INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is widely recognized as a major cause of nosocomial infection worldwide and the risk factors for infection with these pathogens in hospital populations are well established [Reference Lowy1]. Although, traditionally considered to be a nosocomial pathogen, it is evident that the epidemiology of MRSA infections is rapidly changing.
During the 1990s, various reports of community-associated MRSA (CA-MRSA) infections in healthy individuals appeared in the literature, caused by novel strains which were genetically distinct from traditional healthcare-associated MRSA (HA-MRSA) [Reference Chambers and Deleo2]. CA-MRSA were traditionally regarded as MRSA strains causing infection in previously healthy young patients without prior healthcare contact, generally susceptible to non-β-lactam antibiotics, often carrying Panton–Valentine leukocidin (PVL) encoding genes, and of staphylococcal cassette chromosome mec (SCCmec) types IV or V [Reference Naimi3]. By contrast HA-MRSA predominantly caused infections in patients exposed to healthcare settings, exhibited resistance to most non-β-lactam antibiotics and harboured SCCmec types I, II and III [Reference Zetola4].
Consequently, as the microbiology and epidemiology of CA-MRSA have evolved, these traditional definitions have broken down, arguing in favour of genotypic classification of strains. As a result there is a growing consensus to define MRSA strains by combinations of genotyping methods such as multi-locus sequence typing (MLST), spa gene type and/or pulsed-field gel electrophoresis (PFGE) with SCCmec analysis to infer their likely epidemiological origin [Reference Otter and French5].
Numerous lineages of CA-MRSA have since emerged on every continent, several of which have spread internationally [Reference David and Daum6, Reference Deleo7]. Currently, more than 20 distinct genetic lineages have been identified worldwide [Reference Mediavilla8], five of which are globally distributed, including ST1-IV (WA-1, USA400), ST8-IV (USA300), ST30-IV (South West Pacific clone), sequence type (ST)59-V (Taiwan clone), and ST80-IV (European clone) [Reference David and Daum6, Reference Deleo7, Reference Witte9]. Among the latter, ST8-IV and ST30-IV may be considered pandemic, as they have been isolated repeatedly from every continent [Reference Deleo7, Reference Monecke10].
CA-MRSA strains are increasingly implicated in nosocomial infections. Outbreaks of HA infections caused by CA-MRSA strains have been reported from Australia and the USA suggesting that such strains are spreading in healthcare settings and are replacing traditional HA-MRSA strains in some places [Reference Maree11, Reference Valsesia12]. Various lineages typically associated with HA-MRSA, such as ST22-IV (EMRSA-15), are also increasingly identified in CA-MRSA infections [Reference Witte9, Reference Monecke10]. In recent years, ST772-V (Bengal Bay clone) has emerged as a virulent and unusually resistant CA-MRSA strain in Bangladesh and India [Reference D'Souza, Rodrigues and Mehta13–Reference Shambat15], and its spread to the UK and Europe has been documented [Reference Monecke10, Reference Brennan16].
MRSA is a widespread problem in Indian hospitals, where strains have been well characterized [Reference Gadepalli17]. Previous molecular typing of nosocomial MRSA strains recovered from our hospital revealed a similar scenario to that documented for several Indian hospitals where the multi-resistant Brazilian and Hungarian epidemic clone (ST239-MRSA-III) was dominant [Reference Gadepalli17–Reference Abimanyu, Murugesan and Krishnan20]. Recently, MRSA strains carrying SCCmec types IV and V were identified in Indian hospitals in and around Bengaluru and Mumbai where ST772-MRSA-V along with ST22-MRSA-IV are increasingly prevalent and appear to have progressively displaced the previously predominant nosocomial ST239-MRSA-III clone [Reference D'Souza, Rodrigues and Mehta13–Reference Shambat15]. However, these studies did not determine the differences in clinical, demographic and microbiological characteristics of SCCmec type IV/V MRSA strains isolated from patients with and without healthcare exposure. The aim of the present study was to determine whether CA-MRSA SCCmec type IV and type V strains have emerged in our tertiary-care hospital, located in New Delhi, the capital city of India and to analyse the clinical characteristics and clonal diversity of SCCmec IV and SCCmec V MRSA strains from patients with HA skin and soft tissue infections (SSTIs). In addition, we investigated the effects of hospital exposure on SCCmec IV/V MRSA isolates by comparing them with SCCmec IV/V MRSA genotypes isolated from outpatients not exposed to the healthcare setting.
METHODS
This descriptive study was conducted from July 2009 to December 2011 at the All India Institute of Medical Sciences, a 2500-bed teaching hospital providing tertiary care, located in the city of New Delhi, India. The study was approved by the Ethics Committee of the All India Institute of Medical Sciences, New Delhi, India (No. A-25).
Epidemiological definitions
A HA-MRSA SSTI case was defined as one that met one or more of the following criteria: the organism was isolated >48 h after admission to the hospital, the patient had been hospitalized or undergone surgery in the year prior to the MRSA-positive culture results; or an in-dwelling device or percutaneous catheter was present at the time the SSTI specimen for culture was obtained [21]. A case of SSTI infection was classified as CA-MRSA if MRSA was identified in the outpatient setting or <48 h after hospital admission in an individual with no medical history of MRSA infection or colonization, admission to a healthcare facility, dialysis, surgery or insertion of in-dwelling devices in the past year [Reference Otter and French5].
Patients
To find patients with HA-MRSA SSTIs, we identified all skin and soft tissue cultures obtained >48 h after hospitalization that grew as MRSA. A total of 237 patients including both adults and children were eligible for enrolment, but 28 were unavailable to study personnel because of discharge before contacting them; another nine patients refused to give their consent. Thus, a total of 200 patients with HA-MRSA SSTIs were enrolled in the study. To find patients with CA-MRSA SSTIs, we identified all skin and soft tissue cultures obtained from outpatients that grew as MRSA and had no HA risk factor in the past year prior to soft tissue culture. A total of 112 patients were eligible for enrolment; however, seven were unavailable to study personnel because of delay in contacting them and another five patients refused to give their consent. Thus, a total of 100 patients with CA-MRSA SSTIs were enrolled. For analysis, we examined only data from the first positive culture. The medical records of each patient were reviewed for patient demographics and clinical information.
Bacterial strains
A total of 300 MRSA strains (200 HA-MRSA, 100 CA-MRSA) were available for the study. All staphylococci were identified by standard biochemical tests. Susceptibility to oxacillin was determined by the cefoxitin (30 μg) disc diffusion test as recommended by Clinical and Laboratory Standards Institute (CLSI) [22]. Strains were confirmed as MRSA by detection of the femB gene and mecA gene using multiplex PCR [Reference Perez-Roth, Claverie-Martin and Batista23].
Susceptibility testing
Susceptibility testing was performed on all MRSA isolates using the disc diffusion method to 15 antimicrobial agents: amikacin (30 μg), gentamicin (10 μg), netilimicin (30 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), levofloxacin (5 μg), clindamycin (2 μg), co-trimoxazole (1·25/23·75 μg), erythromycin (15 μg), fusidic acid (10 μg), linezolid (30 μg), mupirocin (200 μg), rifampicin (5 μg), teicoplanin (30 μg) and tetracycline (30 μg). Antimicrobial susceptibility was interpreted according to CLSI criteria. A vancomycin (6 μg/ml) agar screen was used to detect intermediate resistance to vancomycin [22]. Multi-drug resistance (MDR) was defined as resistance to ⩾3 non-β-lactam antibiotic classes [Reference Fey24].
Molecular typing
All MRSA isolates were subjected to SCCmec typing and detection of PVL genes. SCCmec elements (I–V) and PVL genes luk F–lukS were identified as described previously [Reference Milheirico, Oliveira and de Lencastre25, Reference Lina26]. Based on SCCmec types, the HA-MRSA isolates were further divided into HA-SCCmec I/II/III MRSA and HA-SCCmec IV/V MRSA. Similarly, CA-MRSA isolates were grouped into CA-SCCmec I/II/III MRSA and CA-SCCmec IV/V MRSA. Multiplex PCR detection of the seh gene (as a marker for CA-MRSA of clonal lineage ST1/USA400 and arcA gene as part of the ACME (arginine catabolic mobile element) cluster for ST8/t008/USA300 were performed on all SCCmec IV isolates as described previously [Reference Strommenger27]. Controls for the assay used were previously characterized reference isolates 05-012 90t127/ST1/she lukPV and 06-011 72t008/ST8/arcA lukPV (kindly provided by B. Strommenger, Robert Koch Institute, Germany).
PFGE was performed using SmaI-digested DNA as described previously [Reference Udo, Al-Mufti and Albert28] with selected MRSA strains of HA-SCCmec III, HA-SCCmec IV/V and CA-SCCmec IV/V having distinct resistance profiles. Comparison and grouping of the PFGE patterns were performed with InfoQuest™ FP Software v. 5·4 (Bio-Rad Laboratories, USA). PFGE patterns were compared on an unweighted pair-group method with averages (UPGMA) dendrogram based on Dice coefficients, where optimization and band position tolerance were set at 1·0% and 2·3%, respectively [Reference Faria29]. A similarity coefficient of 80% was selected to define the patterns [Reference McDougal30].
MLST and spa typing were performed on selected representative isolates of major PFGE patterns in different SCCmec types as described previously [Reference Enright31, Reference Harmsen32]. Isolates were assigned a ST according to the MLST website (http://www.mlst.net) and sequencing scape (Seqscape software, Applied Biosystems, USA).
Spa typing, based on DNA sequencing of the protein A gene variable region was performed, and isolates assigned as described on the Ridom website (http://spa.ridom.de/).
Statistical analysis
Each study variable was compared between SCCmec types. Quantitative variables were summarized as mean ± s.d. and qualitative variables as proportions (%). Odds ratios (ORs) were calculated from logistic regression analysis for SCCmec type IV and type V, along with the 95% confidence intervals (CIs). The ORs for drug resistance were calculated separately for HA-SCCmec I/II/II vs. HA-SCCmec IV/V; and CA-SCCmec IV/V vs. HA-SCCmec IV/V. The proportion of strains resistant to different drugs in different major clones of CA-MRSA was compared using Fisher's exact test. Multivariable logistic regression analysis was performed to identify the variables independently associated with HA-SCCmec IV/V vs. HA-SCCmec I/II/II, and CA-SCCmec IV/V. All tests were two-tailed and a P value ⩽0·05 was considered to be statistically significant. Stata v. 12·1 (StataCorp., USA) was used for all analyses.
RESULTS
SCCmec type, PVL genes and characteristics of patients with HA-MRSA SSTIs
The majority of the 200 HA-MRSA patient isolates investigated carried SCCmec III (n = 116, 58%), followed by SCCmec V (n = 46, 23%) and SCCmec IV (n = 29, 14·5%). Very few isolates carried SCCmec II (n = 5, 2·5%) and SCCmec I (n = 4, 2%). Thus, of this patient cohort classified epidemiologically as having HA-MRSA SSTIs, 125 (62·5%) were infected with HA-SCCmec I/II/III MRSA and 75 (37·5%) with MRSA of HA-SCCmec IV/V lineages. Forty (20%) isolates carried the genes for PVL, which was strongly associated with SCCmec IV and V MRSA strains (86·2% and 26·1%, respectively, carried PVL genes; P < 0·001).
The clinical and epidemiological data of the two groups of inpatients carrying HA-SCCmec IV/V (75) or HA-SCCmec I/II/III (125) were compared to assess associations between carriage of these types and particular risk factors (Table 1). Multivariable analysis confirmed that isolation of HA-SCCmec IV/V MRSA was associated with PVL gene carriage (OR 40·81, 95% CI 11·38–146·32, P < 0·001) and HA-SCCmec I/II/III MRSA was associated with burn wounds (OR 0·09, 95% CI 0·008–1·00, P = 0·050).
Table 1. Characteristics of patients with HA-MRSA, skin and soft tissue infection as per SCCmec type isolated (n = 200)
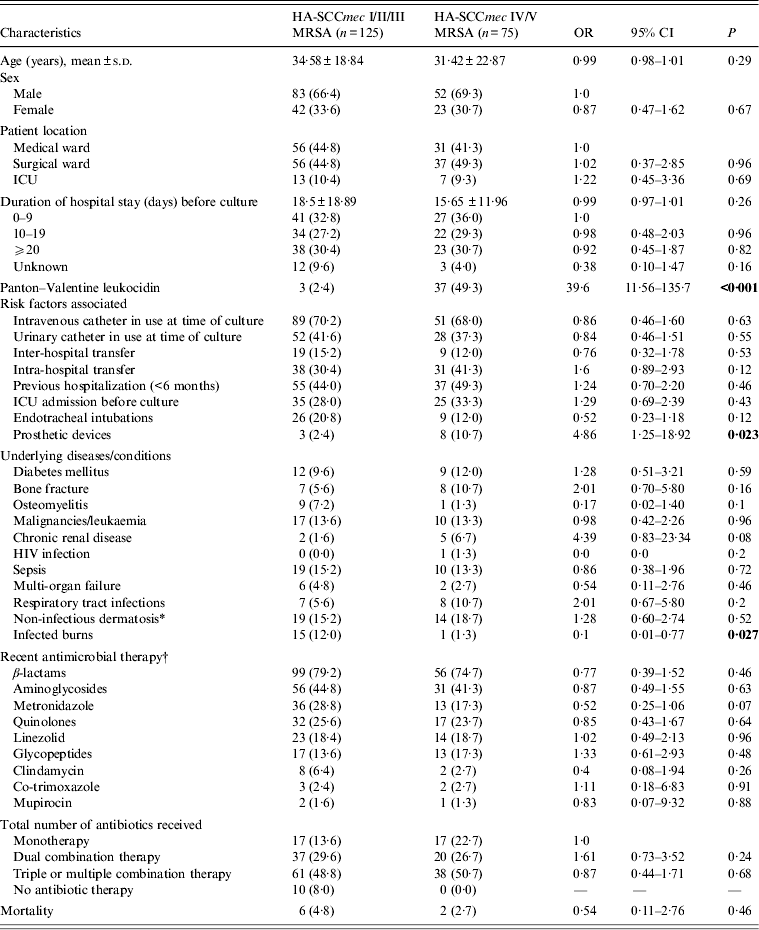
OR, Odds ratio; CI, confidence interval; ICU intensive care unit.
Data are no. (%) of patients, unless otherwise specified.
* For example, psoriasis, eczema, pemphigus vulgaris, etc.
† Preceding antibiotics used for the condition for which the patient was hospitalized.
P values <0·05 are highlighted in bold.
SCCmec type, PVL genes and characteristics of patients with CA-MRSA SSTIs
The most common SCCmec types among 100 isolates from this patient cohort were types V (n = 45), IV (n = 38) and III (n = 17). Interestingly, 65·8% of SCCmec IV isolates carried the PVL gene compared to only 26·7% of type V (P < 0·001). The 83 isolates of types IV and V were designated as CA-SCCmec IV/V MRSA and subjected to further analysis. The demographic and clinical characteristics of these patients were compared with the 75 patients with HA-SCCmec IV/V MRSA SSTIs (Table 2). The mean age of all patients in both cohort groups was 27·95 ± 20·28 years and 104 (65·8%) were male. Multivariable regression analysis identified malignancy (OR 10·57, 95% CI 1·23–90·49, P = 0·03) and bone fractures (OR 12·33, 95% CI 1·47–103·34, P = 0·02) as independent risk factors significantly associated with HA-SCCmec IV/V MRSA SSTIs.
Table 2. Association of study characteristics in patients with CA-SCCmec type IV/V and HA-SCCmec type IV/V infections
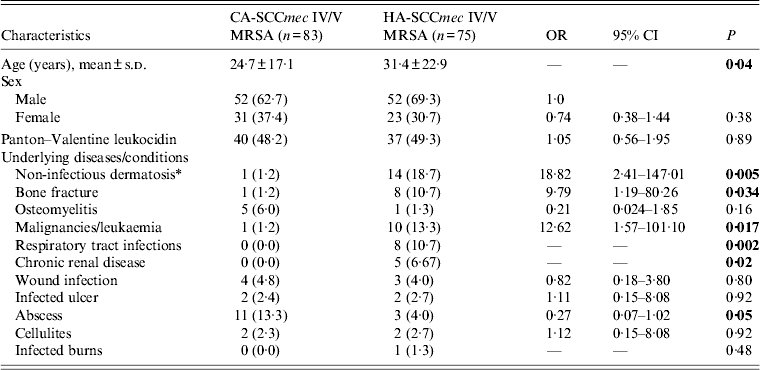
OR, Odds ratio; CI, confidence interval.
Data are no. (%) of patients, unless otherwise specified.
* For example, psoriasis, eczema, pemphigus vulgaris, etc.
P values <0·05 are highlighted in bold.
Antimicrobial resistance
The distribution of resistance to antimicrobials of the MRSA isolates grouped by healthcare exposure status and SCCmec type are presented in Table 3. Compared with HA-SCCmec I/II/III MRSA isolates, a greater proportion of HA-SCCmec IV/V isolates were susceptible to all the antibiotics tested. However, compared to CA-SCCmec IV/V MRSA isolates, a higher proportion of HA-SCCmec IV/V isolates were antibiotic resistant. Ninety-five (76·0%) HA-SCCmec I/II/III isolates and 32 (42·7%) HA-SCCmec IV/V isolates were MDR (P < 0·001) whereas only five (6·0%) of CA-SCCmec IV/V MRSA isolates were MDR (P < 0·001).
Table 3. Antimicrobial resistance patterns of methicillin-resistant Staphylococcus aureus (MRSA) isolates from patients with skin and soft tissue infections grouped by SCCmec type and healthcare exposure status*
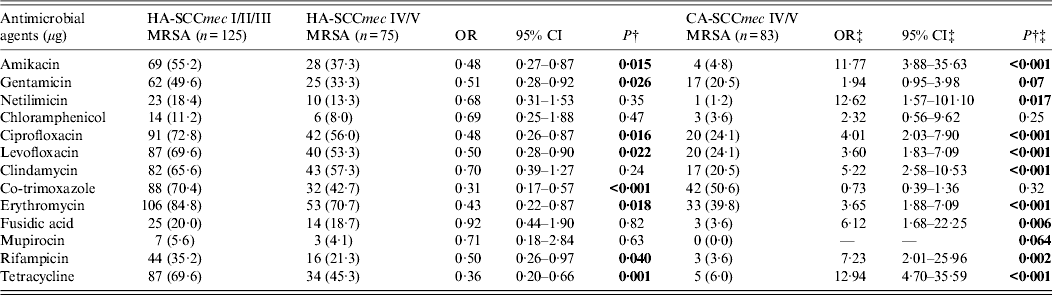
OR, Odds ratio; CI, confidence interval.
* According to the CDC definition [Reference Deleo7].
† All P values determined using odds ratios and not derived from t test or χ 2 test. P values <0·05 highlighted in bold.
‡ Analyses of antimicrobial resistance patterns of HA-SCCmec-IV/V and CA-SCCmec-IV/V MRSA isolates. All strains were susceptible to vancomycin (screen agar method), linezolid and teicoplanin.
P values <0·05 are highlighted in bold.
Clonality of CA-SCCmec IV/V, HA-SCCmec IV/V MRSA and HA-SCCmec III isolates
The SmaI macrorestriction fragment profiles of 48 HA-SCCmec IV/V, 41 CA-SCCmec IV/V MRSA and 20 HA-SCCmec III MRSA isolates revealed 19 clones with four major clusters predominating (clone 4, n = 32, 29·4%; clone 14, n = 22, 20·2%; clone 6, n = 15, 13·8%; clone 1, n = 11, 10·1%) and together representing 80% of all isolates tested (Fig. 1). Each major clone comprised 2–10 subtypes. Except for clone 6, which was exclusive to the HA-SCCmec III MRSA group, the remaining three major clones were found in each patient cohort with SCCmec IV/V MRSA isolates. Clone 4 isolates carried only SCCmec IV and were detected in both CA- (40·6%) and HA- (59·4%) SCCmec IV isolates, often exhibiting PVL carriage (75%). Similarly, clone 14 was highly associated with SCCmec type V (86·4%) and less so with SCCmec type IV (13·2%) isolates; 50% of this clone carried PVL. Clone 1 comprised only SCCmec IV isolates and these often exhibited PVL (81·8%).

Fig. 1. Dendrogram based on similarities derived from the unweighted pair group method with arithmetic mean (UPGMA) and Dice coefficients, using InfoQuest™ FP software (Bio-Rad). The scale at the top represents similarity. Pulsed-field gel electrophoretic patterns of SmaI macrorestriction fragments of EMRSA-1, -15, -16, MW2, WIS, 655 U, Mu-50 along with representative strains of the SCCmec types IV/V major clones 1, 4, 6 and 14.
Comparison of local with international clones
PFGE was performed with epidemic MRSA strains (EMRSA)-1, -15, -16 from the UK, MW2, WIS, 655 U and Mu-50 (vanomycin-intermediate S. aureus) along with representative strains of major clones found here (Fig. 1). The predominant clone 4 showed close similarity (>90%) in DNA pattern to the epidemic UK EMRSA-15 clone. Of note, none of the isolates showed PFGE patterns of the USA400 CA-MRSA lineage. In addition, all HA-SCCmec IV and CA-SCCmec IV MRSA isolates were negative for arcA and seh genes.
Antimicrobial resistance patterns in different clones
The resistance to various antimicrobials varied from clone to clone and there was no correlation with resistance profiles and specific clones. Nevertheless, two broad groups were evident: one with isolates resistant only to β-lactams or 1–3 other antibiotics (clones 1, 2, 4, 12–17), and those resistant to almost all antibiotics tested (clones 3, 5–11, 18, 19). Isolates of clone 6 showed more resistance than other clones, with ~80% resistance to fluroquinolones, tetracycline, erythromycin and clindamycin; clone 4 was uniformly susceptibility to gentamicin, netlimicin, amikacin and co-trimoxazole.
Spa typing and MLST
MLST and spa typing were performed on 38 representative strains of the major clones 1, 4, 6 and 14 (n = 30) and sporadic clones (n = 8). Table 4 shows that the strains were assigned to 14 STs and 17 spa types. The 12 SCCmec V strains belonged to four STs and spa types with eight of them identified as ST772-SCCmec-V-t657 and the remainder were each unique. The SCCmec IV strains (13) clustered in four STs and the dominant ST22 consisted of diverse spa types. The 13 SCCmec III strains were grouped into six STs and spa types with 10 strains belonging to ST239 or the closely related ST241, ST1097 and ST1310.
Table 4. SCCmec, PVL status, MLST and spa typing for selected strains of the major and sporadic PFGE clones
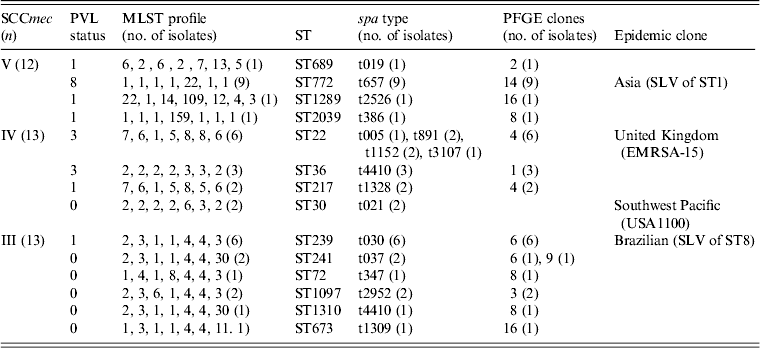
PVL, Panton–Valentine leukocidin; MLST, multi-locus sequence typing; ST, sequence type; PFGE, pulsed-field gel electrophoresis; SLV, single locus variant.
DISCUSSION
Our study documents the emergence of MRSA isolates typical of CA genotypes in patients with HA-MRSA SSTIs in an Indian hospital. Remarkably, at our institute a large proportion (37·5%) of isolates classified epidemiologically as HA-MRSA had a CA genotype. These data confirm the reported spread of CA-MRSA SCCmec IV/V strains in hospital settings in Europe, the USA [Reference Klevens33, Reference Otter and French34] and India [Reference D'Souza, Rodrigues and Mehta13, Reference Shambat15]. Given the vulnerable population within the hospital setting, it is unclear how infections with isolates that contain SCCmec IV/V may differ in symptoms and severity from those caused by the traditional HA-SCCmec I/II/III isolates. The introduction of SCCmec IV/V strains into our hospital population did not result in a change in the spectrum or severity of illness in this group. Compared to patients with CA-SCCmec IV/V SSTIs, it was observed that HA-SCCmec IV/V SSTIs were significantly associated with malignancy and bone fractures. Patients with these conditions are predisposed to use healthcare facilities, are generally exposed to antibiotics, tend to have interventions performed, and hence present opportunities to contract MRSA in the healthcare facility. Exactly why the SCCmec IV/V strains are successful in hospital settings such as ours remains unknown but mathematical models predict the replacement of traditional HA-MRSA strains by CA-MRSA strains, due to their higher growth rate and greater genetic fitness [Reference D'Agata35, Reference Popovich, Weinstein and Hota36].
In this study, as expected, HA-SCCmec IV/V isolates were more susceptible to multiple antibiotics compared to HA-SCCmec I/II/III. However, similar to previous reports [Reference Maree11], HA-SCCmec IV/V strains demonstrated higher antimicrobial resistance rates compared to CA-SCCmec IV/V strains. Differences in antimicrobial susceptibility indicate that, in patients with healthcare contact, SCCmec IV/V MRSA strains exhibit characteristics of traditional HA-MRSA strains. Our findings support the hypothesis of Gonzalez et al. [Reference Gonzalez37] that as typical CA-MRSA strains proliferate in healthcare settings, where antimicrobial selection pressures are high, they will continue to acquire additional antimicrobial resistance genetic elements, causing them to appear similar to more traditional MRSA isolates, with respect to resistance profiles. Our finding of PVL genes in 49% of HA-SCCmec IV/V isolates is not surprising since SCCmec IV/V strains have been associated largely with SSTIs and PVL production [Reference Valsesia12]. However, the sporadic (2·6%) PVL-positive SCCmec III HA-MRSA isolates is an issue of potential concern and could result in the emergence of MDR HA-MRSA isolates with increased virulence, despite the fact that the role of PVL as a virulence factor is a matter of much debate.
Molecular typing revealed that 73% of the isolates belonged to four major genetic lineages, with the remaining sporadic isolates showing a high degree of genetic diversity, suggesting the possibility of new strains being imported into the hospital from the expanding community reservoir. Interestingly, the highly infectious CA-MRSA strains USA300 and USA400 were not detected in our hospital or community. Previous characterization of nosocomial MRSA strains recovered in our hospital from patients with SSTIs identified a major MRSA clone closely related to the ST239:EMRSA-I:III which was MDR [Reference Gadepalli17]. In the present study, selected strains of the major PFGE clone 6 also showed genetic relatedness to the lineage ST239:EMRSA-I:III with spa-type t030. ST239 is the major endemic HA-MRSA clone in many Asian countries, although recent studies show that it is being gradually replaced by emerging CA-MRSA clones [Reference D'Souza, Rodrigues and Mehta13–Reference Shambat15] as was also observed in our study.
The ST22-SCCmec IV MRSA (EMRSA-15) strain is a global pandemic HA-MRSA clone and interestingly it was recovered from both inpatients and outpatients in our hospital. This suggests that outpatients may represent an important reservoir for MRSA dissemination within the hospital, when admitted as inpatients and reinforces the observation of Alcoceba et al. [Reference Alcoceba38] regarding the movement of EMRSA-15 from the community to the hospital setting.
The SCCmec V MRSA isolates that were genotyped were of spa type t657 and ST772, similar to the types reported recently in Mumbai and elsewhere in India [Reference D'Souza, Rodrigues and Mehta13–Reference Shambat15]. The decreasing prevalence of the HA-MRSA strain ST239-MRSA-III in hospital in India since 2006, coupled with an increase in prevalence of ST772-MRSA-V and ST22-MRSA-IV, has led to the suggestion that these strains may be replacing the ST239-MRSA strain in Indian hospitals [Reference D'Souza, Rodrigues and Mehta13–Reference Shambat15]. The emergence of the MDR clone ST772-MRSA-V in our hospital is a worrying development, and is indicative of the need for enhanced surveillance to ensure that these strains do not spread.
Another relevant finding of the present study concerns the presence of the ST36:EMRSA-16:IV (major PFGE clone 1) clones of spa-type t4410. Similar to the finding of Söderquist et al. [Reference Söderquist, Berglund and Strålin39], it was found to have the same ST as the EMRSA-16 (ST36-MRSA-II) strain, which is one of the predominant EMRSA strains in Europe. Furthermore, the described isolate was PVL positive, as were the majority of CA-MRSA strains, but this differs from EMRSA-16 ST36, which is PVL negative. The PFGE pattern of the isolate differed by >6 bands compared to EMRSA-16. To our knowledge, this is the first report of the ST36:EMRSA-16:IV clone in an Asian hospital.
This study had certain limitations. All of the observations were derived from a single hospital and therefore, may only reflect local trends and, thus, might not be representative of other settings in India or other countries. Further, only representative SCCmec IV/V strains of the major PFGE clones were genotyped by MLST and spa typing. Nevertheless, our data clearly demonstrate that the SCCmec type IV and type V epidemic clones of ST22 (EMRSA-15) and ST772 have entered into our hospital and now cause a substantial proportion of serious HA-MRSA infections.
In summary, the high proportions of HA-MRSA strains carrying SCCmec types IV and V, together with the considerable occurrence of PVL-positive MRSA strains, confirm the extensive infiltration of CA-MRSA genotypes in our hospital. We found that, following healthcare exposure, SCCmec IV/V strains have characteristics similar to those of other HA-MRSA strains isolated from these patients. Moreover, HA-SCCmec IV/V strains exhibited an antimicrobial susceptibility pattern in-between those of CA-SCCmec IV/V and HA-SCCmec I/II/III. This finding raises concerns that CA-SCCmec IV/V and HA-SCCmec IV/V strains may exchange genetic material resulting in an organism uniquely adapted to produce aggressive SSTIs similar to CA-MRSA strains which carry PVL genes as well expressing resistance to multiple antimicrobial agents generally associated with the current SCCmec I/II/III strains. The dissemination of these epidemic CA-MRSA clones in both inpatients and outpatients represents a significant challenge to infection control.
ACKNOWLEDGEMENTS
We thank K. Hiramatsu, H. de Lencastre and B. Strommenger for kindly donating some of the prototype and reference strains used in this study. Financial support was received from the Indian Council of Medical Research (ICMR), New Delhi, India.
DECLARATION OF INTEREST
None.








