 (95 % CI 0·97, 1·31), suggesting persistent adverse effects but no longer statistically significant(Reference Goodman, Thornquist and Balmes38). Similarly, Peleg et al. observed that the lowest risk of cancer at all sites was found in participants in quintiles three and four of the baseline retinol levels (mean: about 49 μg/dl) in an Evans County, Georgia study(Reference Peleg, Heyden and Knowles22). The RR for participants in the second, third, fourth and fifth quintiles were 0·9, 0·5, 0·6 and 1·2, respectively, when compared with those in quintile 1(Reference Peleg, Heyden and Knowles22). The study by Willett et al. (Reference Willett, Polk and Underwood20) with a mean retinol value of about 68 μg/dl (67·3 and 68·7 μg/dl, respectively, for total cancer cases and the matched controls) also indicated a non-significant U-shaped relation of serum retinol with total cancer risk, with the lowest cancer risk in participants in quintiles four of the serum retinol levels. The RR for participants in the second, third, fourth and fifth quintiles were 1·0, 0·9, 0·7 and 1·1, respectively, when compared with those in quintile 1(Reference Willett, Polk and Underwood20). Furthermore, for lung cancer, the retinol levels were non-significantly higher in cases than their matched controls(Reference Willett, Polk and Underwood20). However, the mean plasma retinol values for cancer cases and the matched controls were only 41 and 36 μg/dl, respectively, in the study by Coates et al. (Reference Coates, Weiss and Daling21). Accordingly, when serum retinol was treated as tertiles, the RR for participants in the third tertile were 0·3 for breast cancer, and 0·6 for lung cancer, when compared with those in tertile one(Reference Coates, Weiss and Daling21). In consistent with our results, the above studies also suggest that there may be a U-shaped relationship between plasma retinol levels and non-digestive cancers. Nevertheless, both some of the above studies(Reference Willett, Polk and Underwood20–Reference Peleg, Heyden and Knowles22) and the present study were severely underpowered for evaluating the association between plasma retinol and specific sites of cancer. Therefore, our findings should be regarded as hypothesis-generating. Future studies with larger sample size, additional time points of retinol assessment and examination of specific forms of cancer are warranted to confirm and expand our work.
The biological mechanism by which retinol affects cancer risk is not clear. Retinol has been thought to play a role in reducing the risk of cancers by affecting the regulation of cell growth, differentiation and apoptosis(Reference Sporn4–Reference Altucci and Gronemeyer6). Furthermore, retinol has direct anti-inflammatory and antioxidant activities(Reference Palace, Khaper and Qin39). As the gastrointestinal tract is thought to be a major site of antioxidant action(Reference Halliwell, Zhao and Whiteman40), retinol may therefore be especially protective against digestive cancers. The possible mechanisms for the increased risk of non-digestive cancers associated with higher retinol levels are still uncertain. However, it has been suggested that vitamin A may be a double-edged sword. Both a deficiency and an excess of retinol have similar adverse effects in terms of fibrosis and carcinogenesis(Reference Leo and Lieber41). Large doses of vitamin A are associated with embryonic malformations and can cause damage to the skin, bone mineral density, nervous system and internal organs(Reference Rothman, Moore and Singer42–Reference Hathcock, Hattan and Jenkins46). Consistently, our study found that the risk of non-digestive cancers increased with each increment of retinol in those participants with plasma retinol ≥ 68·2 μg/dl. However, more studies are needed to confirm our results and to further investigate the biological mechanisms underlying the associations.
Our study had several strengths. First, the nested case–control design in the context of a large prospective population (20 702 hypertensive adults) reduced possibility of selection bias. Second, we revealed different effects of plasma retinol on different types of cancer, providing new insights in this field. However, our study also had some limitations. First, plasma retinol levels of the study participants were only assessed at baseline. Therefore, we could not evaluate the effect of plasma retinol changing on the incidence of cancer. Second, we did not have the detailed dietary information about the sources of vitamin A. Previous studies suggested that Chinese adults derive vitamin A mainly from plant food sources(Reference Du, Wang and Wang47). Whether our findings could be extrapolated to populations with animal foods as the major sources of retinol should be further examined in future studies. Third, the participants of the present study were all hypertensive Chinese patients; whether our findings can be extrapolated to non-Chinese or populations without hypertension remain to be investigated. For example, for non-digestive cancers, compared with controls, cases had significantly lower DBP levels at baseline and higher time-averaged SBP levels during the treatment period. However, adjustment for baseline blood pressure and time-averaged blood pressure during the treatment period did not substantially change the findings. Fourth, although major potential confounders were controlled for, it is likely that the results may have been affected by unmeasured or unidentified confounders. Lastly, our study had a relatively small sample size. Although we have stratified the analysis by two subtypes, non-digestive and digestive cancers, the number of site-specific cancers was small; thus, this study was severely underpowered for evaluating the association of plasma retinol levels with specific sites of cancer. Therefore, our study was just hypothesis-generating, and all findings need to be further investigated and confirmed in future studies.
In conclusion, our study found a significant inverse dose–response association between plasma retinol levels and the risk of digestive system cancers. However, a U-shaped association was found for non-digestive cancers with plasma retinol turning point of 68·2 μg/dl.
Acknowledgements
This work was supported by the National Key Research and Development Program (2018ZX09301034003, 2016YFE0205400, 2018ZX09739), the Science and Technology Planning Project of Guangzhou, China (201707020010); the Science, Technology and Innovation Committee of Shenzhen (KQCX20120816105958775, JSGG20170412155639040, GJHS20170314114526143, KC2014JSCX0071A); President Foundation of Nanfang Hospital, Southern Medical University (2017C007); and Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009).
X. X., X. Q., H. Z. and X. W. contributed to research idea and study design. L. X., Y. S., T. L., H. G., B. W., G. T., Y. Z., J. L., Y. H., X. W., H. Z. and X. Q. were involved in data acquisition. L. X., Y. S., T. L. and X. Q. were involved in data analysis and interpretation. L. X. and C. L. performed statistical analysis. L. X., Y. S., T. L., H. G., B. W., G. T., C. L., W. H., Y. Y., W. L., Y. Z., J. L., Y. H., X. W., H. Z., X. Q. and X. X. carried out review and revision of the article.
X. X. reports grants from the Science and Technology Planning Project of Guangzhou, China (201707020010) and the Science, Technology and Innovation Committee of Shenzhen (KQCX20120816105958775, JSGG20170412155639040, GJHS20170314114526143, KC2014JSCX0071A). X. Q. reports grants from the President Foundation (2017C007) and Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J009). B. W. reports grants from the National Key Research and Development Program (2016YFE0205400, 2018ZX09739, 2018ZX09301034003). No other disclosures were reported.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S000711451900120X








