Pneumonia is a leading disease and cause of death in children under 5 years in developing countries( Reference Scott, Brooks and Peiris 1 ). It is reported that 14·9 % of 6·3 million deaths of children <5 years old were caused by pneumonia worldwide( Reference Liu, Oza and Hogan 2 ). With advances in medicine, economy and society, pneumonia with diarrhoea and measles was responsible for half of the reduction in mortality of children under 5 years from 2000 to 2013( Reference Liu, Oza and Hogan 2 ). Although great progress was achieved, only a few countries could reach the goal of Millennium Development Goal 4 to reduce under 5 child mortality by two-thirds between 1990 and 2015, and pneumonia is still the leading cause of child mortality( Reference Bryce, Black and Victora 3 ). Therefore, intensive effort should be made in the research of pneumonia.
Under-nutrition has been reported to be strongly associated with impaired immune response,( Reference Scrimshaw and SanGiovanni 4 ) and it has been proven to be responsible for greater severity of pneumonia, prolonged course of disease and increased mortality of pneumonia( Reference Caulfield, de Onis and Blossner 5 ). Zn, as an important micro-element, has an essential role in cellular growth and immune defence, and its deficiency is associated with significant increased susceptibility to various infection pathogens( Reference Basnet, Mathisen and Strand 6 – Reference Shankar and Prasad 8 ). In a randomised-controlled trial (RCT), Brooks et al. ( Reference Brooks, Yunus and Santosham 9 ) first found that adjunct treatment with Zn could reduce the duration of syndromes of severe pneumonia and hospital length of stay (HLOS) in 270 children. This promising finding was consistently confirmed by three other RCT( Reference Valavi, Hakimzadeh and Shamsizadeh 10 , Reference Wadhwa, Chandran and Aneja 11 ). However, the study conducted by Wadhwa et al. ( Reference Wadhwa, Chandran and Aneja 11 ) demonstrated that only very severe pneumonia rather than all severe pneumonia could benefit from Zn. In addition, a relatively large-sample RCT with 610 children found that Zn supplement could reduce the duration of severe pneumonia and the incidence of treatment failure only marginally but not statistically significantly( Reference Basnet, Shrestha and Sharma 12 ). Moreover, some other RCT suggested that adjunct treatment with Zn failed to show any beneficial effect in children with severe pneumonia( Reference Srinivasan, Ndeezi and Mboijana 13 – Reference Sempertegui, Estrella and Rodriguez 17 ).
Given the controversial effect of Zn and accumulating evidence from RCT, a meta-analysis is warranted( Reference Basnet, Shrestha and Sharma 12 ). We, therefore, performed a meta-analysis to evaluate the effect of Zn as an adjunct to antibiotics in children with severe pneumonia.
Methods
This study was conducted and reported in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement( Reference Moher, Liberati and Tetzlaff 18 ) (online Supplementary Material). PROSPERO database registration: CRD42015019798, http://www.crd.york.ac.uk/PROSPERO/
Literature search and search strategy
PubMed, Cochrane library and Embase databases were systematically searched (last search date: October 2015), with the search strategy conducted by combining MeSH terms and free terms related to severe pneumonia, children and Zn. No limitation was imposed. To avoid missing potentially relevant RCT, the reference lists of the retrieved studies and relevant reviews were manually searched. Conference abstracts that met the inclusion criteria were also eligible. Two investigators performed the study selection independently, and any disputes were solved by discussion and judged by the third investigator.
Inclusion criteria
Inclusion criteria were as follows: (1) population: children <5 years of age with severe pneumonia; (2) intervention: standard antibiotics treatment with Zn supplementation; (3) comparative intervention: standard antibiotics treatment; (4) study design: RCT.
Data extraction and outcome measure
Data extraction was performed by two investigators using a pre-designed Excel sheet. The extracted information was as follows: first author, publication year, location, recruitment period, sample size, children age, serum Zn concentration at admission, percentage of male, percentage of wheezing, standard antibiotics therapy, intervention of Zn, intervention of control and reported outcomes. Corresponding authors were contacted repeatedly while essential data were not available. The extracted information was collated by two investigators and rechecked by the third investigator.
The primary outcomes were the time to recovery from severe pneumonia, HLOS, change of antibiotics and treatment failure. Secondary outcomes included time to recovery from severe pneumonia indicators (tachypnoea, hypoxaemia, chest indrawing and fever), the death rate and adverse events (vomiting and deterioration).
Assessment for risk of bias
Risk of bias was evaluated by two authors in adherence to the guideline of Cochrane handbook for systematic review of interventions ( Reference Higgins and Green 19 ), and assessment items included selection bias, detection bias, reporting bias, blinded bias, outcome assessment bias and some other potential bias. Each item was assigned a value of ‘high’, ‘unclear” or ‘low’, and the pooled risk of bias for one study was regarded as high (high risk of bias in one or more items), unclear (low or unclear risk of bias in all items) or low (low risk of bias in all items)( Reference Higgins, Altman and Gotzsche 20 ).
Statistical analyses
Relative risks (RR) with 95 % CI were used for calculating the pooled estimate of dichotomous outcomes. Standard mean differences (SMD) with 95 % CI were used for calculating the pooled estimate of continuous outcomes. Continuous outcomes of time to recovery and HLOS were treated as time-to-event data and expressed as hazard ratios (HR) with 95 % CI in some studies, and thus HR with 95 % CI were also used to pool the results. For continuous data reported as medians with ranges, we used an elementary inequality and approximation to estimate the means and related variances( Reference Hozo, Djulbegovic and Hozo 21 ). Random-effects model was used in all meta-analyses. The Q test and I² statistic were applied to assess the heterogeneity among studies. It was perceived to have high heterogeneity (I²≥75 %), moderate heterogeneity (50 %≤I²<75 %) and low heterogeneity (25≤I²<50 %)( Reference Higgins, Thompson and Deeks 22 ). Sensitivity analysis was performed by excluding one study in each turn and pooling the remaining ones, to detect the influence of a single study on the overall estimate. In addition, intention-to-treat analysis was also conducted to avoid bias from missing participants. In intention-to-treat analysis, we assumed that all the missing participants did not experience the event for dichotomous data and imputed data of missing participants as the average difference according to control and Zn group separately for continuous data. We did not assess publication bias because fewer than ten studies were included. Review Manager version 5.1 (The Cochrane Collaboration, Software Update) was used for all statistical analyses and risk of bias. A value of P<0·05 was considered statistically significant, and P<0·1 for the significance level of the Q test.
Results
Literature search
Study selection and identification process are shown in Fig. 1. Through initial database search, 333 articles were identified after eliminating eighty-two duplications. A total of thirteen studies remained after screening titles/abstracts. Among the thirteen studies, four were excluded, of which one( Reference Shah 23 ) was an advanced abstract of an included study, two( Reference Bansal, Parmar and Basu 24 , Reference Mahalanabis, Lahiri and Paul 25 ) focused on children with severe acute lower respiratory tract infection (LRTI) but not severe pneumonia and one( Reference Coles, Bose and Moses 26 ) used the same data from the included trial. Finally, nine RCT( Reference Brooks, Yunus and Santosham 9 – Reference Sempertegui, Estrella and Rodriguez 17 ) were included.

Fig. 1 Flowchart of study screening in this meta-analysis. LRTI, lower respiratory tract infection; RCT, randomised-controlled trial.
Baseline characteristics and risk of bias
Baseline characteristics of included RCT are summarised in Table 1. The nine studies were published between 2004 and 2014, and all were conducted in developing countries. The sample sizes varied from 117 to 610, with a total of 2926. Severe pneumonia was defined mainly based on clinical syndromes and signs according to the WHO criteria. The detailed definitions of severe pneumonia and outcomes are listed in online Supplementary Table S1. The intervention of Zn or placebo was given accompanied with standard antibiotics therapy. Children received 20 mg of Zn or placebo/d in five studies( Reference Brooks, Yunus and Santosham 9 , Reference Basnet, Shrestha and Sharma 12 , Reference Bose, Coles and Gunavathi 14 , Reference Shah, Dutta and Shah 16 , Reference Sempertegui, Estrella and Rodriguez 17 ), whereas in other three studies( Reference Wadhwa, Chandran and Aneja 11 , Reference Srinivasan, Ndeezi and Mboijana 13 , Reference Valentiner-Branth, Shrestha and Chandyo 15 ) 10 mg of Zn or placebo was given for children <12 months and 20 mg for children ≥12 months. In the remaining one, Zn was given at a dose of 2 mg/kg per d, with a maximum of 20 mg/d( Reference Valavi, Hakimzadeh and Shamsizadeh 10 ). The supplementation duration was 5–14 d or until discharge from a hospital. For primary outcomes, eight studies( Reference Brooks, Yunus and Santosham 9 , Reference Valavi, Hakimzadeh and Shamsizadeh 10 , Reference Basnet, Shrestha and Sharma 12 – Reference Sempertegui, Estrella and Rodriguez 17 ) reported time to recovery from severe pneumonia, with five( Reference Brooks, Yunus and Santosham 9 , Reference Basnet, Shrestha and Sharma 12 – Reference Valentiner-Branth, Shrestha and Chandyo 15 ) reporting HR and 95 % CI; five( Reference Brooks, Yunus and Santosham 9 , Reference Valavi, Hakimzadeh and Shamsizadeh 10 , Reference Bose, Coles and Gunavathi 14 – Reference Shah, Dutta and Shah 16 ) reported HLOS, with three( Reference Brooks, Yunus and Santosham 9 , Reference Bose, Coles and Gunavathi 14 , Reference Valentiner-Branth, Shrestha and Chandyo 15 ) reporting HR and 95 % CI; six( Reference Brooks, Yunus and Santosham 9 , Reference Basnet, Shrestha and Sharma 12 , Reference Bose, Coles and Gunavathi 14 – Reference Sempertegui, Estrella and Rodriguez 17 ) reported treatment failure; and five( Reference Brooks, Yunus and Santosham 9 , Reference Basnet, Shrestha and Sharma 12 , Reference Bose, Coles and Gunavathi 14 , Reference Shah, Dutta and Shah 16 , Reference Sempertegui, Estrella and Rodriguez 17 ) reported change of antibiotics.
Table 1 Baseline characteristics of the included randomised-controlled trials (RCT) (Mean values and mean differences (MD); medians and interquartile ranges (IQR))

NR, not reported; HLOS, hospital length of stay.
The risk of bias of nine RCT is shown in Fig. 2. Risk of bias of allocation concealment was unclear in one study( Reference Brooks, Yunus and Santosham 9 ), as there was no detailed description of the allocation methods. In addition, risk of bias was rated as unclear in detection bias because whether investigators, study nurses and caretakers were masked or not was not stated( Reference Shah, Dutta and Shah 16 ).

Fig. 2 Risk of bias of the included randomised-controlled trial (RCT). (a) Reviewers’ judgments about each risk of bias item; (b) each risk of bias item presented as percentages. ![]() , Low risk of bias;
, Low risk of bias; ![]() , unclear risk of bias.
, unclear risk of bias.
Primary outcomes
Compared with children in the control group, those who received Zn supplementation needed a similar period of time to recovery from severe pneumonia (HR=1·04; 95 % CI 0·90, 1·19; I 2=39 %; P H =0·16; P=0·58; Fig. 3) and HLOS (HR=1·04; 95 % CI 0·82, 1·33; I 2=57 %; P H =0·10; P=0·74; Fig. 3). No significant difference between Zn and placebo groups was found while analysing by pooling with SMD and 95 % CI (Table 2). In addition, no significant difference between the two groups was observed in risks of treatment failure (RR=0·95; 95 % CI 0·79, 1·14; I 2=20 %; P H =0·28; P=0·58; Fig. 3) or change of antibiotics (RR=1·07; 95 % CI 0·79, 1·45; I 2=44 %; P H =0·13; P=0·67; Fig. 3).

Fig. 3 Forest plots of the effects of zinc as an adjunct to antibiotics on outcomes of (a) time to recovery from severe pneumonia, (b) HLOS, (c) treatment failure and (d) change of antibiotics. HLOS, hospital length of stay; HR, hazard ratio.
Table 2 The pooled results of continuous variable as standard mean differences (SMD) (95 % confidence intervals)
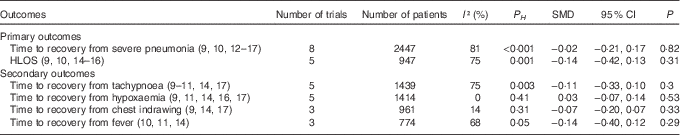
P H , P for heterogeneity.
The primary outcomes were consistently non-significant when intention-to-treat analysis was used (online Supplementary Table S2). Moreover, sensitivity analysis also confirmed consistency and robustness of the pooled results (Table 3).
Table 3 The results of sensitivity analysis for the primary outcomes (Hazard ratios (HR), standard mean difference (SMD), relative risk (RR) and 95 % confidence intervals)

ES, effect size; min, minimum; max, maximum; HR, hazard ratios; HLOS, hospital length of stay; SMD, standard mean difference; RR, relative risk.
Secondary outcomes
Time to recovery from severe pneumonia indicators
The results by pooling HR with 95 % CI manifested that adjunct treatment with Zn could not decrease time to recovery from tachypnoea (HR=1·04; 95 % CI 0·82, 1·33; I 2=65 %; P H =0·06; P=0·74; Fig. 4), hypoxaemia (HR=1·07; 95 % CI 0·89, 1·29; I 2=28 %; P H =0·25; P=0·49; Fig. 4), chest indrawing (HR=1·07; 95 % CI 0·80, 1·45; I 2=58 %; P H =0·12; P=0·64; Fig. 4) or fever (HR=0·97; 95 % CI 0·82, 1·16; I 2=0 %; P H =0·66; P=0·77; Fig. 4). In addition, these results were consistently non-significant while analysed by SMD and 95 % CI (Table 2).

Fig. 4 Forest plots of the effects of zinc as an adjunct to antibiotics on outcomes of (a) time to recovery from tachypnoea, (b) hypoxaemia, (c) chest indrawing and (d) fever. HR, hazard ratio.
Death rate and adverse events
Excluding children with HIV in one study( Reference Wadhwa, Chandran and Aneja 11 ), ten of 905 children in the Zn group and fifteen of 904 in the control group died, and the pooled RR was 0·69 (95 % CI 0·31, 1·52; I 2=0 %; P H =0·83; P=0·36; Fig. 5) for the death rate. The adverse effects were also evaluated, and the results revealed that adjunct treatment with Zn was not associated with risk of clinical deterioration (RR=0·89; 95 % CI 0·59, 1·34; I 2=0 %; P H =0·55; P=0·57; Fig. 5) or vomiting (RR=1·58; 95 % CI 0·99, 2·51; P=0·05; Fig. 5). Consistently, non-significant differences were observed when analysed by intention-to-treat analysis (online Supplementary Table S2).

Fig. 5 Forest plots of the effects of zinc as an adjunct to antibiotics on outcomes of (a) death rate, (b) vomiting and (c) deterioration. RR, relative risk.
Discussion
Main findings
Our meta-analysis of nine RCT with 2926 cases assessed the effect of Zn as an adjunct to antibiotics in children with severe pneumonia. The results suggested that adjunct treatment with Zn failed to reduce time to recovery from severe pneumonia, HLOS, treatment failure or change of antibiotics. In addition, adjunct treatment with Zn was not associated with reduced death rate, adverse events or time to recovery from severe pneumonia indicators, including tachypnoea, hypoxaemia, chest indrawing and fever. The continuous outcomes remained consistent when meta-analysed by pooling SMD and 95 % CI, and all outcomes remained stable in the intention-to-treat analysis.
Comparison with previous studies
A meta-analysis by Das et al. ( Reference Das, Singh and Shafiq 27 ) indicated that no evidence supported the efficacy of Zn as an adjunct to antibiotic in the treatment of severe acute LRTI. The relative broad scope of LRTI could certainly contribute to substantial clinical heterogeneity, and it might be associated with the significant heterogeneity (time to recovery from severe illness: I 2=82 %, HLOS: I 2=82 %). As only a few studies assessed the effect of Zn as an adjunct to antibiotic for the treatment of LRTI, we defined our inclusion criteria as severe pneumonia rather than LRTI, to decrease potential heterogeneity. In addition, the heterogeneities in outcomes of time to recovery from tachypnoea (I 2=77 %), time to recovery from fever (I 2=77 %) and change of antibiotics (I 2=52 %) were statistically significant, and thus it was not appropriate to use a fixed-effects model for meta-analysis. Furthermore, the previous meta-analysis( Reference Das, Singh and Shafiq 27 ) used SMD and 95 % CI for continuous outcomes (time to recovery), and it would be more appropriate to treat them as time-to-event data and calculate with HR and 95 % CI. For dichotomous outcomes, RR, rather than OR, should be used, as the event rates were relatively high in the included RCT.
Another meta-analysis conducted by Theodoratou et al.( Reference Theodoratou, Al-Jilaihawi and Woodward 28 ) was praised to express intervention effect as HR with 95 % CI. However, only two studies with 463 children were included. Moreover, it is unreasonable to exclude children with wheezing in the study by Brooks et al. ( Reference Brooks, Yunus and Santosham 9 ), as wheezing is a common sign of severe pneumonia in children( Reference le Roux, Myer and Nicol 29 ). Furthermore, significant heterogeneity was observed in all outcomes. A Cochrane review was also performed to assess the adjunct effect of Zn in the treatment of severe pneumonia. Nevertheless, only four RCT were included, and three of them were involved in quantitative analysis( Reference Haider, Lassi and Ahmed 30 ). The author found that adjunct treatment with Zn failed to reduce time to recovery from severe pneumonia (HR=1·12; 95 % CI 0·89, 1·41) by combining two studies with 408 children or HLOS (HR=1·04; 95 % CI 0·89, 1·22) by pooling three studies with 707 children. However, substantial heterogeneity was observed (I 2=73 %; P H =0·05 and I 2=56 %; P H =0·1, respectively) and inappropriate fixed-effect model was used. Considering the statistical heterogeneity, random-effects model should be used to give a wider CI. In both previous meta-analyses( Reference Theodoratou, Al-Jilaihawi and Woodward 28 , Reference Haider, Lassi and Ahmed 30 ), clinical outcomes such as treatment failure, changes of antibiotics, death rate and adverse events were not analysed.
Our study generally agreed with and further extended the previous meta-analyses( Reference Das, Singh and Shafiq 27 , Reference Theodoratou, Al-Jilaihawi and Woodward 28 , Reference Haider, Lassi and Ahmed 30 ) in several important aspects. We particularly appraised the effect of Zn as an adjunct to antibiotics in children with severe pneumonia and reinforced the earlier results( Reference Haider, Lassi and Ahmed 30 ) by adding six new RCT with 2207 cases. In our study, the outcomes of time to recovery were meta-analysed as time-to-event data (HR), and the results were consistent with the pooled estimates when analysed as continuous variables (SMD). The consistency undoubtedly consolidated our results. Moreover, sensitivity analysis was performed for the primary outcomes, and intention-to-treat analysis was used for all outcomes, and the null association still remained stable. Furthermore, other meaningful outcomes, such as treatment failure, change of antibiotics, death, vomiting and clinical deterioration, were also analysed.
Potential mechanism
Zn has a crucial role in immune response, including the activation of polymorphonuclear cells, macrophages, natural killer cells, T cell, antibody production, the balance of T helper lymphocyte and immune defence-specific protein synthesis( Reference Brooks, Yunus and Santosham 9 , Reference Bonaventura, Benedetti and Albarede 31 ). Plasma Zn decreases during the acute phase response because of the mobilisation and sequestration of Zn to metallothionein( Reference Brown 32 ), and hence Zn supplementation in the treatment of severe pneumonia might be associated with a robust immune response and consequently a better prognosis. However, no effect of Zn in the treatment of severe pneumonia was observed in current meta-analysis. It might be explained by the following hypotheses. First, respiratory system, different from the digestive system, is sterile below the larynx in normal circumstance, and tissue damage could be caused by Zn-induced robust host response( Reference Tsai and Grayson 33 ). Thus, the decreased inflammatory signs might actually favour the clinical recovery of severe pneumonia( Reference Mizgerd 34 ). Second, Zn might exacerbate the situation by the increased pro-inflammation while eradicating infection. Consistently, it was reported that Zn could decrease the case fatality and treatment failure without improving clinical recovery( Reference Wadhwa, Chandran and Aneja 11 , Reference Bhatnagar, Wadhwa and Aneja 35 ), indicating that the benefit of Zn might be counteracted or alleviated by the detrimental effect from increased pro-inflammation. Third, the duration of Zn supplementation was relatively short, and the impaired immune response could not be reversed by such a short-time supplementation( Reference Sempertegui, Estrella and Rodriguez 17 ). Finally, the varied results from studies and subgroup analyses( Reference Brooks, Yunus and Santosham 9 – Reference Sempertegui, Estrella and Rodriguez 17 ) could be attributed to the differences in population characteristics, intervention of Zn supplementation, outcome measures and location and period of the study, all of which could confound the effect of Zn in the treatment of severe pneumonia.
Limitations
Several limitations should be taken into account. First, several studies expressed the primary outcomes of time to recovery from severe pneumonia and HLOS as continuous variables. Although corresponding authors were contacted repeatedly, results from time-to-event analysis were not obtained in several RCT. The missing data might also induce bias, but the results obtained by pooling HR with 95 % CI were consistent with that obtained from pooling SMD with 95 % CI. Second, significant heterogeneity was found in HLOS and secondary outcomes. To test the robustness of results, sensitivity analysis and intention-to-treat analysis were used, and all the results remained consistent. Third, age, sex and nutrition status of children( Reference Brooks, Yunus and Santosham 9 , Reference Valavi, Hakimzadeh and Shamsizadeh 10 , Reference Sempertegui, Estrella and Rodriguez 17 ), the aetiology, definition, severity and recovery criteria of severe pneumonia( Reference Brooks, Yunus and Santosham 9 , Reference Basnet, Shrestha and Sharma 12 , Reference Srinivasan, Ndeezi and Mboijana 13 ), the dose, timing and duration of Zn supplementation and the location and season of study( Reference Valavi, Hakimzadeh and Shamsizadeh 10 , Reference Bose, Coles and Gunavathi 14 ) have been reported to influence the effect of Zn in treatment of severe pneumonia. However, subgroup analysis was not performed because of the limited number of included studies.
Future direction
Some valuable evidence could be obtained from our meta-analysis. Both previous researches and our results demonstrated that Zn supplementation could increase the incidence of vomiting. However, it is usually limited and slight, and thus proper dose of Zn should be recommended without much concern about the increasing incidence of vomiting. Although there was a reduction of 31 % case-fatality rate in the Zn group, no statistical significance was observed. As only twenty-five deaths were involved, low statistical power might be attributed to, and high-quality and large-scale RCT are still needed. In addition, future studies should take nutrition status of children, breast-feeding time, aetiology of pneumonia, the dose, timing and duration of Zn supplementation into consideration. Moreover, clinical outcomes of vomiting, feeding difficulty, clinical deterioration and need for intensive care should also be evaluated.
Conclusion
In conclusion, our meta-analysis suggested that adjunct treatment with Zn failed to show beneficial effect for the treatment of severe pneumonia in children <5 years old. However, this conclusion should be interpreted cautiously because of clinical heterogeneity across studies, and high-quality and large-scale RCT are still needed before making any definite conclusion. In addition, some confounding factors and valuable clinical outcomes should also be considered.
Acknowledgement
This study was supported by the fund of the National Nature Science Foundation of China (no. 81370744) and the subproject of the National Science & Technology Pillar Program during the twelfth Five-year Plan in China (no. 2012BAI04B05).
The authors’ responsibilities were as follows: H.-T. T. designed the conception, conducted the search, collected the data, assessed the quality of included studies, analysed and interpreted the data and drafted the manuscript. Q. T. conducted the search, assessed the quality of included studies, analysed and interpreted the data and revised the intellectual content. M.-Z. L. collected the data, assessed the quality of included studies and conducted the statistical analysis. Q. L. collected the data and revised the intellectual content. J.-L. Y. designed the conception, analysed and interpreted the data and revised the intellectual content. Q.-C. W designed the conception, analysed and interpreted the data and revised the intellectual content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515005449











