Pyroglutamic acid (PA) is an amino acid that is produced by the formation of a peptide bond between a γ-carboxyl group and an α-amino group of glutamic acid (Fig. 1). PA is found in various foods such as vegetables, fruit and meat, and also in the human brain, spinal cord fluid and blood. For example, canned tomato juice, a processed food known to be rich in PA, contains 100–200 mg PA/100 g(Reference Marconi, Floridi and Montanari1). It has been associated with the activity and generation of the neurotransmitter γ-aminobutyric acid or glycine, and its preventive effects against neuropathy(Reference Silva, Silva and Ruschel2), tumour formation and metastasis(Reference Kimura, Kido and Takaku3) have been reported. However, the effect of PA on type 2 diabetes mellitus (T2DM) has yet to be reported. In the present study, the anti-diabetic effect of dietary PA was examined in two T2DM rodents: non-obese Goto-Kakizaki (GK) rats and obese KK-A y mice. The effects of PA on the expression of genes involved in carbohydrate and lipid metabolism were also examined in the livers of GK rats.
Fig. 1 Chemical structure of pyroglutamic acid.
GK rats are widely used in examining mechanisms for the development of T2DM(Reference Goto, Kakizaki and Masaki4–Reference Bitar, Al-Saleh and Al-Mulla8). These rats were bred by selective breeding of Wistar rats that had high blood glucose levels determined in an oral glucose tolerance test (OGTT)(Reference Goto, Kakizaki and Masaki9). Between the ages of 3 and 4 weeks, the GK rats exhibit mild hyperglycaemia and hyperinsulinaemia. Insulin resistance(Reference Bisbis, Bailbe and Tormo10, Reference Picarel-Blanchot, Berthelier and Bailbe11), impaired insulin secretion(Reference Bisbis, Bailbe and Tormo10), abnormal glucose metabolism(Reference Ostenson, Khan and Abdel-Halim12) and an impaired development of pancreatic islet cells(Reference Movassat, Saulnier and Serradas13) are characteristics of diabetic GK rats. In contrast to other animal models of T2DM, GK rats are not obese and do not develop hyperlipidaemia(Reference Janssen, Vassiliadou and Riley14). By about the age of 24 months, these rats exhibit structural changes such as glomerular hypertrophy and thickening of the glomerular basement membrane, which also characterise the early stages of human diabetic nephropathy(Reference Phillips, Baboolal and Riley15, Reference Mauer, Steffes and Ellis16).
KK-A y mice are the result of cross-breeding between glucose-intolerant black KK female mice and yellow male obese A y mice, and are known to be an excellent model of obese T2DM(Reference Chang, Wyse, Copeland, Serrano-Rios and Lefebure17). These mice also provide a useful model system to study the pathogenesis and prevention of obesity and diabetes, and to investigate therapeutic approaches(Reference Herberg and Coleman18). In addition, these mice show polyphagia, polyposia, polyuria, hyperglycaemia, hyperinsulinaemia, insulin resistance and pancreas islet enlargement. The incidence of diabetes occurrence in 6-week-old male mice reaches 100 %(Reference Iwatsuka, Shino and Suzuoki19).
Experimental methods
Animal care
The present study was performed in accordance with the Animal Experimentation Guidelines of the Laboratory Animal Care Committee of Yamagata University. Rats and mice were individually housed in stainless-steel cages with screen bottoms. All animals were maintained on alternating 12 h light–12 h dark cycles at a humidity of 40–60 % and a temperature of 22 ± 1°C. Diet composition is shown in Table 1. The daily intake of PA used in the present study was in accordance with Kimura et al. (Reference Kimura, Kido and Takaku3); the amount corresponds to a daily intake of about 30 mg PA/kg body weight. This quantity of PA can be obtained from food(Reference Marconi, Floridi and Montanari1). Diet and water were given ad libitum. PA was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
Table 1 Composition of the experimental diets (%, w/w)
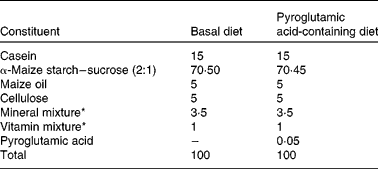
* Mineral mix AIN-93G-MX and vitamin mix AIN-93-VX, which contained 25 g choline bitartrate per 100 g, were obtained from Oriental Yeast Co. Ltd (Tokyo, Japan).
Experiments with Wistar rats
The effect of dietary PA supplementation on non-diabetic animals was investigated by utilising Wistar rats. Male Wistar rats, aged 8 weeks, were purchased from Japan SLC, Inc. (Shizuoka, Japan). After an acclimatisation period of 3 d, rats were placed on a basal diet (Wistar control group (Wistar-CON); n 5) or a diet containing 0·05 % PA (Wistar-PA group; n 6) for 46 d. After feeding for 1 and 6 weeks, rats were fasted for 10 h and blood glucose levels were determined. At the end of the study, blood was collected from rats that had been fasted for 10 h. The serum was prepared by centrifuging the collected blood at 1000 g for 15 min and analysed for lipid analysis and enzyme activities. The liver was removed and stored at − 80°C until needed for analysis. The left kidney and left epididymal adipose tissues were also excised and weighed.
Serum alanine aminotransferase and aspartate aminotransferase activities were measured according to methods in the literature(Reference Matsumoto, Ogata, Inoue, Matsumoto and Uemura20).
Serum total cholesterol (T-chol), HDL-cholesterol (HDL-C) and TAG were measured enzymically with commercial kits (cholesterol E test, HDL-cholesterol test, triglyceride E test; Wako Pure Chemical Industries Ltd, Osaka, Japan). Serum LDL-cholesterol (LDL-C) level(Reference Friedewald, Levy and Fredrickson21) was calculated as:
Atherogenic index(Reference Choi, Yokozawa and Oura22) was calculated as:
Liver lipid was extracted by the method of Folch et al. (Reference Folch, Lees and Sloane Stanley23), and measured by using the same kits as above.
Experiments with Goto-Kakizaki rats
The effect of dietary PA on non-obese T2DM rats was investigated by using the GK rat (GK/Slc). Male GK rats, aged 8 weeks, were purchased from CLEA Japan Inc. (Tokyo, Japan). After an acclimatisation period of 4 d, rats were placed on a basal diet (GK-CON group; n 6) or the diet containing 0·05 % PA (GK-PA group; n 5) for 43 d. As the non-diabetetic control group, five Wistar rats receiving the basal diet were used (Wistar group; n 5).
The fasting blood glucose levels were measured as described above. After 26–27 d of dietary PA supplementation, an OGTT was performed. Before application of an oral glucose load (2 g/kg body weight), blood glucose and serum insulin levels were determined in 10 h fasted rats. Blood glucose and serum insulin levels were measured 15, 30, 60 and 120 min after glucose application. The area under the curve was calculated for glucose during the OGTT. At the end of the study, blood was collected from the fasting rats. Serum was prepared as described above, and analysed for lipid, insulin and TNF-α levels. The liver was removed and stored at − 80°C until needed for assays, including enzyme activities and DNA microarray analysis. The left kidney and left epididymal adipose tissues were also excised and weighed. Serum insulin and TNF-α were determined by using ELISA kits (Levis rat insulin kit, Shibayagi Co., Ltd (Gunma, Japan); TNFα Biotrak ELISA kit, Amersham Biosciences Co. (Uppsala, Sweden)). Serum and liver lipid levels were measured as described above.
Liver enzyme activities including fatty acid synthase (FAS), glucose-6-phosphate dehydrogenase (G6PD), 6-phosphogluconate dehydrogenase (6PGD) and carnitine palmitoyl transferase (CPT) were measured as described below. To measure FAS activity, a liver homogenate was prepared by the Burton method(Reference Burton, Haavis and Potter24). FAS activity was determined in terms of malonyl-CoA- and acetyl-CoA-dependent oxidation of NADPH according to the methods of Kumer et al. (Reference Kumar, Dorsey and Muesing25) and Carey & Dils(Reference Carey and Dils26). The G6PD and 6PGD activities were measured with a Bioxytech G6PD/6PGD-340 kit (Oxis International Inc., Beverley Hills, CA, USA). To measure CPT activities, liver samples were homogenised in a 3 mm-2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)-HCl buffer (pH 7·2) containing 0·25 m-sucrose and 1 mm-EDTA. The reaction mixture was composed of a 58 mm-Tris-HCl buffer (pH 8·0) containing 0·25 mm-5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB), 0·04 mm-palmitoyl CoA, 1·25 mm-EDTA and 1·25 mm-l-carnitine, and the homogenate. CPT activity was determined from the rate of change in absorbance at 412 nm(Reference Guglielmo, Norbert and Hochachka27).
The influence of PA on gene expression related to carbohydrate and lipid metabolism was examined by DNA microarray analysis. RNA was isolated using an RNeasy Mini kit (Qiagen N. V., Hilden, Germany), and the total RNA was converted to cDNA using a WT Sense Target Labeling kit (NuGen Technologies Inc., San Carlos, CA, USA). The cDNA were hybridised onto GeneChip Array (Affymetrix, Inc., Santa Clara, CA, USA), Rat Gene 1·0 ST Array (27 342 genes) for 16 h at 45°C, and scan and image data of arrays were acquired by a GeneChip 3000 Scanner (Affymetrix, Inc.). With a data analysis system utilising GeneChip Operating Software (Affymetrix, Inc.), the array image data of the acquired samples were confirmed. The selected genes were annotated based on NetAffx (linked at http://www.affymetrix.com). The classification of category was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Furthermore, the gene expression of G6Pase, angiopoietin-like 4 and β-actin was also determined quantitatively by RT-PCR. Primers were purchased from Sigma Aldrich Co. (St Louis, MO, USA). These were: glucose-6-phoshatase, sense, 5′-CTACCTTGCGGCTCACTTTC-3′, antisense, 5′-ATCCAAGTGCGAAACCAAAC-3′; angiopoietin-like 4, sense, 5′-CAGAACAG CAAGATCCAGCA-3′, antisense, 5′-CCTCTTTCCCCTCGAAGTCT-3′; β-actin, sense, 5′-ACCCACACTGTGCCCATCTA-3′, antisense, 5′-CGTCACACTTCATGATG-3′.
Reactions were carried out in the LightCycler® 480 System (Roche Applied Science Inc., Basel, Switzerland) using the SYBR Green Qiagen One-Step RT-PCR kit (Qiagen). The program profile was 95°C for 30 s and forty-five cycles of denaturation for 5 s at 95°C, and annealing for 15 s at 55°C and extension for 15 s at 72°C.
Experiments with KK-Ay mice
The effect of dietary PA on obese T2DM animals was investigated in KK-A y mice (KK-A y/TaJcl). Male KK-A y mice, aged 6 weeks, were purchased from CLEA Japan Inc. After an acclimatisation period of 2 d, mice were placed on a basal diet (KK-A y-CON group; n 8) or the diet containing 0·05 % PA (KK-A y-PA group (KKAy-PA); n 7) for 28 d. As non-diabetic control mice, seven C57BL/6J mice receiving the basal diet were used (C57BL group).
Blood glucose levels in fasting (food-deprived for 12 h) rats were measured on the first day of both week 1 and week 4. After 22–23 d of treatment, the OGTT was carried out as described above. Blood glucose levels were measured at 30, 60 and 120 min, and insulin levels were measured at 30 and 60 min after glucose loading. At the end of the feeding period, all mice were bled, and the serum was prepared and stored at − 80°C until analysed for lipid, TNF-α and insulin levels. The liver was removed and stored at − 80°C until required for measuring enzyme activities. The left kidney and left epididymal adipose tissues were also excised and weighed. TNF-α levels were determined by an immunoassay kit (Invitrogen Co., Carlsbad, CA, USA). Serum and liver lipids were measured as described above.
FAS, G6PD + 6PGD and CPT activities of the livers were measured as described above. Glucokinase (GLK) activity was measured spectrophotometrically(Reference Davidson and Arion28). The liver sample was homogenised in an ice-cold buffer (pH 7·5) containing 50 mm-HEPES, 250 mm-sucrose, 100 mm-KCl, 1 mm-EDTA, 5 mm-MgCl2 and 2·5 mm-dithioerythritol, and the homogenate was centrifuged at 105 000 g for 60 min. Hexokinase activity of the supernatant fraction was measured in a buffer (pH 7·4) containing 50 mm-HEPES, 7·5 mm-MgCl2, 100 mm-KCl, 5 mm-ATP, 2·5 mm-dithioerythritol, bovine serum albumin (10 mg/ml), 0·5 mm-NAD+, glucose-6-phosphate dehydrogenase (4 U/ml) (L. mesenteroides) and 0·5 mm-glucose. The total phosphorylating activity was measured by using 100 mm-glucose instead of 0·5 mm-glucose. The reaction was initiated by adding ATP, and the rate of increase in absorbance due to NADH formation was recorded at 340 nm. GLK activity was calculated as the difference between the total phosphorylating activity and hexokinase activity. G6Pase activity was measured by using the microsomal fraction obtained as a precipitate by centrifugation at 105 000 g. The microsomal fraction was re-suspended in a homogenisation buffer and diluted with an ice-cold buffer (pH 6·5) containing 100 mm-HEPES and 0·1 mm-EDTA. The reaction was initiated by adding 10 mm-glucose-6-phosphate at 37°C, and stopped after 20 min by adding 2·2 volumes of a solution containing 3·7 mm-ammonium molybdate and 240 mm-SDS in 270 mm-H2SO4. After adding one-ninth of the volume of 1·2 m-ascorbic acid, the reaction mixture was further incubated for 1 h at 37°C, and the absorbance was measured at 820 nm(Reference Lange, Arion and Burchell29).
Statistical analysis
Data from animals in each group were expressed as mean values with their standard errors. The homogeneity of variance between treatments was verified by Bartlett's test. Data were statistically analysed by one-way ANOVA. A post hoc analysis of significance was made by using Fisher's protected least significant difference test, where differences were considered significant at P < 0·05.
Data of DNA microarray were statistically analysed by Welch's t test and P < 0·01 was considered as statistically significant. False discovery rates were calculated according to Benjamini & Hochberg(Reference Benjamini and Hochberg30), and the threshold of false discovery rate was set at 5 %.
Results
Evaluation in Wistar rats
There were no differences among the two groups in body-weight gains, weight of organs, fasting blood glucose, and serum and liver lipid levels (Table 2). As there were no differences in serum alanine aminotransferase and aspartate aminotransferase among the two groups, no inflammatory reactions in the liver were attributable to PA.
Table 2 Effects of pyroglutamic acid (PA) in Wistar and Goto-Kakizaki (GK) rats
(Mean values with their standard errors)

CON, control; ND, not determined; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
* Mean value was significantly different from that of the GK-CON group (P < 0·05).
† Wistar rats were fed a control diet or supplemented with 0·05 % PA for 46 d.
‡ GK rats were fed a control diet or supplemented with 0·05 % PA for 43 d.
Diabetes alleviation in Goto-Kakizaki rats
The effects of PA in OGTT, which was determined after feeding the experimental diet for 26–27 d, are shown in Fig. 2(A). The blood glucose levels at 15 and 30 min after glucose loading in the PA group did not differ from those of the GK-CON group. However, glucose levels at 60 min in the GK-PA group were significantly lower than in the GK-CON group. The areas under the curve of blood glucose levels are shown in Fig. 2(B). Values for the GK-PA group were significantly lower than for the GK-CON group. Dietary PA supplementation for 26–27 d improved the oral glucose tolerance in GK rats. The serum insulin levels during OGTT are shown in Fig. 2(C). The insulin level in the GK-CON group increased gradually from 0 to 120 min. However, levels in the GK-PA group were significantly lower than those in the GK-CON group.

Fig. 2 (A) Blood glucose during an oral glucose tolerance test (OGTT) in Wistar rats (–○–; n 5), Goto-Kakizaki (GK) rats fed a control (CON) diet (GK-CON; –●–; n 6) and GK rats fed a diet supplemented with 0·05 % pyroglutamic acid (PA) (GK-PA; –□–; n 5) for 26–27 d. (B) Calculated areas under the curve (AUC) of blood glucose in Wistar and GK rats. (C) Insulin levels during an OGTT in Wistar, GK-CON and GK-PA rats. Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
There was no significant difference in the total food intake among the groups; however, the body-weight gains of the GK-PA group tended to be lower than those of the GK-CON group (P < 0·1) (Table 2). Although the liver and kidney weights did not differ, epididymal adipose tissue weights were lower in the GK-PA group than in the GK-CON group. The difference of these adipose weights might have been due to the decrease in body-weight gains of the GK-PA group. Fasting blood glucose levels did not differ between the GK-CON and GK-PA groups. The serum insulin level after the feeding period of 43 d was significantly lower in the GK-PA group than in the GK-CON group (Table 2).
The serum and liver lipid levels are shown in Table 2. The serum T-chol levels in the GK-PA group were significantly lower than in the GK-CON group, but HDL-C level did not differ between the two groups. The serum LDL-C and TAG levels in the GK-PA group were also significantly lower than in the GK-CON group. The liver T-chol and TAG levels in the GK-PA group were lower than in the GK-CON group, and resembled the results observed in the serum.
The ratio of signal intensity of the 27 342 genes in the GK-PA group to those in the GK-CON group was calculated. Genes with a 2-fold ratio increase were defined arbitrarily as up-regulated genes in the GK-PA group, whereas those with a ratio decreased by one-half or more were defined as down-regulated genes. When compared with the GK-CON group, nine genes were up-regulated and twenty-nine were down-regulated in the GK-PA group (see Supplemental table; available at http://www.journals.cambridge.org/bjn). Furthermore, we listed the genes in the two functional categories of carbohydrate metabolism and lipid metabolism (Table 3). Genes involved in glycolysis (GLK) and gluconeogenesis (G6Pase) and the gene for the transcription factor forkhead box O1 were down-regulated; the gene expression of pyruvate dehydrogenase kinase 4 was up-regulated. With respect to the expression of genes concerned with lipid metabolism, down-regulation was found in the following: angiopoietin-like 4, and the fatty acid metabolism enzymes cytochrome P450, family 4, subfamily a. Gene expressions of PPARα (gene symbol: Ppara) and sterol regulatory element-binding protein (gene symbol: Srebf1), which are carbohydrate and lipid metabolism-related, in the GK-PA group did not change when compared with those of the GK-CON group (fold change: Ppara, 0·71; Srebf1, 0·82). Furthermore, gene expressions of insulin receptor substrate 2 (gene symbol: Irs-2) and phosphatidylinositol 3-kinase (gene symbol: Pik3r1), insulin signalling pathway-related genes, also did not differ between the GK-PA and GK-CON groups (fold change: Irs-2, 0·88; Pik3r1, 0·84). Down-regulation of G6Pase and angiopoietin-like 4 in the GK-PA group was also observed when determined by RT-PCR (G6Pase (GK-CON v. GK-PA), 1·00 (sem 0·08) v. 0·44 (sem 0·02); angiopoietin-like 4, 1·00 (sem 0·12) v. 0·52 (sem 0·07)).
Table 3 DNA microarray analysis of Goto-Kakizaki (GK) rat liver after treatment with 0·05 % pyroglutamic acid for 43 d*
(Mean values for three independent experiments)

* Selected genes involved in lipid, glucose metabolism were compared with those from GK rats given the control diet.
† Value relative to control group.
The activities of the liver enzymes FAS, G6PD +6PGD and CPT are shown in Table 4. The GK-PA group showed significantly lower levels of FAS activity than the GK-CON group. On the other hand, CPT activity was significantly higher in the GK-PA group when compared with the Wistar and GK-CON groups. From the DNA microarray analysis, the expression of the CPT gene in the GK-PA group increased in comparison with the GK-CON group (fold change 1·72), and the FAS gene in the GK-PA group decreased (0·62).
Table 4 Liver fatty acid synthase (FAS), glucose-6-phosphate dehydrogenase (G6PD) +6-phosphogluconate dehydrogenase (6PGD) and carnitine palmitoyl transferase (CPT) activities in Goto-Kakizaki (GK) rats and KK-A y mice
(Mean values with their standard errors)
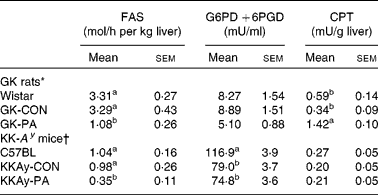
CON, control; PA, pyroglutamic acid.
a,b Within a study, mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Wistar and GK-CON groups were administered the basal diet. The GK-PA group was administered a 0·05 % PA-containing diet for 43 d.
† C57BL and KKAy-CON groups were administered the basal diet. The KKAy-PA group was administered a 0·05 % PA-containing diet for 28 d.
Diabetes alleviation in KK-Ay mice
In OGTT, the blood glucose levels in the KKAy-CON group were higher from 0 to 120 min after glucose loading, but the rises were suppressed at 60 and 120 min in the KKAy-PA group (Fig. 3(A)). The blood glucose level before the glucose load of OGTT in the KKAy-PA group was significantly lower than in the KKAy-CON group. It is considered that the significant, difference relates to the fasting blood glucose level at week 4. Serum insulin level in the KKAy-PA group, which was measured just before glucose loading, was significantly lower than that in the KKAy-CON group (Fig. 3(C)). However, the insulin level at 30 and 60 min after glucose loading did not differ between the two groups. Homeostatic model assessment of insulin resistance level (HOMA-IR), an insulin resistance index, was significantly lower in the KKAy-PA group than in the KKAy-CON group (Fig. 3(D)). These results suggest that the KKAy-CON group became insulin resistant, and that insulin resistance was reduced in the KKAy-PA group.

Fig. 3 (A) Blood glucose during an oral glucose tolerance test (OGTT) in 9-week-old C57BL mice (–○–; n 7), KK-A y mice fed a control (CON) diet (KKAy-CON; –●–; n 8) and KK-A y mice fed a diet supplemented with 0·05 % pyroglutamic acid (PA) (KKAy-PA; –□–; n 7) for 22–23 d. (B) Calculated areas under the curve (AUC) of blood glucose in C57BL and KK-A y mice. (C) Insulin levels during an OGTT in C57BL, KKAy-CON and KKAy-PA mice. (D) homeostatic model assessment of insulin resistance (HOMA-IR) values in C57BL and KK-A y mice. HOMA-IR was calculated by the following formula: ((fasting blood glucose (mg/dl) × fasting serum insulin (μU/ml))/405). Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
The body-weight gains in the KKAy-PA group were significantly higher than those in the KKAy-CON group in spite of the total food intake not differing between the KK-A y (KKAy-CON and KKAy-PA) groups (Table 5). Although the liver and kidney weights did not differ, epididymal adipose tissue weights were lower in the KKAy-CON group than in the KKAy-PA group. The fasting blood glucose level after 4 weeks was significantly lower in the KKAy-PA than in the KKAy-CON group (Table 5). The rises observed in serum insulin and TNF-α levels in the KKAy-CON group were suppressed in the KKAy-PA group (Table 5).
Table 5 Effects of pyrogutamic acid (PA) in KK-A y mice
(Mean values with their standard errors)

CON, control; PA, pyroglutamic acid.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* C57BL and KK-A y mice were given the control diet or supplemented with 0·05 % PA for 28d.
Serum and liver lipid levels are shown in Table 5. The serum T-chol and LDL-C levels in the KKAy-PA group were significantly lower than in the KKAy-CON group. The liver T-chol level in the K-PA group was significantly lower than in the KKAy-CON group. However, unlike the studies in the GK rats, serum and liver TAG levels in the KKAy-PA group were similar to those in the KKAy-CON group.
The activities of the liver FAS, G6PD +6PGD and CPT enzymes are shown in Table 4. FAS activity in the KKAy-PA group was significantly lower than in the KKAy-CON group. On the other hand, G6PD +6PGD and CPT activities were not different between the KKAy-CON and KKAy-PA groups. The liver GLK and G6Pase activities in the KKAy-PA group were significantly lower than those in the KKAy-CON group (Fig. 4). When the GLK:G6Pase ratios, which show the the magnitude of glycolysis and gluconeogenesis, were compared the ratio in the KKAy-PA group was significantly higher than that in the KKAy-CON group.

Fig. 4 Effects of pyroglutamic acid (PA) on liver glucokinase (GLK) (A) and glucose-6-phosphatase (G6Pase) (B) activities, and relative activity of GLK to G6Pase (GLK:G6Pase) (C) in C57BL mice (n 7), KK-A y mice fed a control diet (KKAy-CON; n 8) and KK-A y mice fed a diet containing 0·05 % PA (KKAy-PA; n 7) for 28 d. Values are means, with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05).
Discussion
In the Wistar rats without diabetes, dietary PA did not affect the diabetic markers and lipid levels, including the liver damage marker. These results may suggest that the intake of PA does not have an influence on non-diabetic rats.
Although food intake did not differ among GK rats, body-weight gains in the GK-CON group tended to be higher than in the GK-PA group on day 43. After feeding of the experimental diets for 21 d, the body-weight gains in the GK-PA group were significantly lower than in the GK-CON group, and the body-weight gains in the GK-PA group showed a similar value to that of the Wistar group (data not shown). As the epididymal adipose tissue weight in the GK-PA group was less than that in the GK-CON group, lower body-weight gains may be associated with a decrease in this tissue weight.
The experiments with the GK rats suggested that the feeding of PA resulted in an improvement in glucose tolerance. Reduction of insulin resistance in T2DM by PA may be associated with suppression of G6Pase gene expression, because the gene expression of G6Pase in the GK-PA group was down-regulated as compared with the GK-CON group when measured by DNA microarray analysis. This is considered to be closely related to a lower gene expression of transcriptional glycogenic activators (forkhead box O1). The mRNA levels of GLK in the GK-PA group were lower than in the GK-CON group, supporting the result of a lower blood glucose level in the GK-PA group than in the GK-CON group. In addition, it is considered that a lower expression of angiopoietin-like 4 (Angptl4) in the GK-PA group lowers the TAG level significantly. Li(Reference Li31) and Köster et al. (Reference Köster, Chao and Mosior32) have reported that Angptl4 has been shown to inhibit lipoprotein lipase (LPL) activity and increase plasma TAG. In the GK-PA group, it may be considered that the constitution of the lipoprotein (for example, HDL-C, chylomicron or VLDL-cholesterol levels) was being changed through the activation of LPL.
In the experiment with the KK-A y mice, G6Pase activity in the KKAy-PA group was lower than in the KKAy-CON group, resulting in a higher GLK:G6Pase (activity) ratio in the KKAy-PA group. The higher GLK:G6Pase ratio in the GK-PA group may indicate the regulation of glycolysis by PA. It has been reported that T2DM causes an increase in glucose levels that is associated with hyperglycaemia, and that inhibition of hepatic gluconeogenesis suppresses an increase in fasting plasma glucose levels and also decreases endogenous glucose production in T2DM patients(Reference DeFronzo33–Reference Hundal, Krssak and Dufour35). The higher GLK:G6Pase ratio in the KKAy-PA group further supports the idea that PA may be available to suppress the increase in plasma glucose levels. The mechanism of the anti-diabetes effects in both GK rats and KK-A y mice is related to gluconeogenesis and glycolysis.
The body-weight gains of the KK-A y mice were different from those of the GK rats. Although the total food intakes did not differ among KK-A y mice, the body-weight gains in the KKAy-CON group were significantly lower than in the KKAy-PA group. As the epididymal adipose tissue weights in the KKAy-CON group were less than in the KKAy-PA group, therefore, lower body-weight gains may be associated with a decrease in this tissue weights. In addition, although the serum and liver TAG levels in the GK-PA group were lower than in the GK-CON group, those in the KK-A y mice did not differ significantly between the KKAy-CON and KKAy-PA groups. When the activities of enzymes concerned with lipid metabolism were measured, the FAS activities of the animals fed PA were significantly lower than the CON animals in both GK rats and KK-A y mice. In the GK rats, the CPT activities in the GK-PA group were higher than those in the GK-CON group, but there was no difference between the two groups in the KK-A y mice. These results suggest that PA is effective in inhibiting the accumulation of lipids in animals without severe obesity, such as in GK rats. Higher CPT activities in the GK-PA group compared with the GK-CON group suggest that lower body weight in the GK-PA group was the result of inhibiting the accumulation of fatty acids by PA. However, the reason for the higher body-weight gains in the KKAy-PA group than in the KKAy-CON group in the KK-A y mice study needs to be examined in the future. Hofmann et al. reported that the body weight of KK-A y mice decreased when diabetes accompanied with insulin resistance progressed, and that animals treated with an insulin-sensitising agent (pioglitazone) gained body weight(Reference Hofmann, Lorenz and Braithwaite36). In severe insulin resistance, a condition is induced wherein the intake of glucose is prevented in the liver, muscle and adipose tissue. It is conceivable that protein and lipid are used as energy sources instead of glucose. This metabolic change in the KKAy-PA group might account for the higher weight gain than in the KKAy-CON group.
Effects of PA on TNF-α levels also showed differences between GK rats and KK-A y mice. In the experiment with GK rats, TNF-α levels in the GK-PA group were no different from those in the GK-CON group. However, in KK-A y mice, TNF-α levels of mice fed PA were significantly lower than in the KKAy-CON group, suggesting that TNF-α is produced from macrophages migrated to an enlarged fat cell(Reference Suganami, Nishida and Ogawa37). However, there is no equilateral correlation between TNF-α levels and adipose weights in the present experiment. In the KKAy-PA group, the epididymal adipose weights were higher, but the size of the cells may be smaller than in the KKAy-CON group. Equilateral correlation in TNF-α levels and homeostatic model assessment of insulin resistance in the KK-A y mouse experiment was found. Because TNF-α is one of the factors that causes insulin resistance(Reference Reaven38, Reference Hotamisligil, Shargill and Spiegelman39), dietary PA intake inhibited one factor associated with insulin resistance.
From these results, we conclude that dietary PA is available to mitigate diabetes by diminishing insulin resistance, and serum and liver lipid levels, and by regulating the gene expression of glucose and lipid metabolism. Though non-obesity or obesity type, some mechanisms seem to be different for an anti-diabetic or an anti-hyperlipidaemic effect. In addition, side effects in long-term use and anti-diabetic effects in human consumers are not known. A further study will be necessary in the future.
Acknowledgements
No funding was received.
O. Y. designed and conducted the research, analysed data and wrote the paper. K. I. had primary responsibility for the final content. All authors read and approved the final manuscript.
There are no conflicts of interest to disclose.











