It has been estimated that Zn binds to 3–10 % of all human proteins. Zn finger proteins, of which the majority are transcription factors( Reference Cousins 1 , Reference Andreini, Banci and Bertini 2 ), comprise a large proportion of this total. More than seventy enzymes, including DNA and RNA polymerases, require Zn as a cofactor( Reference Berdanier and Zempleni 3 ). Zn is therefore essential for numerous cellular functions, including processes as fundamental as nucleic acid synthesis and the regulation of gene expression, along with inflammation( Reference Prasad 4 ), immunity( Reference Haas, Rodionov and Kropat 5 ), bone metabolism( Reference Yamaguchi 6 ), taste perception( Reference Wright, King and Baer 7 , Reference Keast 8 ), spermatogenesis( Reference Yamaguchi, Miura and Kikuchi 9 ), skin health( Reference Rostan, DeBuys and Madey 10 ) and defence against free-radical attacks( Reference Powell 11 ). It has emerged relatively recently that Zn acts as an intracellular signalling molecule, facilitating communication between cells, conversion of extracellular stimuli to intracellular signals and controlling a host of intracellular functions( Reference Fukada, Yamasaki and Nishida 12 ).
Zn is not stored in the body and, thus, a constant adequate supply of dietary Zn is required. In man, dietary Zn is absorbed in the proximal small bowel, the distal duodenum and the proximal jejunum( Reference Krebs 13 , Reference Lee, Prasad and Brewer 14 ) and the efficiency of absorption can be affected by the presence of dietary enhancers and inhibitors( Reference Seal and Mathers 15 ). Higher levels of protein enhance Zn absorption( Reference Sandstrom, Arvidsson and Cederblad 16 ) while phytic acid represses it through the formation of insoluble complexes with Zn in the gastrointestinal tract( Reference Couzy, Mansourian and Labate 17 – Reference Hunt and Beiseigel 19 ). Because human and other higher species do not secrete phytases, phytate-bound Zn is excreted in faeces( Reference Lonnerdal 20 ). Decreased dietary absorption efficiency and/or inadequate Zn intake contribute to reduced Zn status and the development of Zn deficiency at the population level( Reference Gibson 21 , Reference Coneyworth, Mathers and Ford 22 ).
In Saudi Arabia there have been few studies of Zn intake and Zn status. However, the limited evidence available suggests that older adults have lower serum Zn status than young adults( Reference Kumosani, Abdul-Jabar and Al-Tazi 23 , Reference Al-Numair 24 ) and that the proportion of free-living elderly males with inadequate Zn intake (below the Estimated Average Requirement) is higher than among young adults( Reference Alissa 25 ). Additionally, low Zn intakes were found in institutionalized females( Reference Sadiq 26 ) but not males( Reference Alenezy 27 ). For free-living older females in Saudi Arabia, there are no data on intakes of Zn or of Zn-absorption modifiers. One reason for this evidence gap is the lack of a suitable tool designed specifically for quantifying intakes of Zn and its absorption modifiers by adults of all ages and both genders in this population group. Most previous studies used either food records (FR) or 24 h recalls( Reference Al-Numair 24 , Reference Sadiq 26 , Reference Alenezy 27 ); an exception was Alissa( Reference Alissa 25 ). However, these methods require a high degree of cooperation from participants and analysis of data is labour intensive( Reference Lee and Nieman 28 ). An FFQ may provide an adequate assessment of usual intake and has the benefit that the demand on respondents and researchers is more modest( Reference Lee and Nieman 28 ). Thus, the aim of the present study was to assess the relative validity and repeatability of an FFQ for estimating dietary intakes of Zn and its absorption modifiers in Saudi adults. In addition, we aimed to use the FFQ to investigate the effect of age and gender on these intakes.
Materials and methods
Participants
One hundred male and female participants aged 20–30 years (younger adults) and 60–70 years (older adults) were recruited from King Abdulaziz University students, staff and their families, via personal contact, by email messages and by telephone. Participants were divided equally into four groups according to gender and age (twenty-five persons per group). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all participants gave written informed consent.
Assessment of dietary intake
A previously validated semi-quantitative FFQ( Reference Samman, Herbert and Petocz 29 ) developed in Australia was modified for use in assessing dietary Zn intake by Saudi adults. Modifications were based on a comprehensive list of foods and drinks consumed by 17 892 Saudis obtained in a previous survey( Reference Almuhaizie and Albehairy 30 ) and we used 24 h recall to collect new data on food and drink consumption. Saudi mixed dishes e.g. rice with milk and rice with tomato sauce that contain Zn in excess of 0·5 mg/100 g of the edible portion( Reference Musaiger 31 ), according to the food composition tables for Arab Gulf countries, were included in the FFQ developed for the present study. Foods with a high Zn content but prohibited or rarely consumed by Saudis, e.g. pork, canned fish, raw oysters and quiche, were excluded. Phytate-rich foods (which inhibit the absorption of Zn) and protein-rich foods (which enhance absorption) were included in the FFQ. Beef, lamb, liver and chicken are examples of high-Zn and -protein foods in the Saudi diet, while chickpeas, broad beans and falafel (deep-fried bean and vegetable patty) are high in phytic acid. The FFQ collected information on foods eaten alone as well as the same foods when consumed in mixed dishes, e.g. white rice alone and rice with milk (saleeq).
Sixty-four food items were classified into ten categories: (i) meat (eight items); (ii) seafood (four items); (iii) egg (one item); (iv) dairy products (seven items); (v) vegetables (nine items); (vi) fruits (one item); (vii) seeds and nuts (two items); (viii) cereals (nineteen items); (ix) beverages (one item); and (x) miscellaneous (twelve items; Table 1). Relatively simple and unambiguous food items such as egg were placed at the start of the questionnaire since this approach may help study participants to get used to the format of the questionnaire and so decrease reporting errors( Reference Cade, Thompson and Burley 32 ). Food items relatively rich in Zn such as meats and seafood were placed in the FFQ shortly after egg because the accuracy of participants’ responses may decline towards the end of the questionnaire due to fatigue or boredom( Reference Cade, Thompson and Burley 32 ).
Table 1 Food items (serving size) included in the FFQ

For each food item, a standard serving (medium serving) was expressed in commonly used portions such as grams, cups, tablespoons, slices or pieces( Reference Alissa 25 , Reference Samman, Herbert and Petocz 29 ). Participants were asked to recall how often, on average, they had consumed each food over the past year and how their usual serving size differed from that of the standard serving (i.e. small or large estimated as 50 and 150 % of the standard serving, respectively). Pictures of food portion sizes were used to aid participant recall( Reference Nelson, Atkinson and Meyer 33 ). The frequency of intake was assessed on an ascending eight-point scale: ‘never’, ‘less than once/month’, ‘1–3 times/month’, ‘once/week’, ‘2–4 times/week’, ‘5–6 times/week’, ‘once/day’ and ‘twice or more/day’. This scale was used because it covers the usual range in frequency of consumption of foods by Saudis; a few foods such as meat or cheese pie are consumed two to four times weekly, whereas others such as rice and breads are consumed more than once daily. Finally, participants were asked to record any food items that were usually eaten and that were not included in the list. Between 2 and 6 weeks (average 4 weeks) after the first administration of the FFQ (FFQ1), all participants completed the FFQ for the second time (FFQ2) to test its repeatability.
Zn intake from the FFQ was calculated by multiplying the amount of Zn (mg) in a medium serving by a serving size factor and a frequency factor. Serving size factors were 0·5, 1 and 1·5 for small, medium and large serving, respectively. The frequency of intake per day was calculated as follows: 0, 1/60, 2/30, 1/7, 3/7, 5·5/7, 1 and 2 for the eight-point scale, respectively. Total daily absolute Zn intake for each participant was calculated by summing the intakes from each food item. Phytic acid and protein intakes were calculated in the same way.
An open record of food intake (food record; FR) on two sequential weekdays and one weekend day was used as a reference method for validating the FFQ and was completed in the same week as, but after, the administration of FFQ1. All participants were given instruction about completing the FR. In addition, each participant was provided with a booklet of serving size pictures to aid them in recording the quantity of the foods and drinks consumed. Zn, phytic acid and protein intakes from the FR and FFQ were analysed using the Nutrition Data System for Research software version 2012, developed by the Nutrition Coordinating Center (NCC), University of Minnesota (Minneapolis, MN, USA) after entering the nutritional analysis of Saudi dishes( Reference Musaiger 31 ).
Statistical analysis
To assess the validity of the FFQ, differences in mean intake of dietary components between the FFQ and FR were examined by using Student’s paired t test. ANOVA was used to test the differences in dietary component intakes between age and gender groups. Correlations between values obtained from the FFQ and the FR were tested using Spearman’s correlations because the data distributions were skewed. Bland–Altman analysis was undertaken to investigate the agreement between the two methods for estimating intakes of dietary components. Moreover, cross-classification was used to evaluate the ability of both methods to classify individuals similarly into equal thirds of the distribution of dietary component intake. The cut-off points were determined separately for the FFQ and the FR.
To test the repeatability of the FFQ, the same statistical tests were performed between FFQ1 and FFQ2 with the exception of the cross-classification. Additionally, intraclass correlation coefficients were calculated for each of the dietary components for data from FFQ1 and from FFQ2.
All statistical analyses were performed using the statistical software package IBM SPSS Statistics version 19 with the exception of Bland–Altman analysis, which was performed using MedCalc Statistical software version 12·6·1. Statistical significance was taken as P<0·05.
Results
Study participants
One hundred volunteers participated in the present study. All completed two FFQ and a 3 d FR. The majority of the younger participants were healthy while half and a third of the older adults had diabetes and high blood pressure, respectively. The mean BMI was 28·30 kg/m2. More than 50 % of the participants were non-smokers and 40 % had a high education level (Table 2).
Table 2 Characteristics of the study participants; males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100)

† Sisha/argela consists of tobacco, molasses or honey and dried fruits.
Relative validity
We assessed the validity of the FFQ by comparing data collected using the first administration of the FFQ (FFQ1) with data from the 3 d FR. Table 3 shows the mean daily intakes of Zn, phytic acid and protein estimated by FFQ1 and the 3 d FR. Mean intakes of Zn and protein from FFQ1 were significantly higher than those from the FR (P<0·001 and P=0·013, respectively). In contrast, there were no statistically significant differences between estimates obtained by FFQ1 and the FR for the mean intake of phytic acid (P=0·792). Significant correlation coefficients were found between Zn and protein intakes obtained from FFQ1 and the FR (Table 4).
Table 3 Mean daily intakes of zinc, phytic acid and protein assessed by the first administration of the FFQ (FFQ1), the 3 d food record (FR) and the second administration of the FFQ (FFQ2) among males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100)

Mean values are significantly different from those of FFQ1: *P<0·05, ***P<0·001.
† Paired differences between FFQ1 and FR.
‡ Paired differences between FFQ1 and FFQ2.
Table 4 Spearman correlation coefficients (r) between intakes estimated from the first administration of the FFQ (FFQ1) and the 3 d food record (FR; validity) and between FFQ1 and the second administration of the FFQ (FFQ2; repeatability) among males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100)

Significance of the correlation: ***P<0·001.
Bland–Altman analysis was performed to reveal if use of FFQ1 compared with the 3 d FR resulted in any bias in measured intake values across the range of values and to obtain values for limits of agreement between the two methods. For this purpose, the differences in the estimates of Zn, protein and phytic acid intake between the two methods (FR – FFQ1) were plotted as a function of the mean Zn, phytic acid and protein intakes estimated by the two methods (FFQ1 + FR)/2). Limits of agreement and mean differences, respectively, were: for Zn, −10·6 to +5·9, mean difference −2·3 mg/d (Fig. 1(a)); for phytic acid, −715 to +696, mean difference −9·5 mg/d (Fig. 1(b)); for protein −67·5 to +52·1, mean difference −7·7 g/d (Fig. 1(c)). For zinc and phytic acid, the difference in intake estimated by FFQ1 and the FR increased with increasing mean intake (Fig. 1(a) and (b), respectively). However, the difference in protein intake as measured by the two different methods was consistent across the full range of intakes (Fig. 1(c)).
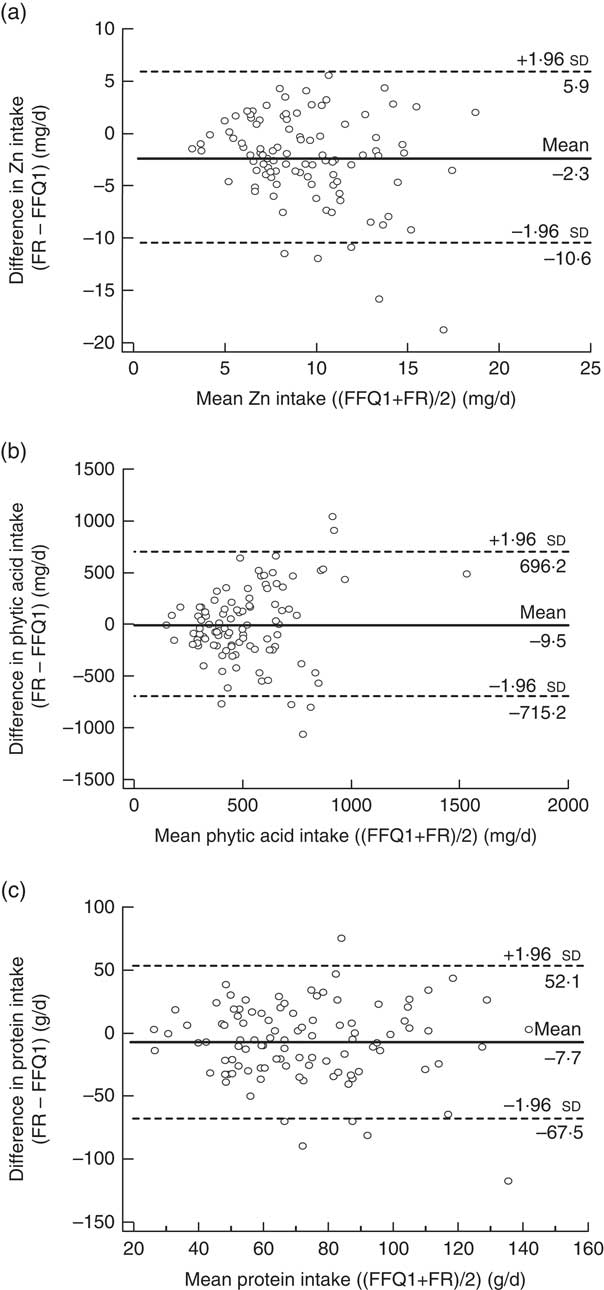
Fig. 1 Bland–Altman plots showing the relationship between the differences in daily intake of (a) zinc, (b) phytic acid and (c) protein estimated by the first administration of the FFQ (FFQ1) and the 3 d food record (FR) and the corresponding mean daily intakes estimated by the two methods; males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100). ——— represents the mean difference and ‑‑‑‑‑‑‑‑ represent the lower and upper 95 % limits of agreement
The extent of agreement between measurement methods in classifying individuals into the same or extreme tertiles of the intake distribution is shown in Table 5. The proportion of participants correctly categorized in the same tertile ranged from 55 % (phytic acid) to 62 % (Zn). Zn and protein intakes showed the lowest proportion of misclassification (2 %), whereas the highest degree of misclassification was observed for phytic acid intake (8 %).
Table 5 Cross-classification of daily intakes derived from the first administration of the FFQ (FFQ1) and the 3 d food record (FR) among males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100)

Repeatability
We assessed the repeatability of the FFQ by comparing data collected using FFQ1 and data collected using the second administration of the same FFQ, FFQ2 (Table 3). Daily Zn and protein intakes from FFQ2 were slightly but significantly lower than those from FFQ1 by ~0·5 mg and ~4 g (P=0·024 and P=0·040), respectively, whereas estimates of phytic acid intake were not significantly different (P=0·130). Intakes of Zn, phytic acid and protein from FFQ1 and FFQ2 were highly correlated (Table 4).
Figure 2 presents the limits of agreement and the mean differences in estimated dietary intakes obtained from FFQ1 and FFQ2. For all dietary components, the limits of agreement were narrower than those obtained for the comparison between FFQ1 and FR. For Zn and protein, the mean differences between the two FFQ were smaller, and for phytic acid the mean difference was larger, than those obtained between FFQ1 and FR.
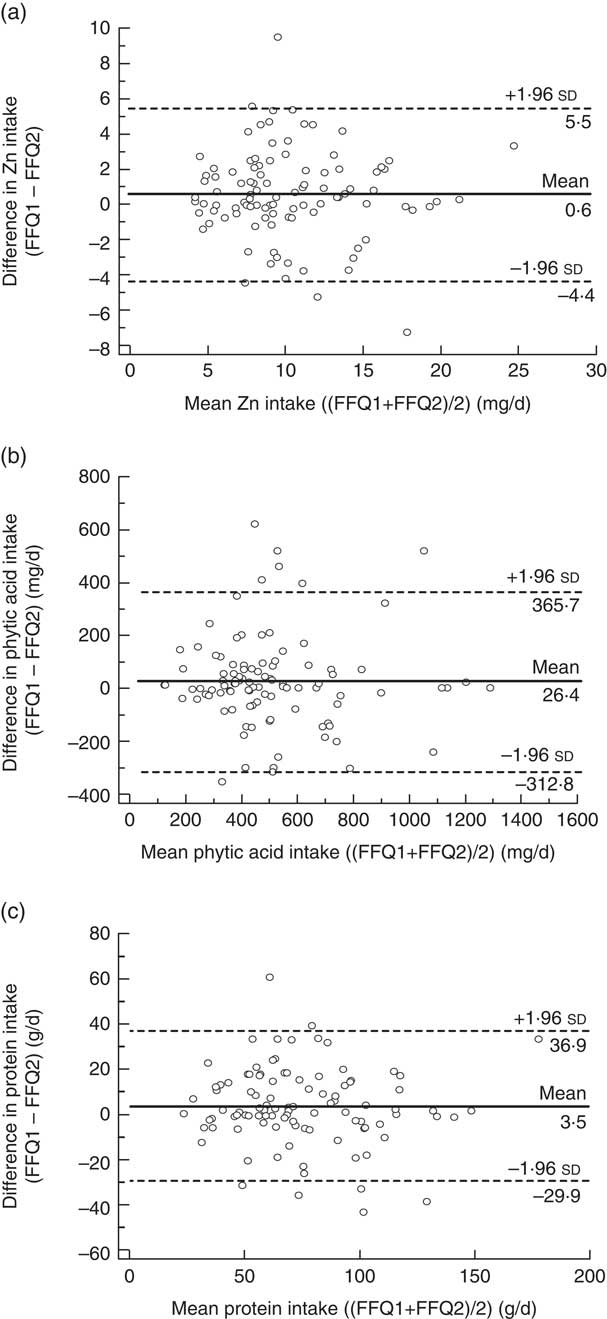
Fig. 2 Bland–Altman plots showing the relationship between the differences in daily intake of (a) zinc, (b) phytic acid and (c) protein estimated by the first administration of the FFQ (FFQ1) and the second administration of the FFQ (FFQ2) and the corresponding mean daily intakes estimated by the two methods; males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100). ——— represents the mean difference and ‑‑‑‑‑‑‑‑ represent the lower and upper 95 % limits of agreement
Effects of age and gender on intakes of Zn and Zn-absorption modifiers in Saudi adults
The mean intakes by age and gender age groups estimated using each of the two methods are displayed in Table 6. Irrespective of whether measured using FFQ1 or the 3 d FR, we observed that young adults consumed more Zn and protein than older adults. There were no detectable effects of age on phytic acid intake by either dietary assessment method. In contrast, for other dietary components, differences in intakes between groups were statistically significant only when measured by the FR. Intake of phytic acid was 100 mg higher in males than females (P=0·033). Females consumed ~2 mg less Zn daily than males (P=0·002). Similarly, females’ intake of protein was lower, by approximately ~22 g/d, than that of males (P<0·001).
Table 6 Mean daily intakes of zinc, phytic acid and protein estimated by the first administration of the FFQ (FFQ1), the 3 d food record (FR) and the second administration of the FFQ (FFQ2), according to age and gender groups, among males and females aged 20–30 years and 60–70 years, Jeddah, Saudi Arabia (n 100)

Both FFQ indicated that older adults consumed less Zn and protein than young adults (P=0·002 and P=0·006, respectively; Table 6). Other differences in intakes between groups were statistically significant only when measured by FFQ2. We observed that males consumed more Zn and protein than females (P=0·021 and P=0·045, respectively).
Discussion
The sixty-four-item FFQ developed for the present study was used successfully to obtain estimates of intakes of Zn, protein and phytic acid by all 100 young and older Saudi adult participants.
Relative validity
The FFQ yielded higher estimates of Zn intake than were obtained from the FR. A similar difference in estimates of Zn intake between a seventy-four-item FFQ and a 7 d weighed record was also reported by Samman et al.( Reference Samman, Herbert and Petocz 29 ). Although FFQ can both under- and overestimate nutrient intake, many validation studies have reported that FFQ overestimate nutrient intakes when compared with FR or 24 h recalls( Reference Segovia-Siapco, Singh and Jaceldo-Siegl 34 – Reference Schaefer, Augustin and Schaefer 36 ). Of course, whether estimates of intake obtained using an FR are reliable cannot be known with certainty, but it is evident that our FFQ yielded consistently higher estimates of Zn intake than did the FR (as confirmed by Bland–Altman analysis). A potential reason for this difference is the inclusion within the FFQ of a relatively large number of food items focusing on specific dietary components of interest, which may lead to overestimates of intakes. Other factors include measurement errors caused by over-reporting of frequency of food consumption and serving sizes( Reference Segovia-Siapco, Singh and Jaceldo-Siegl 34 ) and decreases in participants’ accuracy toward the end of the questionnaire.
The correlation coefficients observed between the FFQ and FR for estimates of Zn, phytic acid and protein intakes compare favourably with those reported in other studies using the same reference method (multiple days of FR)( Reference Barrat, Aubineau and Maillot 37 – Reference Lee, Pan and Liu 40 ). The higher correlations between methods reported by Samman et al.( Reference Samman, Herbert and Petocz 29 ) and Heath et al.( Reference Heath, Skeaff and Gibson 41 ) may result from the use of a different reference instrument, i.e. weighed diet records rather than estimated FR.
In the present study, the limits of agreement between the FFQ and the FR and the mean difference between them were not unreasonable for any dietary component measured. When compared with the results obtained by Samman et al.( Reference Samman, Herbert and Petocz 29 ), the limits of agreement between the two methods in estimating Zn intake were wider and the mean difference was slightly higher. Conversely, our estimate of the limits of agreement for protein intake was narrower than that reported by Pakseresht and Sharma( Reference Pakseresht and Sharma 42 ) and the mean difference was lower. The authors of the latter study chose the 24 h recall as a reference method, which shares with the FFQ some of the same sources of potential measurement error including recall bias, conceptualization of portion sizes and distortion of reported diet( Reference Barrat, Aubineau and Maillot 37 ). Bland–Altman plots of phytic acid intake showed that the agreement between the two methods was better for participants with lower phytic acid intake. This indicates that participants with higher intakes may be over-reporting their intake in the FFQ( Reference Pakseresht and Sharma 42 ) or that the FR method does not capture accurately the intakes of high consumers. To our knowledge, the present study is the first one to use Bland–Altman analysis to evaluate estimates of phytic acid intake. The cross-classification analysis revealed that more than 50 % of participants were correctly classified into the same third, and less than 10 % were misclassified into opposite thirds, of dietary component intakes. These results are in line with recommendations( Reference Masson, McNeill and Tomany 43 ) and consistent with other studies assessing the ability of FFQ and FR to classify nutrient intakes into tertiles( Reference Barrett and Gibson 38 , Reference Lee, Pan and Liu 40 ). The favourable tertile classifications that were obtained suggest that our FFQ is suitable for ranking individuals correctly according to their nutrient intake.
Repeatability
Several studies have reported lower estimates of nutrient intake when an FFQ was used a second time with the same participants( Reference Barrat, Aubineau and Maillot 37 , Reference Lee, Pan and Liu 40 ). In our study, intake estimates with FFQ2 were consistently lower than with FFQ1 but the differences were small for Zn and for the Zn-absorption modifiers investigated (1·0–5·5 % only; Table 3). Barrat et al.( Reference Barrat, Aubineau and Maillot 37 ) suggested that such decreases may be due, in part, to a learning effect (i.e. participants responded more accurately in the second FFQ and thus real dietary habits were reflected). Alternatively, the chore of completing the FFQ on a second or subsequent occasion may lead to less careful completion of the questionnaire. None the less, Lee et al.( Reference Lee, Pan and Liu 40 ) found that correlations between the first FFQ and the best estimate from dietary records were slightly lower than those between a third application of the FFQ and the dietary records, confirming the possibility of a learning effect( Reference Barrat, Aubineau and Maillot 37 ). This trend is confirmed by findings from the present study in which correlations between the second FFQ and the FR were slightly higher than those obtained from the first FFQ (results not shown).
Correlations coefficients between FFQ1 and FFQ2, observed in the current study, were very similar to those reported in a short-term repeatability study by Barrat et al.( Reference Barrat, Aubineau and Maillot 37 ) and slightly higher than those reported by Jia et al.( Reference Jia, Craig and Aucott 44 ), Barrett and Gibson( Reference Barrett and Gibson 38 ), Alissa( Reference Alissa 25 ), Fernandez-Ballart et al.( Reference Fernandez-Ballart, Pinol and Zazpe 45 ) and Lee et al.( Reference Lee, Pan and Liu 40 ). It should be noted that the time periods between the two FFQ in the latter studies were longer (from 3 months up to 1 year, compared with 1 month in the present study), which increased the likelihood of real changes in dietary habits and thus reduced correlation coefficients( Reference Barrat, Aubineau and Maillot 37 ).
Effects of age and gender on intakes of Zn and Zn-absorption modifiers in Saudi adults
The mean daily Zn intake assessed by the FFQ (10·6 mg) is very similar to the mean values (10·50 mg) obtained by Samman et al.( Reference Samman, Herbert and Petocz 29 ) in Australia and Alissa( Reference Alissa 25 ) (10·23 mg) in Saudi Arabia but substantially lower than that reported by Barrett and Gibson( Reference Barrett and Gibson 38 ) (15·3 mg) for Australian participants using an FFQ method. The mean phytic acid intake of Saudi adults (521·33 mg) was lower than intake of British (917 mg)( Reference Amirabdollahian and Ash 46 ) and New Zealand adults (1498 mg)( Reference Heath, Skeaff and Gibson 41 ) when using a 4–7 d weighed intake method and FFQ, respectively. The mean daily intake of protein was 76·4 g, which is comparable with the result of another Saudi study (72·53 g)( Reference Alissa 25 ) and lower than those reported in Taiwanese (87 g)( Reference Lee, Pan and Liu 40 ), Spanish (103·1 g)( Reference Fernandez-Ballart, Pinol and Zazpe 45 ) and Australian (105·9 g)( Reference Barrett and Gibson 38 ) studies when using FFQ methods.
Several studies found that mean Zn and protein intakes were lower in older adults than young adults when estimated by various dietary methods (e.g. FFQ and 24 h recall), in agreement with our findings irrespective of the method of measurement( Reference Alissa 25 , Reference Adamson, Collerton and Davies 47 ). Lower intakes of phytic acid, Zn and protein in women than in men as observed in our study have been reported previously by McDaid et al.( Reference McDaid, Stewart-Knox and Parr 48 ), Amirabdollahian and Ash( Reference Alenezy 27 ), Coulibaly et al.( Reference Coulibaly, Turgeon O’Brien and Galibois 49 ) and Adamson et al.( Reference Adamson, Collerton and Davies 47 ). These differences may due to differences in energy intake between the age and gender groups; we observed that total energy intake was lower in older than in young adults and in female than male participants (data not shown).
Strengths and limitations
The use of an FFQ reduces the burden associated with dietary intake estimation for both study participants and researchers. Such an approach may be particularly useful in some cultural settings because it lowers the cognitive complexity and difficulty for participants not involved in cooking. Since including too few items has been shown to lead to underestimation of intake using an FFQ( Reference Cade, Thompson and Burley 32 ), the present study used a relatively large number of food items (sixty-four) to assess intake of Zn and its absorption modifiers. Additionally, we used a relatively large number of frequency categories to reduce participants’ frustration if unable to find the correct response( Reference Cade, Thompson and Burley 32 ). However, in common with all conventional approaches to estimating dietary intake, FFQ have potential limitations due to subjectivity in intake recording( Reference Penn, Boeing and Boushey 50 ). Newer developments, including the use of metabolomics approaches, may provide more objective estimates of dietary exposure( Reference Fave, Beckmann and Lloyd 51 ). The participants recruited to the study were a convenience sample and were not intended to be representative of the whole Saudi population. This limitation should be borne in mind when considering any extrapolation of the data obtained beyond the parameters of this study population.
Conclusion
The FFQ developed and tested in the present study demonstrated reasonable relative validity and high repeatability in estimating and ranking intakes of Zn, phytic acid and protein of Saudi adults living in the western region of Saudi Arabia and is likely to have wide utility in epidemiological and population surveys. Its use demonstrated clear gender- and age-related differences in Zn intake and in intakes of Zn-absorption modifiers in this convenience population sample, which may have implications for Zn status and health particularly among older people.
Acknowledgements
Acknowledgements: The authors are grateful to all of the volunteers who participated in the study. Financial support: This work was supported by the Joint Supervision Program (JSP), King Abdulaziz University, Jeddah, Saudi Arabia. JSP had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: As part of a PhD research programme, H.A.M. designed the study, worked in all stages of data collection and analysis, and wrote the manuscript. F.Y. collected data. T.K., D.F. and J.C.M were responsible for supervising the project, designed the study, contributed to interpretation of the data and reviewed the draft. Ethics of human subject participation: This project was approved by the research ethical committee at King Abdulaziz University, Jeddah, Saudi Arabia.










